Introduction
The seventh meeting of the European Bifurcation Club (EBC) was convened in Lisbon on October 14-15, 2011, with the agenda of reaching a consensus on the current state of the art of percutaneous bifurcation treatment. The present report represents a synthesis of the findings from this meeting and also incorporates a literature review from the field of bifurcation interventions. Topics covered in this Consensus document include the state-of-the-art in percutaneous treatment of unprotected left main coronary artery, discussion of anatomical and functional assessment of bifurcation lesions and solutions for difficult side branch access.
Unprotected left main coronary artery (LMCA)
Two important meta-analyses were published in 2011 with the purpose of determining the safety and efficacy of percutaneous coronary interventions (PCI) compared with coronary artery bypass grafts (CABG) in patients with LMCA disease. These two meta-analyses1,2 of 1,611 patients randomised in the LEMANS3, SYNTAX left main cohort4, PRECOMBAT5 and by Boudriot et al6 reached similar conclusions, that the primary endpoint of one-year MACCE was non-significantly different in the PCI group compared with CABG (PCI 14.5% vs. CABG 11.8%; Odds Ratio [OR] 1.28; 95% CI: 0.95-1.72; p=0.11). As in each of the individual studies, the rate of stroke was higher amongst those treated with CABG (PCI 0.1% vs. CABG 1.74%; OR 0.15; 95% CI: 0.03-0.67; p=0.013). Conversely, higher rates of TVR were observed in the PCI cohort (PCI 11.4% vs. CABG 5.4%; OR 2.25; 95% CI: 1.54-3.29; p<0.001). The authors concluded that PCI is comparable to CABG for the treatment of unprotected LMCA (ULMCA) with respect to the composite of major adverse cardiovascular or cerebrovascular events at 12-month follow-up, as well as having a lower risk of stroke and a higher risk of target vessel revascularisation (TVR) compared with CABG1,2.
In most of the present studies, provisional stenting of the main branch has been the preferred approach, with additional side branch (SB) intervention if angiographic results are suboptimal. This strategy has largely been driven by lower TVR, procedure time and contrast exposure, not by hard clinical endpoints. However, certain bifurcations with severe disease that extends several millimetres beyond the ostium of a large SB may still require stenting of both vessels, and a recent randomised DKCRUSH-II trial7 in true bifurcation lesions (Medina classification 1,1,1 and 0,1,1) demonstrated that a two-stent approach, when optimally performed with final kissing balloon inflation, may reduce the rate of TLR and TVR as compared with the provisional approach and is associated with more favourable long-term clinical outcomes8.
Importantly, several studies (SYNTAX left main cohort4, LEMAX9, FRENCH10, the Italian Society of Invasive Cardiology ULMCA registry11, DISTAL study12) demonstrated that PCI with DES has a favourable outcome, comparable to CABG, when the lesion is ostial or mid-shaft of the left main, while distal left main predicts worse outcomes, mainly driven by increased TLR after DES. However, outcomes in the left main are dependent on the complexity of downstream anatomical disease, which is a major confounding factor. The European Society of Cardiology and the European Association for Cardio-Thoracic Surgery Guidelines on myocardial revascularisation provide a Class IIa (Level of Evidence: B) recommendation for PCI of left main ostial or shaft disease when it exists in isolation or in combination with one-vessel disease; a Class IIb (Level of Evidence: B) recommendation for left main distal bifurcation disease when it exists in isolation or in combination with one-vessel disease; a class IIb recommendation for any left main disease with concomitant two- or three-vessel disease and a SYNTAX score ≤32; and a Class III recommendation for left main disease with concomitant two- or three-vessel disease and a SYNTAX score ≥33. CABG is the favoured approach for all of these scenarios (Class I, Level of Evidence: A)13.
