Case Summary
BACKGROUND: A 60-year-old man with history of coronary bypass surgery in 2000 with three saphenous venous grafts (SVG) to left anterior descending artery (LAD), diagonal and obtuse marginal branches, presented by worsening chest tightness of one month duration. He had a clinical history of hypertension, dyslipidaemia and chronic renal impairment.
INVESTIGATION: Physical examination, electrocardiography, laboratory tests, echocardiography and coronary angiography.
DIAGNOSIS: Significant aorto-ostial stenosis of the SVG to LAD, left main stenosis, chronic total occlusion of LAD from its origin and significant stenosis of the mid portion of left circumflex artery.
TREATMENT: Percutaneous intervention re-do bypass surgery, dual anti-platelet therapy.
KEYWORDS: coronary artery bypass grafting, left main intervention, chronic total occlusion, transradial, bilateral approach, intravascular ultrasound
Presentation of the case
A 60-year-old man was admitted to our hospital in November 2009 for assessment of recurrent worsening chest tightness of one month duration. He had undergone coronary artery bypass graft (CABG) surgery nine years ago with three saphenous venous grafts (SVG) to the mid segment of left anterior descending (LAD) artery, diagonal and first obtuse marginal (OM) branches. The patient’s related comorbidities included long standing hypertension, dyslipidaemia and chronic kidney disease with a serum creatinine level of 1.9 mg/dl. In 2005, he suffered from acute myocardial infarction and he had history of two prior transradial percutaneous coronary interventions (PCIs) with stenting for a mid-right coronary artery (RCA) lesion in 2003 and for the RCA posterolateral branch in 2005. Clinical examination was unremarkable. Twelve-lead resting electrocardiogram (ECG) showed normal sinus rhythm with poor R wave progression in the precordial leads. Transthoracic echocardiography showed anteroseptal wall hypokinesia with mild impairment of left ventricular function (ejection fraction of 48%). The patient refused transfemoral approach for coronary angiography and intervention and preferred the transradial access.
After the patient had been pre-treated with proper hydration, coronary angiography (CAG) was performed via the left radial approach with a 5 Fr 3.5 Ikari left 3.5 guiding catheter (GC) (Terumo, Tokyo, Japan) after failure to advance a 6 Fr arterial sheath in a small calibre left radial artery probably due to the effect of the previous multiple PCI. The same catheter was used for engagement of the right and left coronary arteries and for the cannulation of the SVGs. The CAG revealed distal left main (LM) 50% tubular stenosis, and a chronic total occlusion (CTO) of the LAD from its origin. The left circumflex (LCX) had a 70% stenosis just after the origin of the first OM (Figure 1A and Figure 1B). The RCA showed no significant in-stent restenosis. The SVGs to the diagonal and to OM branches were patent (Figure 1B and Figure 1C). However, the SVG to LAD was markedly degenerated with presence of a 74 % stenosis in its ostium and a delayed distal flow (Figure 1D). The logistic EuroSCORE was 11.77%. The case was discussed with the patient’s family, the cardiac surgeon and the interventional cardiology team.
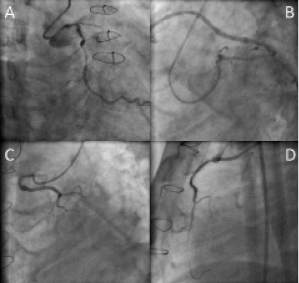
Figure 1. Baseline angiogram of the left coronary artery (LCA). Panel (A) shows a 50% tubular stenosis in the LM, a totally occluded LAD from its orifice and a 70 % stenosis in the mid segment of LCX. Panel (B) shows patent SVG to the OM branch in the Left anterior oblique view (LAO) with caudal angulation. Panel (C) shows patent SVG to the diagonal branch in the right anterior oblique (RAO) view with cranial angulation. Panel (D) shows SVG to LAD in the lateral projection with significant ostial stenosis.
Reference
How would I treat?
The Invited Expert’s opinion
A complete proximal left anterior descending (LAD) artery occlusion could become a major problem in a patient with whom the saphenous vein graft (SVG) on the LAD has a 70% ostial stenosis as well as a 50% stenosis on the distal left main (LM); in this particular situation an aggressive treatment could be justified.
