
Abstract
The advent of intracoronary stents has greatly increased the safety and applicability of percutaneous coronary interventions. One of the drawbacks of drug-eluting stents (DES) is the increased risk of late and very late stent thrombosis (ST). It was anticipated that the risks of ST after DES implantation would be solved with the advent of fully biodegradable scaffolds, which offer the possibility of transient scaffolding of the vessel to prevent acute vessel closure and recoil while also transiently eluting an antiproliferative drug to counteract constrictive remodelling and excessive neointimal hyperplasia. In spite of the enthusiasm for the concept of bioresorbable scaffolds, current clinical data on the Absorb bioresorbable vascular scaffold (BVS) have generated concerns about scaffold thrombosis (ScT) in both the early and late phases. However, the causes of ScT in both the early and late phases have yet to be fully elucidated. This article seeks to provide insights into the possible mechanical causes of ScT in the early and late phases with data stemming from intracoronary imaging (intravascular ultrasound and optical coherence tomography) of the currently published ScT cases following the implantation of BVS and reviews the practical recommendations for implantation of the BVS made by a group of experts.
Introduction
The advent of intracoronary stents has greatly increased the safety and applicability of percutaneous coronary interventions (PCI). Large-scale randomised trials and all-comer registries have shown excellent results of PCI with drug-eluting stents (DES) in terms of the need for repeat revascularisation. However, one of the drawbacks of DES is the increased risk of late and very late stent thrombosis (ST). Registries of all-comers treated with DES have shown late ST rates of 0.53% per year, with a continued increase to 3% over four years1. Post-mortem pathological specimens of DES revealed significant numbers of uncovered struts with evidence of a persistent inflammatory reaction around the stent struts2. Second-generation DES have solved some aspects of these problems, and the frequency of ST in second-generation DES (everolimus-eluting stent [EES]) has been reduced to 0.7% at a mean follow-up of 21.7 months3.
It was anticipated that ST in the late and very late phases after DES implantation would be solved with the advent of fully biodegradable scaffolds, which offer the possibility of transient scaffolding of the vessel to prevent acute vessel closure and recoil while also transiently eluting an antiproliferative drug to counteract constrictive remodelling and excessive neointimal hyperplasia. As drug elution and scaffolding are temporary until the vessel has healed, no foreign material potentially triggering very late scaffold thrombosis (ScT), such as non-endothelialised struts and drug polymers2, would remain in the vessel.
In spite of the enthusiasm for the concept of bioresorbable scaffolds, current clinical data on the Absorb bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA) have generated concerns about ScT in both the early and late phases4-8. Recently published meta-analyses have revealed that patients treated with a BVS had a higher risk of definite or probable ScT than those treated with a metallic EES (odds ratio [OR]: 1.99-2.09)9. However, the causes of ScT in both the early and late phases have yet to be fully elucidated.
The purpose of this review article is to provide insights into the possible mechanical causes of ScT in the early and late phases, with data stemming from intracoronary imaging (intravascular ultrasound [IVUS] and optical coherence tomography [OCT]) of the currently published ScT cases following the implantation of BVS.
DEFINITIONS OF SCAFFOLD THROMBOSIS
The sensitivity and specificity of the definition of ScT depend on the level of certainty required (a better judgement can be made in the presence of angiography or autopsy studies), and also on the accuracy of data available during adjudication. A standardised definition of ScT was proposed by the Academic Research Consortium (ARC) (Table 1). The ARC definition acknowledges these issues by establishing a gradation of certainty (definite, probable, and possible), and standardising the timing of ScT (acute, subacute, late, and very late), which may have different pathophysiological mechanisms and clinical implications. Acute or subacute can also be replaced by the term early ScT. Although ARC definitions added uniformity, they remain an imperfect balance of sensitivity and specificity: “definite” ScT is highly specific but probably underestimates true frequency, whereas “possible” ScT, although more sensitive, lacks diagnostic certainty. Most contemporary studies exclude the category of “possible” ScT and select the endpoints of “definite” or “probable” ScT to provide a balance of specificity and sensitivity. In this review article, we focus only on the definite ScT case reports to provide some insights into the mechanical causes of ScT using intracoronary imaging.
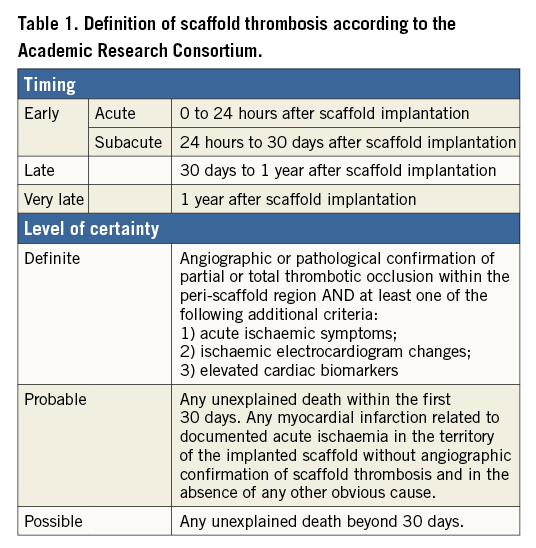
INCIDENCE OF SCAFFOLD THROMBOSIS
The incidence of ScT has been evaluated in clinical trials, registries and meta-analyses. The interpretation of data may vary depending on the study population and study design. As shown in Table 2-Table 4, the estimated rates of early definite, and definite or probable ScT ranged from 0.42% to 1.37%. The highest incidence of early ScT was observed in acute coronary syndrome (ACS) (1.37%) (Table 3) followed by an all-comer population (1.08%) (Table 4) and stable patients undergoing elective PCI (0.86%) (Table 2). This observation is similar to that of metallic DES; patients with ACS (0-3.1%) had a higher risk of early ST compared with stable patients (0.3-0.4%)10.