The ongoing international multicentre EXCEL (Evaluation of XIENCE Prime versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularisation) trial, aiming to recruit 2,600 patients with ULMCA disease and a SYNTAX score <33 – randomised to surgical (n=1,300) or percutaneous (with the XIENCE PRIME or XIENCE V EECSS PRIME DES [n=1,300]) revascularisation – will ultimately answer the question of the most appropriate revascularisation modality for patients with ULMCA disease14. The primary endpoint is the composite incidence of death, MI or stroke at a median follow-up duration of three years, powered for sequential non-inferiority and superiority testing. Questions remain unanswered regarding the need to include revascularisation in the primary endpoint as well as the exclusion of patients with extensive triple vessel disease, in addition to left main stenosis. Based on meta-analyses, and awaiting the results of the EXCEL trial, the following consensus for LMCA treatment was reached:
For LMCA treatment, PCI and CABG have similar safety outcomes at 12-month follow-up. PCI has a lower risk of stroke but a higher risk of repeat intervention.
PCI with DES has excellent outcomes for ostial or mid-shaft left main lesions, but has a higher incidence of TLR for distal left main stem bifurcation lesions.
Specific areas of interest regarding the treatment of the unprotected LMCA bifurcation were also discussed in detail during the EBC meeting.
PREDICTING OUTCOME BY PLAQUE DISTRIBUTION
It was reported that plaque distribution could be a contributing factor for outcomes following LMCA intervention. By identifying three regions of the LMCA bifurcation (through LMCA-LAD; through LMCA-LCX and at the point of bifurcation), lesions could be classified as “whole bifurcation” (1,1,1 or 0,1,1) and “partial bifurcation” (1,1,0 or 1,0,1 or 0,1,0 or 0,0,1) involvement. In a total of 329 patients who underwent PCI with DES, there was a higher cumulative target lesion revascularisation (TLR) in those with “whole bifurcation” (HR 3.12; 95% CI: 1.59-6.11; p=0.001), independent of the stenting technique or degree of stenosis15.
It has been previously reported that there is a relationship between lesion location and plaque distribution: ostial LMCA stenosis has less plaque burden and larger lumen area and more negative remodelling than distal-bifurcation LMCA stenosis16. In addition, Oviedo et al showed the limitations of an angiographic rather than IVUS classification of LMCA bifurcation lesions17. IVUS evaluation verified that bifurcation disease is rarely focal, mostly involving both LAD and CX ostia, and that both sides of the flow divider are disease-free. Plaque distribution was not influenced by the LAD/LCX angiographic angle, lesion severity, LMCA length or remodelling. Importantly, Kang et al showed that lumen loss at the LCX ostium frequently occurred after crossover stenting from the distal LM to the LAD18. The main mechanism was carina shift that was associated with a narrow angle between the LAD and LCX. Therefore, the assessment of the LCX ostium by direct LCX pullback is necessary to evaluate accurately the mechanisms of lumen loss during stent implantation.
Left main stem plaque distribution may help determine optimal revascularisation strategy.
THE LMCA ANGULATION
The 3-D measurement of coronary bifurcation angles prior to stenting can predict changes in bifurcation geometry after stenting and the decrease in the bifurcation angle after stenting is a predictor of less favourable outcomes19. Godino et al reported that in both LMCA and non-LMCA bifurcations there are significant differences in the angle between the proximal main vessel and the side branch (proximal bifurcation angle - A) and the angle between the two distal branches (distal bifurcation angle - B) pre- and post-PCI20. That difference was driven by the two-stent technique and was most evident with a baseline bifurcation angle ≥70 degrees (the crush technique caused the largest angle variation post-procedure, particularly in non-true LMCA bifurcations)20. A study from the Asian Multicentre DES-LMCA registry demonstrated that DES implantation in low angled LMCA bifurcation lesions showed a lower incidence of cardiac events compared with high angled bifurcation lesions at five-year clinical follow-up21. Although the bifurcation angle affects the selection of stenting strategies there are still controversies in terms of the effect of these angles on outcomes, primarily because all studies were underpowered for this clinical endpoint. In a substudy from the SYNTAX trial, Girasis and colleagues evaluated using three-dimensional quantitative coronary angiography (3-D QCA) the bifurcation angle parameters of the LMCA, the effect of PCI on this angulation and the impact of bifurcation angles on clinical outcome22. This study showed that both proximal and distal bifurcation angles were affected by cardiac motion and that PCI modifies the distal angle. Importantly, there was no clear difference in MACCE rates across tertiles of pre-PCI distal bifurcation angle values. Chen et al in their study also demonstrated that, when utilising crush stenting techniques for coronary bifurcation lesions, the bifurcation angle does not influence clinical outcomes23. The result of this study is in disagreement with the report of Dzavik et al that a bifurcation angle >50° is an independent predictor of MACE after crush stenting24. Interestingly, Chen et al found significant correlations among high bifurcation angles, final kissing balloon inflation (FKBI) and MACE23. The MACE-free survival rate was lower in high bifurcation angle patients without FKBI than in high bifurcation angle patients with FKBI.