In this case, the right coronary artery was stented in 2003 and 2005 and functioned normally, while the left marginal branch was bypassed by a still patent SVG. Reopening the native LAD could be hazardous because of the 50% LM stenosis and interventional treatment of an ostial stenosis in a degenerated graft would probably not be the best option. Therefore, this patient needs a new revascularisation in the LAD by a left internal mammary artery (LIMA) graft on the beating heart without extracorporeal circulation (ECC). This new arterial graft will protect the LV anterior wall and allow further interventional treatment on the LM.
The authors noted a logistic EuroSCORE of 11.7% which is relatively high for this kind of situation. The operative risks factors were marked by re-operation, moderate LV dysfunction and moderate kidney failure (creatinine: 1.9 mg/dl), but the EuroSCORE1 takes into account this point only if it’s over 2 mg/dl (200 µl/l). In fact, in this particular 60-year-old patient, the logistic EuroSCORE was 3.8% and not 11.7% as mentioned by the authors (unless some data are missing in this presentation).
The EuroSCORE has been established for coronary surgery under ECC, so it could overestimate the operative risk in off- pump surgery2.The estimated operative risk is twofold in repeat coronary bypass surgery on-pump; this is one of the reason’s why some surgeons chose to operate off-pump and to perform a less invasive surgery without ECC. In a study by Dewey of 453 patients for repeat coronary surgery, 153 were bypassed off-pump and he successfully demonstrated significantly less mortality in the group of patients treated off-pump (2.6%) vs. (9.6%) on-pump3. He also observed a significant decreased morbidity due to a decreased need for blood products (25% vs. 67%, p<0.0001) and a decreased incidence of prolonged ventilation.
Technically, this operation needs a resternotomy to access the heart and to harvest a LIMA. It’s usually easy to follow the SVG on LAD to find an area to open the LAD. After moderate heparinisation (1 mg/kg), the LAD is opened distally to the previous anastomosis; a small shunt is introduced inside LAD to avoid ischaemia and bleeding in the operative field and to facilitate the confection of anastomosis between LAD and LIMA which takes 10 to 15 minutes.
Thus, I conclude this patient is a candidate for off-pump surgery, requiring a LIMA on LAD for all his prolonged life.
Conflict of interest statement
The author has no conflict of interest to declare.
How would I treat?
The Invited Experts’ opinion
Simplex Sigillum Veri
The case presented poses a challenge, as the patient has had previous coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) for triple vessel coronary disease. Furthermore, he has chronic kidney disease (CKD) with a high logistic EuroSCORE. He now presents with a history of over one month of unstable angina with no clear evidence as to the culprit area of ischaemia. There is clearly significant disease in the saphenous vein graft (SVG) to the left anterior descending (LAD) coronary artery, with additional disease in the circumflex artery and left main stem; the significance of the latter is uncertain from the current images and may require further functional testing.
Initially in this case, following angiography, we would discuss with our multidisciplinary team the possible option of further CABG (the internal mammary arteries were not used in the initial surgery). It is common for graft failure to develop following CABG1 and in view of the high-risk associated with redo-surgery, PCI often remains the revascularisation method of choice.2 Moreover, in view of the comorbidities of the patient in this case, it is likely that the chosen treatment route would be PCI.
In consideration of the unstable clinical presentation, anterior hypokinesia on echocardiography and angiographic findings, we presume that the culprit lesion in this patient is the LAD territory, rather than the circumflex or left main stem. Traditionally, PCI of the native vessel is preferable to that of a graft, especially SVGs, which are often degenerate, since this does carry higher acute and long-term risks.3 However, PCI to the SVG may be preferred in the case of complex lesions, including chronic total occlusions (CTO) in native vessels of the same territory.
Therefore in this case, we have two choices to consider percutaneously. The first is to try to recanalise the CTO of the native LAD. This would be complex as the CTO is long-standing (present for at least nine years) with the additional high-risk of developing contrast-induced nephropathy in a patient with CKD. Furthermore, from the limited images, the CTO stump appears blunt and proximal with no clear point of entry, with no information regarding collaterals to allow for a retrograde approach. PCI to the SVG is the alternative option, with a recent retrospective study demonstrating that SVG PCI constituted an increasing proportion of post-CABG PCI in patients more than five years following surgery, due to progression of native coronary artery atherosclerosis.4
Taking the above into consideration, our choice in the first instance would be to perform PCI to the LAD-SVG.
