CASE SUMMARY
BACKGROUND: A 68-year-old male with a history of two prior coronary artery bypass grafting (CABG) procedures presented with a non-ST-segment elevation myocardial infarction (NSTEMI), and post-myocardial infarction angina. He had severe bilateral aorto-iliac atherosclerotic disease, and had undergone a prior bilateral aortofemoral artery bypass grafting, and femoral-femoral crossover bypass grafting. He underwent urgent coronary angiography given the high-risk features including persistent angina on medical therapy.
INVESTIGATION: Physical examination, ECG, laboratory investigation, diagnostic coronary angiography, percutaneous coronary intervention, and invasive haemodynamic assessment.
DIAGNOSIS: Significant calcific LMCAD, and left anterior descending (LAD) artery atherosclerotic disease in the setting of occluded coronary bypass graft to the LAD, and a depressed left ventricular ejection fraction. The patient was deemed to be inoperable for a third CABG.
MANAGEMENT: High-risk percutaneous coronary intervention to the calcific left main and LAD stenosis, with the mechanical support of an Impella 2.5 left ventricular assist device inserted through a left axillary artery approach.
KEYWORDS: heart assist device, Impella 2.5, left main, percutaneous coronary intervention, peripheral vascular disease, stents
PRESENTATION OF THE CASE
A 68-year-old male, 100 pack-year tobacco smoker, with a profound history of coronary artery disease including a history of coronary artery bypass grafting (CABG) in 1987, and a re-do CABG in 2000, presented to the emergency department with a non-ST-segment elevation myocardial infarction (NSTEMI), and post-myocardial infarction ongoing angina. His past medical history was notable for severe bilateral aorto-iliac atherosclerotic disease, and he had undergone a prior bilateral aortofemoral artery bypass grafting, and femoro-femoral crossover bypass grafting. Other pertinent past medical history entities included hypertension, dyslipidaemia, type 2 diabetes, chronic atrial fibrillation, severe chronic obstructive pulmonary disease (COPD), and cerebrovascular disease with multiple transient ischaemic attacks (TIAs) for which he had undergone a left carotid endarterectomy for significant left internal carotid stenosis.
On arrival at the emergency department, he was noted to be suffering from ongoing chest pain, and diaphoresis. He was haemodynamically stable, and his electrocardiogram did not demonstrate evidence of ST-segment elevations. The patient was started on a nitroglycerine infusion with persistent chest pain. His initial troponin was noted to be 20 ng/dl. Bedside echocardiography revealed a depressed left ventricular ejection fraction below 25% with moderate to severe anterior wall hypokinesia.
He was subsequently referred for urgent transradial coronary angiography given the high-risk features including persistent angina on medical therapy.
On arrival at the catheterisation laboratory, the right radial artery was accessed and coronary angiography demonstrated serial complex and calcified LM and LAD stenoses (Figure 1A- Figure 1D, Moving image 1A-Moving image 1C). The Ramus intermedius artery and left circumflex artery were diffusely diseased. The right coronary artery was dominant with chronic total occlusion in the mid segment. All coronary bypass grafts were found to be occluded except the saphenous vein graft to the right posterior descending artery (RPDA) which was patent and supplied faint collaterals to the obtuse marginal and LAD arteries.
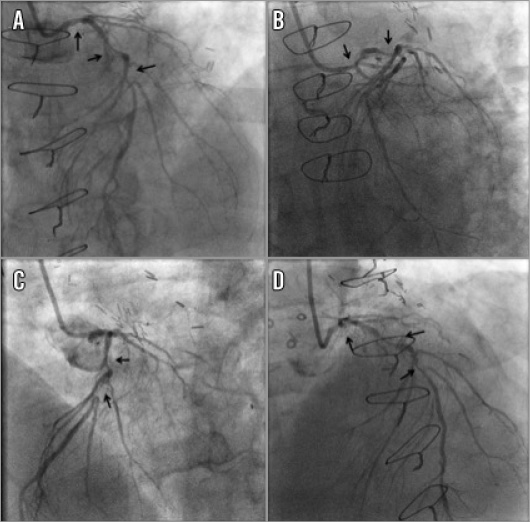
Figure 1. Left coronary angiography. A) Left anterior oblique-cranial view: diffusely calcified, severe left main (LM), proximal and mid left anterior descending artery (LAD) stenoses (arrows). B) Left anterior oblique-caudal view: diffusely calcified, severe LM and proximal LAD stenoses (arrows). C) Left anterior oblique 60 degree view: diffusely calcified, severe proximal and mid LAD stenoses (arrows). D) Anteroposterior cranial view: diffusely calcified, severe LM, proximal and mid LAD stenoses (arrows).