The distal bifurcation angle affects the selection of left main stenting strategies. However, there is still controversy as to whether or not bifurcation angles might affect clinical outcomes.
ADJUNCTIVE TECHNOLOGY
Other technical issues were discussed at the EBC meeting, including an emphasis on the use of fractional flow reserve (FFR), intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to provide information to reduce restenosis. In the case of intermediate LMCA stenosis, pre-intervention IVUS may accurately assess plaque distribution and severity as well as the adequate sizing of stents25. Kang et al showed that pre-procedural angiographic percent diameter stenosis did not correlate with the IVUS MLA at the LCX ostium18. Therefore, direct imaging of both the LAD and LCX is suggested to identify the minimal lumen area (MLA) in the LMCA and the presence of disease in the LAD and/or LCX.
Although the optimal cut-off of the MLA for identifying significant LMCA disease and its accuracy remain debatable, it has been demonstrated that in isolated LMCA disease an IVUS-derived MLA <4.8 mm2 is a useful criterion for predicting FFR <0.8025. The advantages of absolute lumen cross-sectional area (CSA) are that it does not depend on finding a disease-free reference segment, and works even in the setting of significant LAD or LCX stenoses. Kang et al have evaluated the optimal IVUS stent area to predict angiographic ISR after sirolimus-eluting stent implantation for LMCA disease26. The minimal stent area (MSA) cut-offs that best predicted ISR on a segmental basis were 5.0 mm2 (ostial LCX ISR), 6.3 mm2 (ostial LAD ISR), 7.2 mm2 (ISR within the polygon of confluence - POC) and 8.2 mm2 (ISR within the LMCA above the POC). Post-stenting underexpansion was an independent predictor for two-year MACE, especially repeat revascularisation, while stent malapposition did not predict ISR or MACE. Park et al have reported that long-term mortality among patients undergoing IVUS-guided left main stem stenting may be better than for those undergoing the procedure with angiographic guidance alone27.
However, it is not clear whether the same IVUS criteria for a “significant” LMCA stenosis should be used for ostial LMCA lesions as they are for mid-shaft/distal bifurcation lesions, for positively vs. negatively remodelled lesions or for unstable vs. stable morphology (plaque ruptures vs. no plaque ruptures).
IVUS is also helpful in assessing possible stent underexpansion and malapposition. This is extremely important in the setting of LMCA intervention, considering that stent underexpansion has been correlated to stent thrombosis28-30.
IVUS guidance for LMCA bifurcation intervention is recommended.
DEDICATED STENTS FOR LMCA
A final discussion on LMCA stenting with dedicated stents was opened also to Industry, and a “show of hands” demonstrated that more than 60% of the attendees believed that within five years we will have dedicated bifurcation stent systems in routine clinical use. This may be particularly true for the left main stem, where bulkier devices may be delivered with greater ease31. Numerous novel dedicated systems are in development, and we can expect these to play an important role in the coming years32.
Dedicated stents may play an important role for LMCA stenting in the future.