Percutaneous management
In view of the baseline CKD, the patient should be adequately pre-hydrated and the renal function optimised. It is noted that for the diagnostic coronary angiogram that the transfemoral approach was refused by the patient, however this would be preferable as repeat radial access would be difficult. We would utilise a standard 6 Fr sheath and guiding catheter providing adequate support, such as an AL1 (Cordis, Johnson & Johnson, Warren, NJ, USA). As anticoagulation, unfractionated heparin 100 IU/kg should be administered, however, bivalirudin has been to shown to have similar outcomes in SVG PCI.5 Additionally, glycoprotein IIb/IIIa inhibitors could be considered if there was any evidence angiographically of thrombus, but this has been shown not to improve outcomes when used in conjunction with embolic protection devices.6
Firstly, we would use a workhorse coronary guidewire to secure access to the LAD-SVG and then pass a filter wire parallel to this. It has been shown that distal balloon tipped guidewires reduce major adverse cardiovascular events (MACE),7 with similar results demonstrated with the use of filter wires.6,8 We would then prepare the lesion adequately with predilatation, before implanting a drug-eluting stent (DES) (removing the buddy-wire first) which have better outcomes than bare metal stents (BMS),9,10 including in the recently published ISAR-CABG, which randomised over 600 patients to BMS or DES.11 Post-dilatation with a non-compliant balloon is not necessary unless concerns remain regarding stent under-expansion.
Finally, regarding whether complete revascularisation should be performed during the index procedure, it is still debated.12-14 In this case, if the contrast load was not too high or the duration of fluoroscopy not excessive, we could consider completing the revascularisation with PCI to the circumflex lesion and then PCI to the left main stem after assessing the significance of the lesion that appears “intermediate” by the limited angiographic images with the support of adjunctive tools (intravascular ultrasound and/or fractional flow reserve). Otherwise, we could consider complete revascularisation in a second procedure at a later date.
Conflict of interest statement
The authors have no conflicts of interest to declare in relation to this manuscript.
How did I treat?
Actual treatment and management of the case
Percutaneous intervention was decided to be the therapeutic option for this case. The strategy was to attempt recanalisation of the native LAD CTO retrogradely through the SVG after dilatation of its ostium, and to treat the LCX lesion antegradely after dilatation of the LM lesion. A 7 Fr Amplatz left 1 (AL1) (Cordis, Johnson & Johnson, Warren, NJ, USA) GC was used to cannulate the SVG to LAD via the high right radial approach about 10 cm above the styloid process, and a 5 Fr Ikari-left 3.5 GC (Terumo, Tokyo, Japan) to engage the LM ostium. A 0.014 inch Runthrough™ NS floppy guidewire (Terumo) was advanced through the SVG into the distal LAD and a 2.5×20 mm Maverick balloon (Boston Scientific, Natick, MA, USA) was used to dilate the ostium of the SVG (Figure 2A). A Fielder FC guidewire (Asahi Intecc Co. Ltd., Osaka, Japan) with the support of a Finecross microcatheter (Terumo) was advanced retrogradely to the proximal LAD, then the Finecross was exchanged to a 1.25×10 mm Ryujin plus over-the-wire (OTW) balloon (Terumo) for a better support. The Fielder FC guidewire was replaced by a Miracle 3 (Asahi Intecc Co. Ltd.) guidewire, and with the use of the non-coaxial anchoring balloon technique by inflating a 2.5×20 mm Maverick balloon in the distal LAD, we were able to advance the wire across the LAD CTO segment (Figure 2B). Further advancement was achieved through the diseased LM and