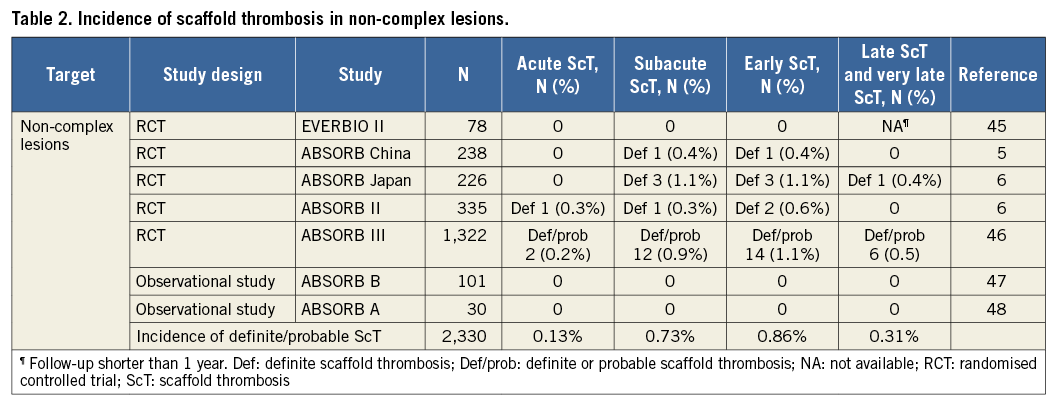
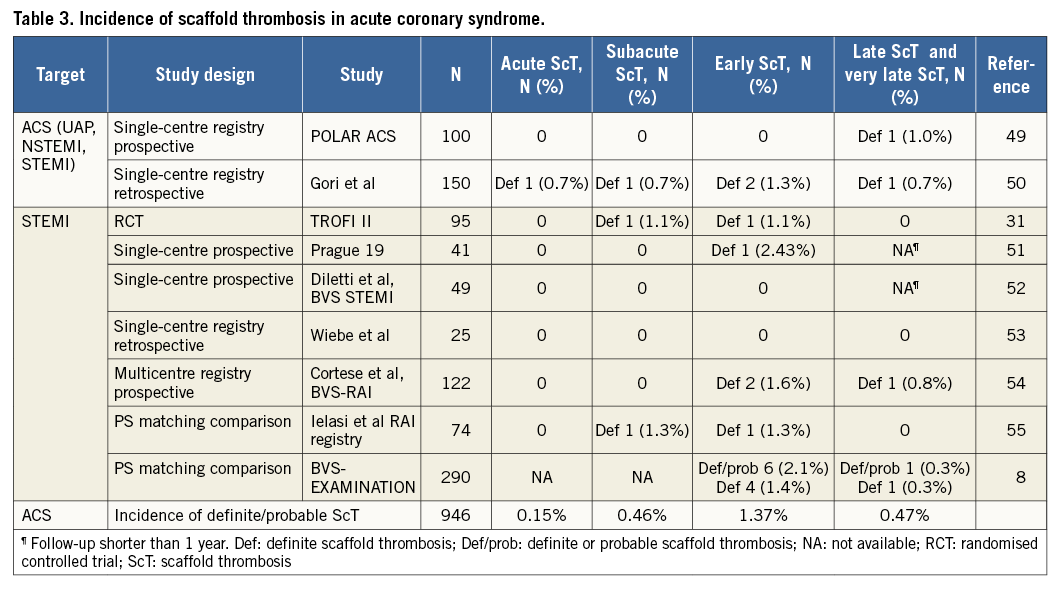
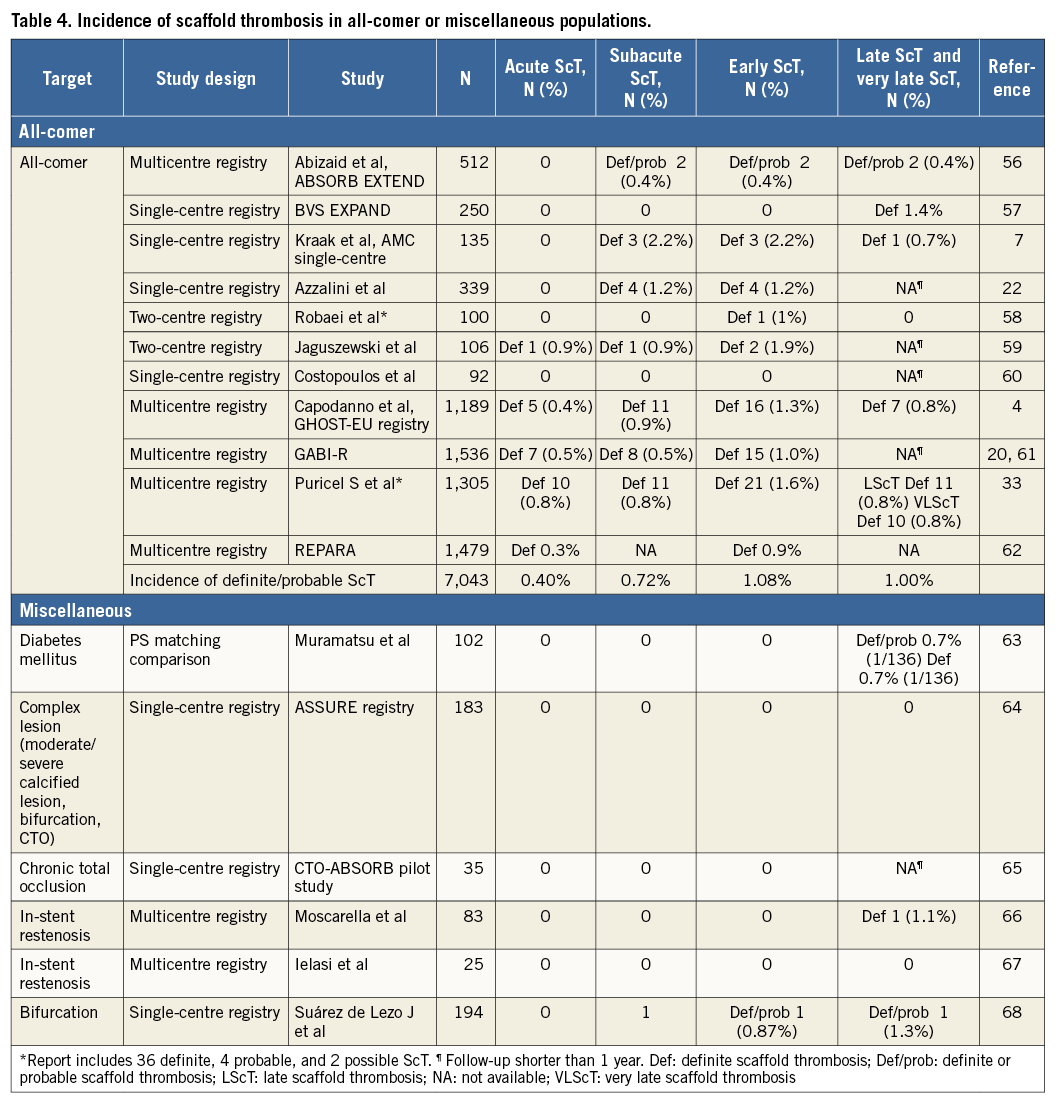
The incidence of late and very late ScT ranged from 0.31% to 1.00%. Of note, the incidence of ScT in non-complex and ACS lesions decreased over time (non-complex lesions, early ScT 0.86% vs. late and very late ScT 0.31% [Table 2]; ACS, 1.37% vs. 0.47% [Table 3]), whereas it was stable in an all-comer population (all-comers, 1.08% vs. 1.00% [Table 4]). The theoretical risk reduction of ScT in the late phase needs to be evaluated in further investigations with long-term follow-up.
Possible mechanical causes of scaffold thrombosis: insights from case reports with intracoronary imaging
Two independent reviewers (Y. Sotomi and P. Suwannasom) systematically searched (March 2016) MEDLINE/PubMed and available abstract data, applying the search terms “bioresorbable scaffold” and “thrombosis”. Data were limited to human studies using the Absorb BVS. Presentation slides of late-breaking clinical trials, including EuroPCR 2015 and TCT 2015 meetings in TCTMD.com, were also obtained. From the studies obtained, only the case reports and case series of ScT were focused upon.