VIRTUAL BENCH FOR LMCA STENTING (“JOHN DOE” SESSION)
Digital simulation has developed into an increasingly reliable alternative to bench testing33. Genuine normal or diseased coronary bifurcations can be captured by 3-D angiography or MSCT. They are subsequently transformed into “finite elements” each being attributed the mechanical properties obtained from autopsy studies. Similarly, the structure and mechanical features of stents can be simulated as well as their deployment against potential vessel wall resistance. However, proper experimental validation is needed to determine whether all model assumptions are justified. If the assumptions cannot be justified, the validity of the model can be challenged34. An example of comparison between the real case and the virtual simulation is shown in Figure 1 and Figure 2.
A new way of thinking is now open to us with the possibility of simulating PCI from patient data.
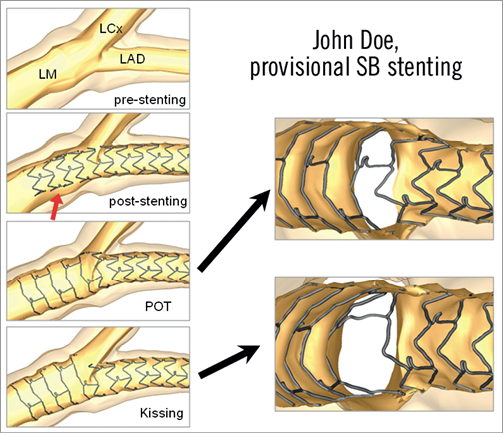
Figure 1. Provisional side branch stenting procedure using finite element simulations. Courtesy of Peter Mortier, EBC 2011.
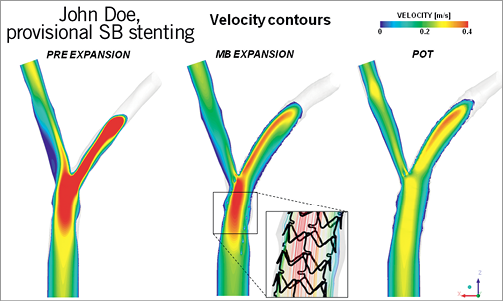
Figure 2. Flow dynamics comparison during provisional side branch stenting. Courtesy of Gabriele Subini, EBC 2011.
Anatomical and functional assessment of bifurcation lesions
CONTRIBUTION OF IVUS TO THE MANAGEMENT OF CORONARY BIFURCATION LESIONS
Invasive imaging of coronary arteries is presently based on the triad of digital flat-panel detector X-ray angiography, intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
Coronary bifurcation lesion imaging aims to enable successful angioplasty of coronary bifurcations, assessing lesion severity, atherosclerosis distribution, reference lumen diameters, stent length, post-stenting geometry, any strut malapposition, distortion of any part of the stent(s) as well as associated arterial complications.
The stent-boost technique improves X-ray imaging quality by increasing local spatial contrast resolution. Contrast enhancement of the implanted stent allows rapid and precise detection of pre-procedural presence and distribution of calcium, change in stent geometry and, in two-stent (Y or T) procedures, local destructuring or strut protrusion, especially in the carina, and gaps. Stent-boosted images seem to correlate well with IVUS for evaluation of adequate stent deployment.
The two cross-sectional imaging systems –IVUS and OCT– differ in spatial resolution, which is very satisfactory with 40 MHz IVUS (90 µm radial resolution) and excellent with OCT (around 13 µm radial resolution). The main limitation of OCT, intrinsic to the physics involved, is its weak signal penetration beyond 0.5-1 mm into the arterial wall. Another difference is in pullback speed: 1 mm/sec for IVUS (30 images/sec) and 10-20 mm/sec for OCT. On the other hand, OCT and IVUS present the same strengths and weaknesses with regard to plaque composition analysis35.
Whether or not to use IVUS or OCT guidance in treating coronary bifurcation lesions remains a subject of great controversy. The criteria sought are now well-defined:
– Before percutaneous coronary intervention (PCI) 1) longitudinal plaque distribution, 2) plaque composition, 3) mother and daughter vessel reference diameters36, 4) precise stent landing zone analysis, and 5) side-branch ostium analysis (diseased or not).