into the 5 Fr Ikari-left GC with the support of Ryujin plus OTW balloon inflated in the proximal LAD (Figure 2C). The retrograde wire was trapped inside the antegrade GC by inflating a 2.5×20 mm Maverick balloon advanced through an antegrade Runthrough floppy-wire in the distal LCX (Figure 2D). The Ryijin plus balloon was exchanged for a 1.5×10 mm Ottimo balloon (Kaneka, Osaka, Japan) which was inflated retrogradely through the proximal LAD to the LM up to 16 atm. Stepwise retrograde dilatations were conducted by 2.0×20 mm and a 2.5×20 mm Maverick balloons inflated to 18 atm. Intravascular ultrasound (IVUS) study using the Volcano S5 imaging system, was performed by advancing the IVUS catheter retrogradely through the SVG into the proximal LAD and the LM. The pre-stenting IVUS confirmed the true lumen passage of the retrograde guidewire and provided the full data needed for proper selection of the stent sizes and lengths. Before the LM stenting, we shifted to treat the LCX lesion using a Maverick 2.5×20 mm balloon inflated at 16 atm, followed by the implantation of a Vision 3.0×23 mm stent (Abbott Vascular, USA) (Figure 3A). With the support of a 2.5×20 mm Maverick balloon inflated in the antegrade catheter to anchor the retrograde wire, a 3.0×28 mm Xience V stent (Abbott Vascular, Redwood City, CA, USA) was advanced retrogradely through the SVG and deployed from LM to LAD at 14 atm (Figure 3B). This was followed by the implantation of a 2.5×28 mm and a 2.5×23 mm Xience V stent in the proximal (Figure 3C) and mid LAD, respectively. Post-stenting IVUS study showed under-expansion of the LM to proximal LAD stent, which necessitated high pressure inflation with a 2.5×12 mm NC Sprinter balloon (Medtronic, Inc., Minneapolis, MN, USA) at 26 atm for proximal LAD, followed by a 3.0×10 mm Durastar balloon (Cordis) inflated at the proximal LAD to LM up to 28 atm. After rewiring into the LCX with a Runthrough guidewire, the LM stent strut was opened with a 1.5×10 mm Ottimo balloon, followed by a Maverick 2.5×20 mm balloon inflated from LM to LCX at 16 atm.
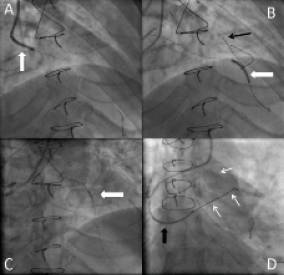
Figure 2. Panel (A) shows balloon dilatation of the ostium of SVG to LAD. Panel (B) RAO cranial view shows a 2.5×20 mm balloon anchored in the distal LAD (white arrow) to help advancing the retrograde wire (black arrow) across the LAD occlusive lesion. (Panel C) shows a Ryujin plus 1.25×10 mm OTW balloon inflated in the proximal LAD to give the retrograde wire sufficient support for crossing into the antegrade guiding catheter (GC). Panel (D) shows the retrograde wire (white arrows) trapped in the antegrade GC by inflating the 2.5×20 mm balloon inside the catheter (black arrow).
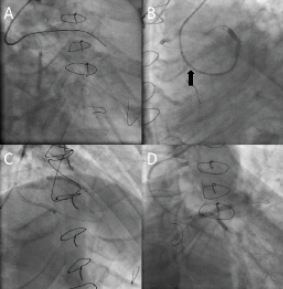
Figure 3. Panel (A) shows stent deployment across the LCX lesion. Panel (B) LAO caudal view shows deployment of LAD to LM stent via the retrograde wire which was trapped by inflating a 2.5×20 mm balloon inside the antegrade GC (black arrow). Panel (C) RAO cranial view shows stent deployment of proximal to mid LAD lesion. Panel (D) shows bi-directional kissing balloon inflation with LM to LAD balloon via the retrograde wire and LM to LCX balloon via the antegrade wire.
Final kissing balloon inflation was performed with a Maverick 3.0×20 mm balloon advanced retrogradely through the SVG to the LM-LAD, and a Maverick 2.5×20 mm balloon advanced antegradely across the LM to the LCX (Figure 3D). Simultaneous balloon inflation was performed up to 16 atm with excellent angiographic results (Figure 4A and Figure 4B). Final IVUS showed good expansion of the LM-LAD stents with full apposition of the stent struts to the adjacent vessel wall (Figure 5). The patient was discharged uneventfully three days later, and remained angina free at the follow-up period.