In the current literature, 100 case reports of definite ScT (acute and subacute ScT, n=63; late and very late ScT, n=37) were identified4,6,11-31. Out of these cases, imaging insights with IVUS and OCT were available in five and 38 cases, respectively. The other 57 cases did not include intracoronary imaging assessment at the time of the ScT event. Table 5 and Table 6 summarise the imaging findings of the 17 early ScT cases and 26 late ScT cases assessed by IVUS and OCT. Representative examples of underlying possible mechanisms of ScT explored by OCT are summarised in Figure 1.
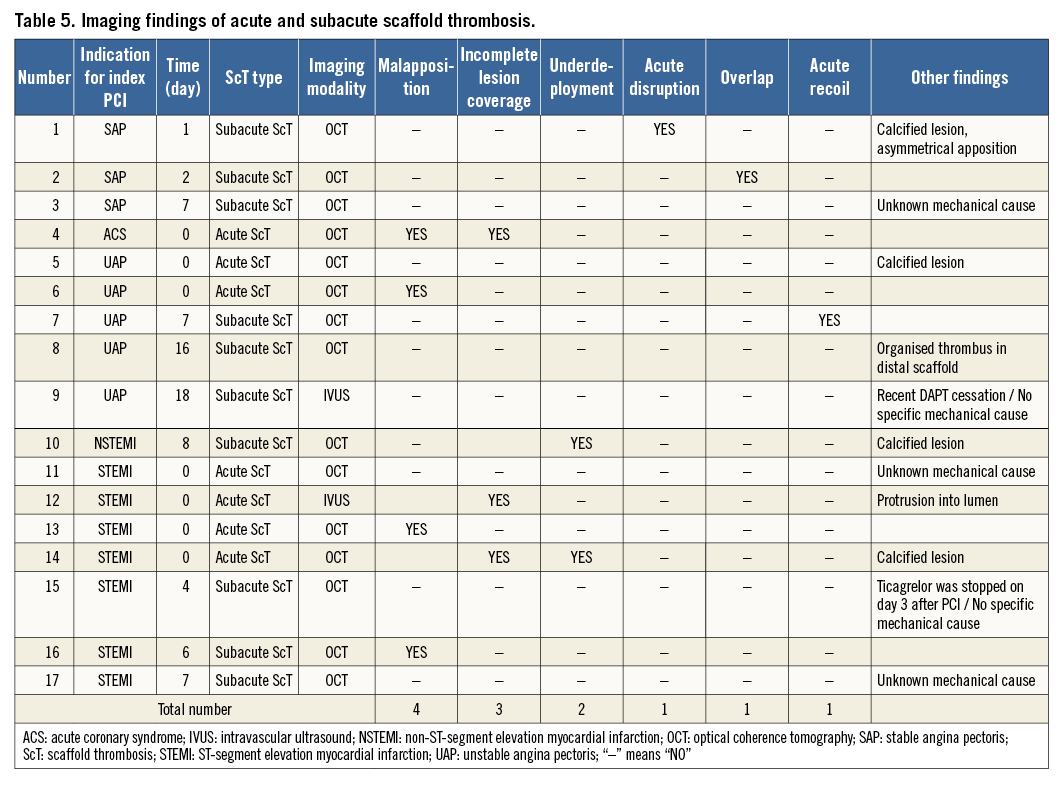
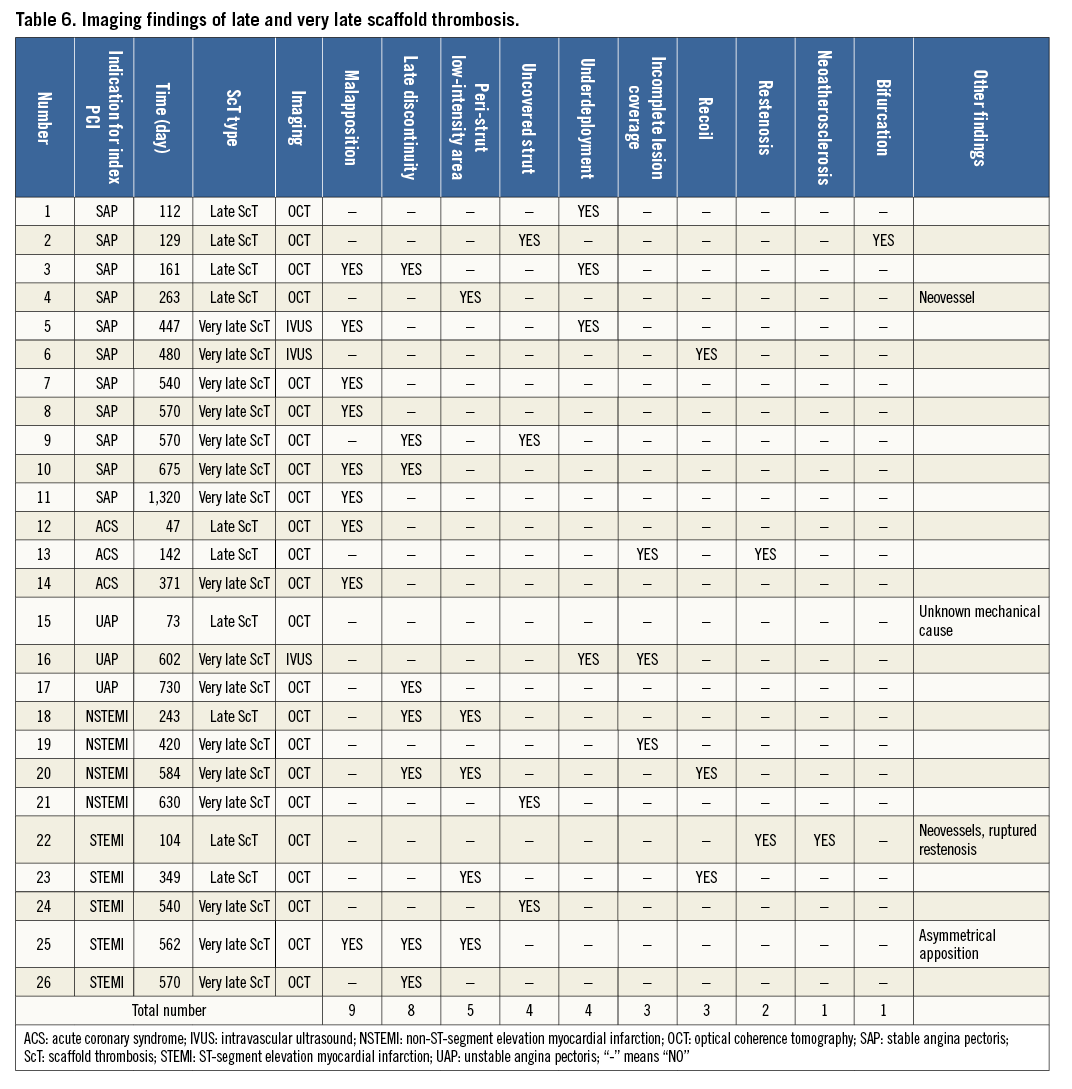
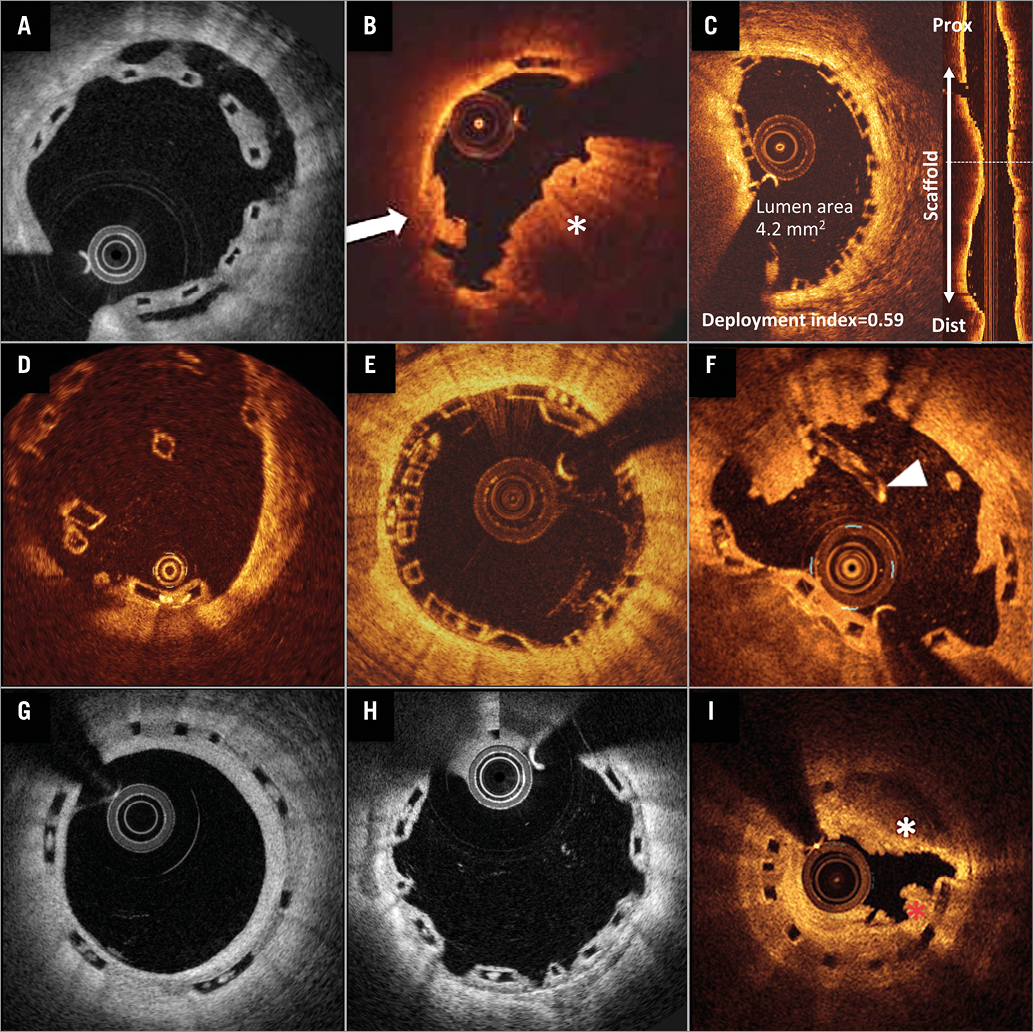
Figure 1. Representative examples of scaffold thrombosis underlying mechanisms explored by optical coherence tomography. In acute and subacute ScT, strut malapposition (A), incomplete lesion coverage (B, possible ruptured plaque [white arrow], uncovered thrombus [white asterisk]), and underdeployment (C) were the leading mechanical causes, followed by acute disruption (D) and overlap (E). In late and very late ScT, malapposition (A), late discontinuity (F, white arrowhead), and peri-strut low-intensity area (G) were the leading features, followed by uncovered struts (H) and neoatherosclerosis (I), mural thrombus (red asterisk) with highly attenuating area (white asterisk). Panel B reprinted with permission from Wolters Kluwer Health, Inc., Copyright (2015)14. Panel F reprinted with permission from Elsevier, Copyright (2015)18. Panel I reprinted with permission from Elsevier, Copyright (2015)44.
Acute and subacute scaffold thrombosis (Figure 2A)
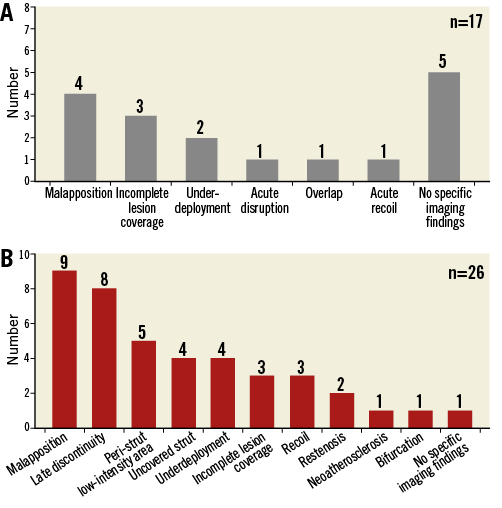
Figure 2. Imaging findings on scaffold thrombosis. Imaging findings on scaffold thrombosis in the acute/subacute (A) and late/very late (B) phases summarised in Table 5 and Table 6 are presented as bar graphs. The vertical axis shows the number of each finding observed in the 43 case reports (acute and subacute: n=17; late and very late: n=26).
MALAPPOSITION
In the current publications, malapposition (Figure 1A) was the most frequent imaging finding of early (acute and subacute) ScT. This could be induced by undersizing of the device, insufficient lesion preparation, inadequate post-dilatation, etc. Imaging-guided scaffold implantation is relatively uncommon in current clinical practice. Moreover, scaffold overexpansion has been intensively prohibited in order to avoid acute disruption. Therefore, it is highly probable that such situations lead to insufficient scaffold expansion in relation to the vessel, resulting in a higher rate of strut malapposition. Several reports have identified undersizing as a key factor for ST in both bare metal stents and drug-eluting stents10, which can mean that malapposition is a key factor for ST. As with the reports on metallic stents, the undersizing of polymeric scaffolds probably leads to scaffold strut malapposition which is the underlying possible mechanical cause of ScT.
INCOMPLETE COVERAGE OF LESIONS (GEOGRAPHICAL MISS)
Incomplete lesion coverage (Figure 1B) was observed either because of mismatch of the predilated segment and the scaffolded segment or because of incomplete coverage of the thrombosed segment in acute coronary syndrome (ACS). Due to the inherent mechanical properties of polymeric devices, BVS require rigorous lesion preparation, potentially translating to a higher risk of incomplete coverage of the injured segment, compared with direct stenting often applied with metallic stents. Incomplete coverage of thrombogenic plaque with altered flow dynamics due to the protrusion of polymeric struts into the lumen would synergistically contribute to the formation of thrombus17.