– After PCI 6) stent expansion and apposition, 7) side-branch ostium assessment, 8) final vessel sizes (stent over- or under-expansion), 9) proximal or distal dissection, and 10) peri-adventitial haematoma.
Several of these criteria (criteria 1, 4, 9 and 10) cannot be objectively assessed on OCT, which is therefore unsuited to interventional guidance. IVUS seems preferable, improving the safety of coronary bifurcation stenting using drug-eluting stents (DES) and in bifurcations in general as well as in left coronary artery bifurcations in particular27,37. It is still controversial whether routine use of IVUS in conventional lesions leads to improvement in clinical outcomes after PCI. Park SJ et al reported in the propensity matched study that the three-year incidence of mortality was lower in patients with LMCA lesions treated with DES when IVUS guidance was used as compared with angiography guidance27, while Zhang et al published a meta-analysis which demonstrated that IVUS-guided coronary DES implantation is associated with a significant reduction in death, MACE and stent thrombosis compared to angiography guidance38. However, appropriately powered randomised trials are necessary to confirm the findings from this meta-analysis. In addition, the recent propensity matched study of Park KW et al showed that there were no significant advantages of IVUS guidance for non-left main lesions, but rather a significant increase in periprocedural enzyme elevation, reflecting more aggressive procedures performed with IVUS guidance39. Binary or purely quantitative assessment criteria pose no problem (e.g., randomised studies of the use of fractional flow reserve); the situation in IVUS and/or OCT imaging, however, is much more complex, as final interpretation involves a wide range of distinct data that are difficult to reduce to a single quantitative variable.
A major issue highlighted in recent years concerns in-stent neo-atherosclerosis after bare metal (BMS) and DES implantation, the main anatomopathologic imaging signs of which have now been described40. Neoatherosclerosis is a heterogeneous recomposition of the in-stent neointimal process with a necrotic lipid core, rich in microvessels, frequently associated with disrupted neointima and thrombus, giving rise to acute atherothrombotic processes, the incidence of which is greater in DES (36%) than BMS (16%) and with earlier onset in DES (>2 years)41-43.
IVUS illuminates all aspects of bifurcation stenting. However, there is no strong evidence that use of IVUS improves outcomes.
CONTRIBUTION OF OCT TO THE MANAGEMENT OF CORONARY BIFURCATION LESIONS
Frequency domain imaging has greatly increased the speed of acquisition of OCT images and has attenuated the negative impact of the need to examine the vessel clear from blood by replacing it with a crystalloid solution44. High-speed optical frequency domain imaging (OFDI) can be used to create 3-D reconstructions of implanted stent structures with excellent quality and at resolutions much higher than what is currently possible using IVUS45. OCT may be used to guide the procedure preserving SB patency without compromising the MV, obtaining the optimal vessel dimensions and reducing malapposition of stent struts and the amount of unplanned floating struts.
SPATIAL PERCEPTION OF OCT OF CORONARY BIFURCATIONS
While the analysis of OCT in straight coronary segments is relatively straightforward, the interpretation and reporting of OCT in bifurcation lesions is a far bigger challenge. The investigator must try to rebuild the wealth of 2-dimensional cross-sections into a 3-dimensional structure. While the sequential 2-dimensional cross-sections are the base for detailed assessment and quantification of expansion, apposition and long-term coverage of stent struts, it is felt that dedicated software to develop 3-D reconstructions of the bifurcation anatomy before and after intervention (Figure 3) would be of great help for the interventionalist providing insight into the take-off of side branches as well as aid in the understanding and planning of optimal treatment strategy46,47.