The cardiothoracic surgery department was consulted for consideration of urgent surgical revascularisation. However, his Society of Thoracic Surgeons (STS) score for a third CABG was elevated at 13.5, given the history of re-do CABG, severe COPD, previous TIAs and severe peripheral arterial disease. The patient was considered inoperable for a third CABG.
How would I treat?
THE INVITED EXPERTS’ OPINION
Complex coronary interventions are a challenging endeavour, especially when required for the treatment of patients who present not only with complex coronary artery disease but also with impaired left ventricular function and relevant comorbidities, rendering them high-risk. The patient presented by Ibrahim et al exhibits all three of these high-risk features. Based on the clinical presentation and the complexity of the coronary disease, revascularisation appears to be urgently required in this scenario. However, what are the reasonable options for this particular high-risk patient who is considered inoperable for a third CABG by Heart Team consensus?
If an inoperable NSTEMI patient with such coronary artery disease and depressed left ventricular function presented at our institution, complete revascularisation of LM and LAD would be performed under left ventricular support using the Impella LP 2.5 or CP1 device via transfemoral access with the intention to maintain haemodynamic stability during potentially extensive lesion preparation and subsequent stent implantation in order to lower the periprocedural risk.
While such a “protected” PCI generally seems a reasonable and feasible approach, the true dilemma in the presented case remains the access for the Impella device, since a transfemoral approach is apparently not eligible. In such a case, we would opt for a completely percutaneous left axillary approach with direct puncture and suture device-mediated closure in the so-called “preclosure” technique. In order to facilitate direct puncture of the axillary artery and as a safety net in case of closure device failure, we would place a wire via the ipsilateral brachial artery access into the subclavian artery which is then used as a fluoroscopic landmark during puncture and may be used for bail-out stenting in case of an access-site complication. Still, the anatomic feasibility of this approach needs to be confirmed prior to embarking on this strategy. Since the case represents an emergent setting, we would do this angiographically rather than by CT, which would be our standard approach for access evaluation in elective cases.
A second option would be a surgical approach, which would even allow the insertion of the larger-bore Impella LP 5.0 device for more extensive haemodynamic support. This approach can be either a simple surgical cut-down or even a side graft cannulation of the right axillary artery. The latter approach would also enable long-term support, if required after the intervention, allowing rehabilitation of the patient. However, this approach would add further procedural and logistical complexity.
If both of the above approaches were not feasible, we would probably need to proceed without protection. Theoretically, a transfemoral caval-aortic access, as recently described for transcatheter aortic valve implantation2, could eventually be considered as an ultimate option. Nevertheless, this approach would require the availability of special interventional equipment as well as special expertise of the operator.
Conflict of interest statement
Philipp Kahlert has received modest speaker’s fees from Abiomed Europe GmbH. The other authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
This case nicely highlights different challenging issues we face in such patients. First, patients with a prior history of coronary artery bypass grafting (CABG) (twice here) are challenging patients to be treated either by surgery or by percutaneous coronary intervention (PCI). Also, patients with critical peripheral arterial disease (PAD) represent a difficult population when presenting with impaired left ventricular (LV) function, while some of the LV assist devices cannot be used as in standard patients (e.g., intra-aortic balloon pump [IABP]).
The first discussion point about the method of revascularisation is quite straightforward here, while such patients with prior re-do CABG, severe COPD and PAD are definitely bad candidates for surgery, which will be associated with very high perioperative mortality. Therefore, the PCI strategy seems reasonable for this patient.