UNDERDEPLOYMENT/DEVICE-VESSEL MISMATCH
This may highlight the importance of lesion selection, optimal lesion preparation before bioresorbable scaffold implantation, and optimal scaffold sizing (Figure 1C). The implantation of a large Absorb scaffold in a relatively small vessel results in a relative underexpansion of the scaffold termed “underdeployment”, defined as the ratio of minimal scaffold area to the nominal area of the device deployed in vitro at a nominal pressure less than 0.80. Scaffold “underexpansion” is defined as the ratio of minimum scaffold area to the average reference lumen area less than 0.90. We need to use these two terms quite differently since these two conditions are completely dissimilar. The underdeployment of scaffolds could cause a high density of polymer in small vessels, which could be associated with an increased thrombogenicity and disturbance in the haemodynamic microenvironment around the struts32,33. Device oversizing has been associated with a higher risk of MACE at one-year follow-up after BVS implantation34.
ACUTE DISRUPTION
BVS pattern irregularity at baseline is considered as acute disruption of the BVS scaffold (Figure 1D)35. This irregularity ranged from local overhanging single struts shifted out of their expected pattern position during deployment, to complete pattern disruptions possibly involving structural discontinuities. At the time of implantation, the bioresorption process does not influence the mechanical integrity of the scaffold at all, so that any disrupted struts observed immediately after the procedure are the consequence of a mechanical disruption caused by extreme overexpansion of the scaffold. The reported incidence of scaffold pattern irregularities (acute disruption) was 3.9-5.8%35. However, the degree of disruption might also be important. Complete structural disruption could lead to loss of scaffold integrity, malapposition of struts, acute recoil of the device, resulting in ScT.
OVERLAP
Overlapping scaffolds (Figure 1E) inherently create malapposed struts at the inner scaffold, which potentially cause ScT. In addition, the structural complexity of overhanging and stacked struts at overlapping segments could be a potential nidus of thrombus due to the blood flow disturbance and alteration of endothelial shear stress.
SUMMARY
ACS was the predominant presentation in patients in the publications with early ScT. Mechanical features of strut malapposition, incomplete lesion coverage, and scaffold underdeployment could cause disturbance of laminar blood flow and thus alteration of endothelial shear stress, which synergistically induce early ScT with the inflammatory microenvironment in ACS lesions as discussed below.
Late and very late scaffold thrombosis (Figure 2B)
MALAPPOSITION
As observed in early ScT, strut malapposition was frequently observed in cases with late and very late ScT (Figure 1A). In addition to persistent incomplete strut apposition, recoil of scaffold, positive remodelling of the vessel (late acquired incomplete strut apposition), evagination, and late discontinuity35 could result in malapposition in the late phase. Struts at the ostium of a side branch can also be recognised as malapposed struts in a mechanical perspective.
LATE DISCONTINUITY
So-called late discontinuity is the programmed phenomenon in the bioresorption process of the polymeric device (Figure 1F). The incidence of late discontinuity was reported to be 42% in the ABSORB cohort B trial35. Six months after device implantation, the polymeric scaffold starts losing its mechanical integrity which can lead to expected late discontinuity. Theoretically, this is a benign change during the bioresorption process and does not cause any problems if struts are well covered. However, in cases where struts are not covered by neointima and late discontinuity allows protrusion of part of the struts into the lumen and brings thrombogenic proteoglycan into contact with blood, late discontinuity could be a malignant potential cause of ScT. “Uncovered” late discontinuity could be critical, whereas late discontinuity itself would not be a culprit of ScT. Therefore, enhancement of neointimal coverage would be a key to prevent ScT associated with late discontinuity. Prevention of malapposition by either a BVS-specific implantation strategy (described later) or OCT-guided implantation, and new-generation BVS with thinner struts could contribute to early neointimal coverage and a consequent reduction of the incidence of late and very late ScT.
PERI-STRUT LOW-INTENSITY AREA
Peri-strut low-intensity areas were defined as homogenously appearing, non-signal-attenuating zones around struts of lower intensity than the surrounding tissue (Figure 1G). Although the causes and consequences of peri-strut low-intensity areas remain uncertain in BVS, a trial in 26 coronary swine segments treated with everolimus-eluting DES demonstrated a direct correlation between the degree of peri-strut low-intensity area and peri-strut inflammation at histology36. Otsuka et al compared the vascular responses to the implantation of BVS versus a metallic everolimus-eluting cobalt-chromium stent in non-atherosclerotic swine37. Although there was no inflammation at one month for both devices, the inflammation scores were greater for the BVS at six to 36 months. Cuculi et al reported that the predominant OCT finding in late and very late ScT was peri-strut low-intensity area, which might be the correlate of vascular oedema, thus enhancing vascular vulnerability. However, the direct association of the peri-strut low-intensity area pattern with the occurrence of late and very late ScT is still unclear and needs to be evaluated in future studies.
UNDERDEPLOYMENT/ASYMMETRICAL APPOSITION/RECOIL RESTENOSIS
Lumen narrowing at the time of late and very late ScT was one of the predominant features of late and very late ScT. The mechanism of the restenosis cannot be precisely determined in the absence of serial intravascular imaging and visualisation of the external elastic membrane. Restenosis in the absence of significant neointimal proliferation can be explained by chronic recoil, plaque growth, or shrinkage of the external elastic membrane. Although neointimal hyperplasia was the cause of restenosis with metallic DES, restenosis in the absence of significant neointimal proliferation may occur late during the BVS bioresorption process. Careful examination with serial OCT assessments in future studies is warranted.