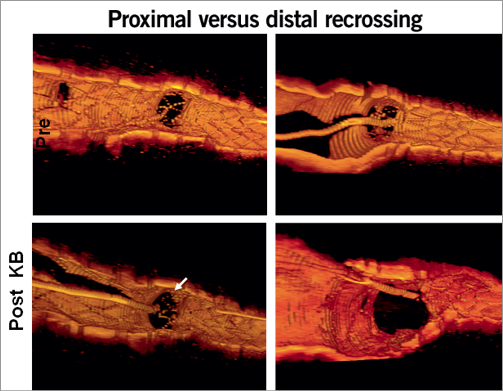
Figure 3. Guidance of cell recrossing locations using 3-D OCT. Courtesy of N. Foin, EBC 2011.
OFF-LINE ANALYSIS METHODOLOGY
Off-line analysis of bifurcation OCT should be preceded by a detailed evaluation of coronary angiography images of bifurcation lesion and quality assessment of OCT pullbacks in the main vessel and the side branch, both before and after intervention. The region of interest is defined as a stented segment plus 5 mm at both edges, and analysis is performed in the proximal MV, distal MV and SB at 0.6 mm intervals and inside the bifurcation segment at 0.2 mm intervals48.
CLINICAL STUDIES
An OCT study of 38 sirolimus-eluting stents, 50 zotarolimus-eluting stents, 33 biolimus-eluting stents and 57 everolimus-eluting stents confirmed that coverage of the incomplete stent apposition and non-apposed side branch struts is delayed with respect to well-apposed struts at 9-13 months of follow-up49.
POSSIBLE CLINICAL APPLICATIONS
The detailed assessment of the bifurcation by OCT pre-intervention may aid tailoring the treatment strategy. The expected ability to assess the risk of carina shift or side branch closure may influence the decision to protect a side branch with a wire and whether or not to predilate it. The exact determination of vessel dimension and distribution of disease in the bifurcation segment could influence the decision whether to plan a simple or a complex approach up front, ensuring adequate coverage of all diseased areas where needed. A final OCT pullback after a complex bifurcation procedure often points to areas of underexpansion, malapposition or an excessive amount of free floating struts, which are not visible on angiography and can be corrected with additional high-pressure post-dilation or kissing balloon inflation. Several groups are focusing on employing 3-dimensional optical coherence tomography (3D-OCT) image reconstruction to ensure recrossing in the side branch after MV stenting in the most distal cell of the bifurcation, providing better coverage at the ostium of the side branch and avoiding accumulation of metal at the region of the flow divider47,50,51. In order to visualise the stent clearly in 3D-OCT, an enhancement of the stent strut is necessary and a novel automatic strut detecting programme allows rapid 3-D stent image reconstructions (about 10 minutes) during procedure51.
The EBC found that there was a need to develop an initiative to create a consensus document on how to analyse and report OCT examinations in bifurcation lesions. The aim was to ensure standards aiding comparisons between studies and to facilitate standardised scientific and clinical use of OCT in bifurcation assessment and treatment. The International Working Group for Standardisation and Validation of Intravascular Optical Coherence Tomography (IWG-IVOCT) recently published an extensive consensus document on acquisition, interpretation and analysis of OCT in general52.
OCT is likely to become increasingly important in the procedural management of patients with coronary bifurcation lesions.
2-D and 3-D OCT are complementary imaging tools and reassessment of the understanding of 2-D OCT imaging may be warranted in light of the 3-D findings.
CONTRIBUTION OF FFR TO THE MANAGEMENT OF CORONARY BIFURCATION LESIONS
Several studies revealed the angiographic inaccuracy in the assessment of bifurcation lesions. However, a recent study showed that a dedicated quantitative coronary angiography (QCA) system had less inter- and intra-observer variability and better correlation with FFR compared to conventional 2-dimensional QCA53,54. However, the limitations of a pure anatomical evaluation to determine the appropriateness of revascularisation in bifurcation lesions still remain. Currently under development, 3-dimensional QCA or QCA with finite element analysis may improve the accuracy of angiographic assessment of bifurcation lesions.