Although complete revascularisation should be the aim in patients with multivessel disease, for this specific patient the situation is different. He still has a patent graft to the right posterior descending artery supplying collaterals to the marginal. We would therefore probably only consider a PCI approach for the left main and the left anterior descending (LAD) to make it simple. Given the critical PAD, we would, of course, re-use the radial route for PCI with a 6 Fr sheath. In conclusion, the PCI strategy would be PCI of the LM and LAD through radial access using a new-generation metallic drug-eluting stent (DES). Indeed, the benefit of a DES over a bare metal stent is obvious here, and the use of a bioresorbable scaffold (BRS) in a 68-year-old man with complex and calcified lesions is not an option. LM and LAD lesions are calcified here, but we would only use a rotablator in case conventional PCI were not effective either to cross or to open the lesion. One specific question in patients undergoing complex PCI with impaired LV function is the use of an LV assist device such as an IABP or Impella during the procedure. The use of such devices is not regular practice in our centre for high-risk PCI but is mainly for patients with cardiogenic shock in the acute setting of ST-elevation myocardial infarction. Also, the critical PAD here makes the femoral access unsuitable for these devices, and considering another vascular access would also increase the risk of vascular complication (e.g., stroke), assuming that PAD is a “systemic disease”. Therefore, we would not consider an LV assist device for this patient. Finally, this patient was admitted initially for NSTEMI, and antithrombotic therapy should be optimised to improve short and long-term results of high-risk PCI. Between diagnostic angiography and PCI, this patient would be pre-loaded with both aspirin and new P2Y12 blockers (prasugrel here for a diabetic patient) at least one hour before PCI and given full anticoagulation during the procedure. GP IIb/IIIa antagonists would be used only as bail-out therapy. Finally, concerning long-term and optimal DAPT duration, we would treat this patient with 12 months of DAPT with aspirin and prasugrel and probably maintain DAPT beyond one year with aspirin and clopidogrel if there were no safety concerns during the first 12 months. In conclusion, our strategy would be PCI revascularisation through radial access with DES and no LV assist device, and long-term DAPT.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A 68-year-old male with a profound history of coronary artery disease including a history of re-do coronary artery bypass grafting (CABG) presented to the emergency department with a non-ST-segment elevation myocardial infarction (NSTEMI), and post-myocardial infarction ongoing angina. After medical stabilisation, urgent coronary angiography demonstrated serial complex and calcified LM and LAD significant stenoses with occluded coronary bypass grafts to the LAD. In the light of the elevated STS score of 13.5 for a third CABG, percutaneous coronary intervention with rotational atherectomy to the left main and LAD lesions was pursued. Given the high-risk nature of this intervention and the depressed left ventricular ejection fraction (EF), haemodynamic support during the procedure was considered. This was performed with the mechanical support of the Impella LP 2.5 litre left ventricular assist device. It was delivered through a left axillary chimney (Figure 2A-Figure 2D). The axillary artery was cut down and an 8 mm diameter Gelweave™ graft (Vascutek Ltd, Inchinnan, United Kingdom) was attached as a chimney. A 14 Fr sheath was inserted into the graft followed by an Impella delivery catheter. The Impella was successfully delivered into the left ventricle and provided appropriate haemodynamic support (Figure 3, Moving image 4). The presence of extensive aorto-iliac atherosclerotic disease precluded the peripheral insertion of the Impella device through the femoral access.
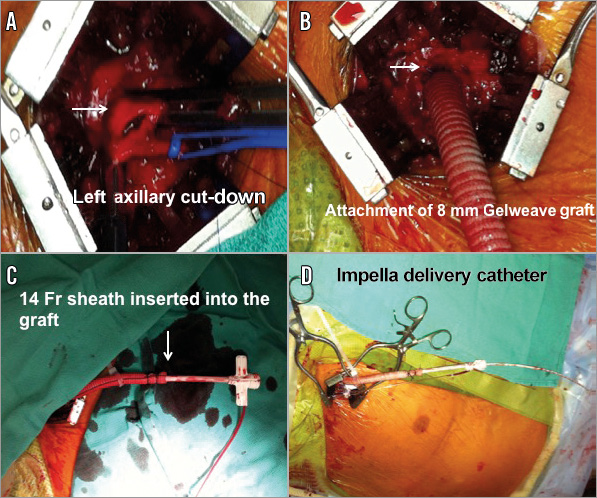
Figure 2. Axillary artery approach for Impella insertion. A) Left axillary artery cut-down. B) Attachment of 8 mm Gelweave graft as a “chimney”. C) 14 Fr sheath inserted into the graft. D) A 2.5 Impella delivery catheter inserted through the 14 Fr sheath.
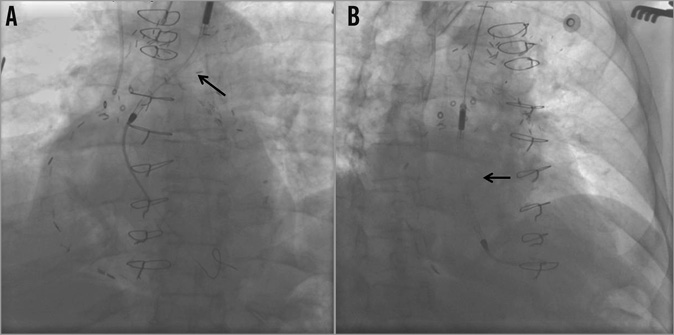
Figure 3. Impella delivery. 2.5 Impella delivered via the left axillary “chimney” (A) into the left ventricle (B).