UNCOVERED STRUTS
A lack of strut coverage caused by delayed healing has been identified as a possible mechanism of late/very late ST after implantation of early-generation DES. It has also been observed that delayed neointimal coverage of BVS is a possible mechanical cause of late and very late ScT (Figure 1H). The one-year follow-up OCT study of BVS and EES demonstrated similar rates of uncovered struts (5.3% EES vs. 4.5% BVS; p=0.11)38. However, when the neointimal coverage of polymeric struts is delayed, the biodegraded products of polymeric struts can be an additional enhancer of ScT. The hydrolysis of a polymeric strut starts immediately after the device comes into contact with water. Afterwards, the polymer is progressively replaced by a malleable provisional matrix of proteoglycan which reportedly has a higher thrombogenicity than the polymeric material itself39. The exposed proteoglycan without neointimal coverage could be a hazardous cause of ScT.
OVERLAP
In the late phase, an overlapping scaffold could inherently create two potential mechanical causes, malapposition and incomplete coverage. Theoretically, the struts of the inner device at the overlap site cannot be apposed to the vessel wall. In a juvenile porcine model, overlapping BVS scaffolds showed more delay in tissue coverage than non-overlapping scaffolds. It is likely that the larger strut thickness of the stack-like BVS scaffolds (approximately 300 μm) in overlapping segments led to a greater neointimal response compared with that in the non-overlapping segments. Delayed coverage of overlapped struts presumably results from that greater neointimal response which is long-lasting.
NEOATHEROSCLEROSIS
Neoatherosclerosis has been identified as a mechanism related to very late ST in intracoronary imaging cohort studies. Macrophage accumulations are considered the earliest manifestations of neoatherosclerosis. In some case reports, neoatherosclerosis was observed in the scaffolded vessels, but not as the direct cause of ScT (Figure 1I). No obvious correlation between neoatherosclerosis and thrombus formation was observed except for one case from Cuculi et al25. In that case, ruptured plaque was identified by OCT as a cause of ScT. Theoretically, the plaque sealing effect of BVS was expected to reduce neoatherosclerosis. Although it might be a rare event, neoatherosclerosis could be considered as a potential cause of ScT after BVS implantation.
SUMMARY
In line with the findings of early ScT, strut malapposition was the most frequently observed finding of late and very late ScT. In contrast to early ScT, strut malapposition was more frequently observed in patients with stable lesions than in those with ACS. In ACS lesions, peri-strut low-intensity area and late discontinuity are the predominant imaging findings (Table 6), indicating that the inflammatory microenvironment around the ACS lesions could play an important role in late and very late ScT. The imaging findings in the late phase, however, should be interpreted with caution due to the “snapshot” nature of the imaging assessment. All the potential mechanical causes of ScT in the late phase are putative in the absence of serial imaging assessments.
Pathophysiology of scaffold thrombosis
All the possible mechanical causes of ScT in the early (acute and subacute) and late (late and very late) phases can translate into three fundamental pathophysiological mechanisms: 1) the disturbance of laminar blood flow and thus the alteration of endothelial shear stress, 2) high thrombogenicity of the biodegradation products, and 3) an inflammatory milieu around the polymeric struts.
ALTERATION OF ENDOTHELIAL SHEAR STRESS
Malapposition, acute disruption, underdeployment, device-vessel mismatch, incomplete coverage of lesion (geographical miss), overlap, and late discontinuity all cause the disturbance of laminar blood flow. In a flow simulation of a microenvironment computed by OCT/angiography fusion in a human coronary artery, the relatively high endothelial shear stress on top of the strut and low endothelial shear stress measured behind and between the BVS struts were demonstrated40. The larger alteration of shear stress around the malapposed struts than around the apposed/embedded struts was also demonstrated by computational fluid dynamic simulation32. Low endothelial shear stress attenuates the endothelial expression of nitric oxide, prostacyclin I2, and tissue plasminogen activator, shifting towards a prothrombotic state. Additionally, low endothelial shear stress may promote ScT by inhibiting endothelial cell proliferation and retarding re-endothelialisation of the arterial and strut surface. Endothelial shear stress peaks over the strut surface edges and activates platelets that release thromboxane A2 and adenosine diphosphate, two potent mediators of platelet activation. Erythrocytes exposed to high endothelial shear stress also release adenosine diphosphate. Activated platelets enter flow separation zones downstream to the struts and reach high concentrations due to delayed flow in conjunction with low endothelial shear stress, resulting in triggering of the coagulation cascade. This shear stress disturbance could be a nidus for thrombus in the microenvironment around struts.
THROMBOGENICITY OF BIODEGRADATION PRODUCTS
In addition to flow disturbance, high thrombogenicity of biodegradation products may play a critical role in thrombus formation, especially in the late phase. The thrombogenicity of a malleable provisional matrix of proteoglycan during the biodegradation process of polymer is higher than that of the polymeric material itself39. Farb et al described 50 consecutive cases of sudden cardiac death attributable to coronary thrombosis, in which 22 had superficial erosion of a proteoglycan-smooth muscle cell-rich plaque without plaque rupture39. Heterogeneous endothelialisation of the scaffold struts or failure of degradation due to incomplete integration into the vascular wall could cause exposure of biodegradation products into the blood flow, resulting in a high risk of thrombus formation. Moreover, biodegradation products of polymer during the bioresorption process and minimal inflammatory milieu (discussed in the next paragraph) could synergistically induce ScT, especially in the late phase.
INFLAMMATORY MILIEU
Arterial thrombosis in atherosclerotic lesions is prone to occur in the presence of activated plaque-derived tissue factor, and has historically been proposed to be the predominant activator of coagulation cascade. An inherent role of tissue factor derived from blood-borne inflammatory cells in the context of arterial thrombosis and its specific contribution to enhanced coagulation has been shown in an experimental study on human leukocytes. Human blood-derived neutrophils and monocytes have been shown to be an important source of tissue factor to initiate coagulation pathways. In an ex vivo porcine arteriovenous shunt model, Koppara et al demonstrated increased adherence of acute inflammatory cells in thick-strut BVS as compared with thin-strut EES in the acute phase (<28 days)41.