In two recent studies, the clinical outcomes of intravascular ultrasound (IVUS)-guided intervention for bifurcation lesions were better than angiography-guided intervention37,55. Although it has a limitation to assess the functional significance of a stenosis56, IVUS can provide detailed anatomical information which is very helpful in planning the treatment strategy and assessing the procedural success. In addition to lumen, plaque and vessel areas, IVUS can provide the distribution of plaque and the location of carina, which are useful to predict the severity of side branch jailing after main branch stent implantation57,58.
Fractional flow reserve (FFR) is a pressure-derived flow index, which represents the amount of a flow reduced by a specific stenosis. FFR-guided revascularisation strategy is known to be better than angiography-guided revascularisation in various lesion subsets. FFR can also be used in bifurcation lesions but the rate of possible complications during FFR evaluation in the side branch is not negligible59,60. Further study is required to examine the feasibility, practical clinical application and safety of this technique.
However, there are some tips we need to know before we apply this parameter to complex bifurcations lesions:
First, when the side branch FFR is measured, it should be remembered that measured FFR reflects the plaque burden of both the proximal main branch and side branch. Second, the pressure wire should not be jailed by a stent during percutaneous coronary intervention (PCI). Third, pre-intervention of the side branch FFR is not that helpful in predicting jailed side branch FFR due to the dynamic geometric changes at the ostium of the side branch during main branch intervention. Fourth, as the amount of ischaemic burden is clinically more important than the presence of ischaemia, FFR should be measured in large side branches.
The combination of computational fluid dynamics and coronary CT angiography has allowed for the non-invasive anatomical and physiologic assessment of coronary artery disease. As this novel technology starts with a 3-dimensional anatomic model from conventional CT images, no additional imaging processes or medications are needed. A first-in-human clinical trial (the DISCOVER-FLOW study) showed that CT-derived computed FFR (FFRCT) had good correlation with invasively measured FFR (Spearman’s rank correlation=0.717, p<0.0001; Pearson’s correlation coefficient=0.678, p<0.0001), and FFRCT was superior to CT for the diagnosis of lesion-specific ischaemia (per-patient discrimination AUC 0.92 vs. 0.70, p=0.0001)61. However, this novel technology has yet to be tested in complex bifurcation lesions.
Both anatomical and physiological information are important in the assessment and treatment of complex bifurcation lesions.
Adequate knowledge of the strengths and weaknesses of IVUS and FFR are mandatory for proper interpretation of data per procedure.
Solutions for difficult side branch access
The SB access session was based on the concept that, in spite of the various advancements achieved in bifurcation PCI, effective guidewire placement in the side branch (SB) at the beginning of the intervention (“primary wiring”) and eventually after stent implantation (“rewiring”) are critical technical steps for successful procedures.
Regarding the management of primary wiring in “very complex” SB access, the possible need for “advanced” wiring techniques, additional tools to facilitate wire manipulations and “last resort” strategies have been discussed.
When the SB take-off angle is >90% and its stenosis is sub-occlusive, the task of primary wiring may be difficult and the conventional technique of antegrade wiring (by pushing the wire directly into the SB from proximal MV) may be unsuccessful62. A possible alternative is to use the “reverse wire technique”. This technique was described by Kawasaki et al63 several years ago, and is based on the preparation of the guidewire tip by creating two sharp curves: a longer proximal one which should create a loop in the distal MV and an opposite short distal one which should engage the SB ostium during the wire pullback (Figure 4). To use this technique successfully, the operator should not only know how to shape and manipulate the wire, but also how to insert it properly into the guiding catheter.