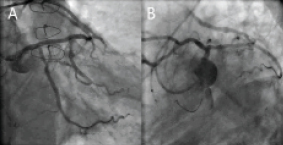
Figure 4. LCA in antero-posterior (Panel A) and LAO caudal (Panel B) projections revealed satisfactory angiographic results.
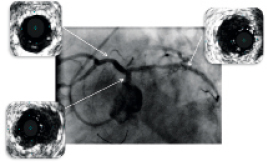
Figure 5. Post-stenting angiogram with intravascular (IVUS) images demonstrated significant gain of the minimal stent area (MSA), with MSA of mid LCX 6.9 mm2, MSA of LM 10.2 mm2 and MSA of proximal LAD 8.4 mm2, with adequate stent apposition to the vessel wall .
Discussion
The long-term benefits of coronary artery bypass surgery (CABG) are limited by the frequency of vein grafts degeneration. Five years after CABG, about 25% of the vein grafts are occluded, and >50% of vein grafts are occluded at 10 years1. In addition to a higher morbidity and mortality, redo bypass surgery is associated with less complete relief of angina and reduced graft patency2.
For our patient who had undergone CABG with SVGs to LAD, diagonal and OM branches nine years ago, the expected mortality rate of the redo bypass surgery was 11.8% according to his calculated EuroSCORE. To avoid re-operation for the aorto-ostial stenosis of the SVG to LAD and with the increased likelihood of occlusion of the SVGs feeding the other vessels in the near future, we intended to perform percutaneous revascularisation of the native coronary arteries. Moreover, the native coronary circulation was targeted for percutaneous intervention because of the greater success, lower complications and lower restenosis rate than that of angioplasty of vein grafts3.
The retrograde approach was adopted to eliminate the risk of damaging the LCX ostium during antegrade wiring of the totally occluded LAD through a diseased LM stem. Moreover, the true ostial LAD occlusion without a stump is one of the anatomical features that would be more suitable for the retrograde approach4.
IVUS has been employed in this procedure to confirm the true lumen wire course through the totally occluded LAD segment, to optimise the stenting process particularly in the left main and to limit the amount of contrast used in a patient with renal impairment.
Retrograde stenting of the LM through a SVG has been reported by Silvestri et al5. They had to deeply intubate the GC into the SVG, almost to the anastomosis of the graft to the LAD in order to get adequate backup support to advance the stent in the LM lesion. However, this method may be not applicable in all cases. We dilated the SVG-LAD ostial stenosis to provide stability for the GC during this complex procedure. Different anchoring balloon techniques had been employed for a similar purpose. For advancing the retrograde wire across the proximal LAD CTO lesion through the severe angulation between the distal SVG and the mid LAD, we used the non-coaxial anchoring balloon technique by inflating a balloon in the distal LAD to hold the retrograde GC in place (Figure 2B). The coaxial anchoring balloon technique with the use of an over-the-wire balloon also helped the guidewire to cross the LAD CTO lesion through the LM into the antegrade GC (Figure 2C). To provide the successful wiring with the sufficient support for the retrograde passage of sequential balloons into the lesion, we inflated a 2.5×20 mm Maverick balloon inside the antegrade catheter in order to trap the retrograde wire, helping the subsequent advancement of the IVUS catheter and stent deployment across the target lesions.
We intended to treat the downstream distal LCX lesion before stenting of the LM, to avoid difficulties that could originate from delivery of the stent into the LCX lesion through the struts of the LM stent. After deployment of the LM stent cross-over from the LCX to the LAD, we opened the LM stent struts into the LCX ostium.
The antegrade 5 Fr GC was too small to accommodate the two balloons for KBI. So, we employed the retrograde-antegrade approach by advancing one balloon through the SVG to LAD-LM and the other one antegradely from LM to LCX. Final KBI was performed to correct any stent deformity that might happened during dilatation of struts into the LCX ostium, and ensure full expansion of the stent in LM segment. This was confirmed by final IVUS which revealed full apposition of the stent struts into the adjacent vessel wall throughout the whole length of the diseased segment.
We conclude that, different innovative methods and techniques should be employed to make PCI more feasible and safe and to avoid re-operation in facing of increased number of patients with CABG failure presenting with recurrent symptoms.
Conflict of interest statement
The authors have no conflict of interest to declare.