In early ScT, strut malapposition combined with ACS is the most predominant feature of ScT, whereas in late and very late ScT peri-strut low-intensity area and late discontinuity rather than malapposition are more frequently observed in ACS patients. Although late discontinuity itself is a programmed benign phenomenon in the biodegradation process, the late discontinuity observed in ScT cases could translate into the intraluminal mass which hinders the laminar flow and results in the alteration of endothelial shear stress. Pathophysiologically, this is a condition similar to strut malapposition. The inflammatory milieu around the polymeric struts observed in the acute phase of ACS and during the biodegradation process after device implantation (six to 36 months) could induce a more thrombogenic status. The pathophysiological meaning of peri-strut low-intensity area still remains unclear and needs further investigation.
Inflammation associated with BVS might be related to polymer resorption42, which suggests that the minimal inflammation lasts until the complete bioresorption (<36 months). After complete bioresorption, the inflammatory response would theoretically disappear and the coronary vessel would return to its original status.
Clinical implications
A recently published study demonstrated that decreased left ventricular ejection fraction and ostial lesions were independent predictors of ScT in an all-comer multicentre European cohort (n=1,305)33. Implantation of metallic stents in patients with decreased left ventricular function, treatment of ostial and/or type B2/C lesions, and interruption of DAPT have all been previously reported to be associated with ScT. Recent data on the bioresorbable scaffold were in line with the data on metallic stents. Of note, a BVS-specific implantation strategy significantly reduced the rate of ScT in the cohort. When a BVS-specific implantation strategy was implemented, 12-month ScT rates fell from 3.3% to 1.0%, an effect that remained significant when adjusted for multivariable propensity score (p=0.012; hazard ratio: 0.19; 95% confidence interval: 0.05 to 0.70). The BVS-specific implantation strategy can be summarised as follows33:
1. Predilatation with a non-compliant balloon up to the same size as the reference vessel diameter.
2. BVS implantation only in case of full expansion of the non-compliant percutaneous transcatheter coronary angioplasty balloon as demonstrated by angiography in two orthogonal planes.
3. Implantation of a BVS of the same size as the reference vessel diameter at 10 to 12 atm.
4. Post-dilatation with non-compliant balloons up to a maximum of 0.5 mm larger at 14 to 16 atm.
The possible mechanical causes described in this article are theoretically treatable and avoidable by the BVS-specific implantation technique. Operators need to stay on top of the advantages and limitations of bioresorbable scaffolds, and to follow strictly the specific strategy recommended in the expert review33,43. In addition, we can expect new-generation BVS with thinner struts to lower the risks of scaffold thrombosis associated with a number of the mechanical causes described in the current article. Thinner struts could theoretically contribute to less flow disturbance and earlier neointimal coverage compared to the thick struts of the current BVS.
Limitations
This review article presents some limitations. We focused on coronary imaging insights from current publications, resulting in an unavoidable selection bias and publication bias. The true relative and absolute frequency of each of these potential mechanisms remains unknown. Although the present article reviews each interpretation of the mechanical cause in each report, some causes might have been affected or created by the procedure of thrombectomy, IVUS or OCT: malapposition, discontinuity, and lack of coverage of struts might have been iatrogenically created by the insertion of thrombectomy, IVUS or OCT catheters. The “snapshot” nature of the intracoronary imaging investigations precludes any dynamic interpretation of the ongoing mechanical cause of ScT. All the possible mechanical causes, especially in the late phase, are hypothetical in the absence of serial imaging assessments. Lastly, the current review only provides insights into the Absorb BVS from Abbott Vascular. The findings might be different with other bioresorbable scaffolds.
Conclusions
Malapposition, incomplete lesion coverage, and underdeployment are frequently observed in cases of early ScT, whereas, in late ScT, malapposition, late discontinuity and peri-strut low-intensity area are the predominant features of intracoronary imaging. The mechanical causes raised in this article, however, could theoretically be treatable and avoidable by using the BVS-specific implantation technique. New-generation BVS with thinner struts can also be expected to lower the risks of scaffold thrombosis associated with a number of the mechanical causes described in the current article. Future trials focusing on the imaging features are warranted.
Guest Editor
This paper was guest edited by Stephan Windecker, MD; Department of Cardiology, Bern University Hospital, Bern, Switzerland.
| Impact on daily practice The present systematic review of published case reports of bioresorbable scaffold thrombosis (ScT) with intracoronary imaging demonstrated that malapposition, incomplete lesion coverage, and underdeployment are frequently associated with early ScT, whereas, in late/very late ScT, malapposition, late discontinuity and peri-strut low-intensity area are the predominant features of intracoronary imaging. Early and late/very late ScT could have different mechanical causes. To minimise the potential risk of early and late/very late ScT, it is important that operators try to avoid such abnormalities at the time of implantation. The impact of a BVS-specific implantation technique on the incidence of ScT still needs to be investigated in future prospective trials. |
Conflict of interest statement
Y. Sotomi received speaker honoraria from Abbott Vascular Japan and research grants from the Fukuda Memorial Foundation for Medical Research and the SUNRISE Lab. Y. Onuma and P.W. Serruys are members of the Advisory Board for Abbott Vascular. P. Suwannasom has no conflicts of interest to declare. The Guest Editor declares research grants to the institution from Abbott, Biotronik, Boston Scientific, Medtronic, Edwards and St. Jude.