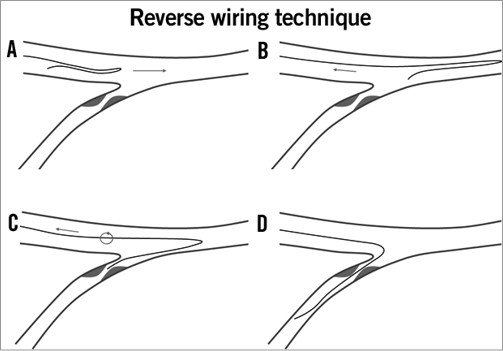
Figure 4. Complex side branch wiring, the “reverse wire” technique. A) A Medina 0,0,1 bifurcation with an extreme angulation (150°) of the side branch. B) A guidewire with a hairpin bend at about 5 cm from its distal tip is advanced in the main vessel. C) When in the distal main vessel, the guidewire is pulled back toward the bifurcation. D) Owing to the straight bend, the distal tip of the guidewire engages the side branch. E) Gentle turning counter-clockwise advances the guidewire in the side branch. Adapted from F. Burzotta et al. EuroIntervention. 2010;6:J72-J80.
Of note, since the ability to place the bent wire into the distal main vessel is the key point for successful reverse wiring technique63, use of a dedicated single lumen microcatheter or a dual lumen microcatheter is recommended in order to navigate unfavourable main vessel anatomies.
Finally, when all the attempts to wire the SB successfully have failed due to the inability of placing the wire with an appropriate bend in the correct position due to plaque burden in the MV, the role of “last resort” strategies has been recognised62. They consist in the use of plaque modification techniques (ballooning or rotablation) in the MV axis in order to create more suitable anatomic condition for SB wiring. Among these, many of the participants agreed on the possible key role of rotablation in their practice.
Moving forward to the rewiring phase, the possible multiple advantages of the POT technique (post-dilation of the proximal segment of the MV) have been highlighted (Figure 5)33. Indeed, POT may be extremely useful in procedures performed using various techniques, far more than simply providing better remodelling of the MV to allow the stent to fit in relation to its size in the proximal MV segment. In particular, the POT technique may facilitate SB access by minimising the risk of rewiring “under the stent” (due to better apposition of stent struts to proximal MV segment) and by facilitating SB rewiring and ballooning through the distal MV stent’s side cells (due to MV stent strut protrusion and opening at SB ostium level).
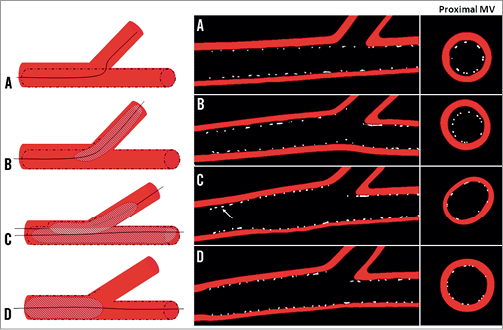
Figure 5. Effect of the provisional approach and kissing inflation on strut distribution. Strut malapposition and stent underexpansion during stenting of the MV (A) and dilatation of the SB (B). Longitudinal and cross-sectional views show the asymmetrical distortion of the stent in the proximal MV after KB inflation (C). Final Proximal Inflation (FPI), e.g., final Proximal Optimisation Technique (POT) with a post-dilatation of the proximal part of the MV corrects for the stent distortion induced after KB while ensuring complete apposition of stent struts (D). Adapted from N. Foin et al. EuroIntervention 2011;7:597-604.
Accordingly, the POT technique has been recognised as the first line technique when any trouble in SB rewiring or balloon advancement is faced. Moreover, it can also be considered as a possible essential step of the “simple” strategy.
If the rewiring of a severe SB stenosis is impossible despite all these recommendations, the jailed wire can be used to access the SB64. The technique consists of using the jailed wire to dilate the occluded side branch. Initial dilatations are performed with a low profile, 1.25 mm diameter balloon catheter. A regular balloon is then inflated through the same wire to open the side branch, crushing the proximal part of the MV stent. At this point, a second stent is implanted at the side branch, finishing the procedure as an inverted crush stenting64.
Conclusion
The EBC annual meeting remains concentrated and dedicated to a single topic. As such, it is able to bring together clinicians, engineers and physicists for detailed discussions. The consensus statements from the 7th EBC meeting reflect this unique opportunity.
Conflict of interest statement
All authors have read and agreed to the manuscript as written and have reported that they have no conflicts of interest to declare.