Subsequently, a 6 Fr EBU 3.75 guiding catheter with side holes was used to engage the left main via a right femoral approach. The left main and proximal LAD calcific lesions were treated with rotational atherectomy using a 1.5 mm burr rotablator catheter with three passes at 145,000 rpm rotablation (Figure 4A). The lesion was then serially predilated with a 2.5×20 mm balloon from the mid LAD up to the left main artery. Serial stenting was then performed using 2.25×28 mm, 3.0×38 mm and 4.0×8 mm drug-eluting stents (DES) from the mid LAD up to the left main artery (Figure 4B-Figure 4F). The final angiogram revealed good distal flow with an excellent result (Figure 5A-Figure 5B, Moving image 5).
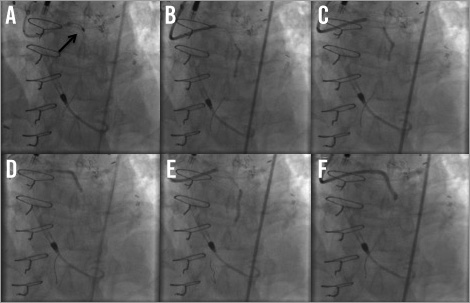
Figure 4. Percutaneous coronary intervention (PCI). A) Rotational atherectomy with 1.5 mm burr. B) & C) The LAD was serially predilated with a 2.5×20 mm balloon from the mid LAD to the left main. E) & F) Drug-eluting stents of 2.25×28 mm to mid LAD, 3.0×38 mm to proximal LAD, and 4.0×8 mm to the left main.
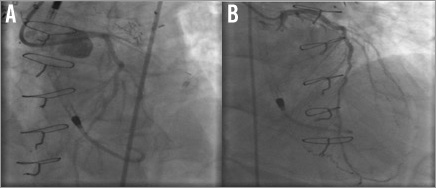
Figure 5. Final angiogram. A) Left anterior oblique view; B) posterior anterior view.
Immediately post procedure, the Impella catheter was removed. The Gelweave® graft was clipped and stapled near the anastomoses, and the overlying skin was sutured. The patient was haemodynamically stable, post-procedurally and throughout his hospital stay. Two-month follow-up was uneventful, with significantly improved quality of life and resolution of his angina. His transthoracic echocardiogram revealed a left ventricular ejection fraction of 40%. He is alive and well three years later.
Several studies and case series have been conducted revealing encouraging results with regard to the safety and feasibility of using Impella support in high-risk percutaneous coronary intervention (PCI). Recently, results from the USpella Registry concluded that the use of Impella 2.5 in high-risk PCI appeared feasible and safe in the real-world setting. The utilisation of the Impella 2.5 was successful, resulting in favourable short-term and midterm angiographic, procedural and clinical outcomes3.
At least two other case series have reported safety and feasibility for an axillary artery alternative approach for implantation of the Impella LP 2.5 and LP 5.0 in different situations involving PCI. The former case series reported successful percutaneous use of the Impella 2.5 L device via the left axillary artery in two patients undergoing high-risk PCI with concomitant severe aorto-iliac disease4. The latter reported a percutaneous and surgical approach for the Impella 5.0 L for circulatory support in ST-segment elevation myocardial infarction (STEMI) patients presenting with acute heart failure5.
The PROTECT II study enrolled patients with three-vessel disease and LVEF ≤30% or with unprotected left main or last patent conduit and LVEF ≤35%. They were randomised in a 1:1 fashion to Impella or intra-aortic balloon pump (IABP) for haemodynamic support during the PCI procedure. The 30 and 90-day outcomes were a composite endpoint of major adverse events (MAE). The 30-day incidence of MAE was not different for patients with either Impella or IABP haemodynamic support. However, when more extensive revascularisation was performed, the use of an Impella improved outcomes as compared to IABP at 90 days6. The Impella 2.5 system recently gained approval by the United States Food and Drug Administration (FDA) for elective and urgent high-risk PCI procedures.
The evidence behind the use of Impella support in high-risk PCI using the axillary approach is limited, and there are no randomised clinical trials. According to the 2011 ACCF/AHA/SCAI guidelines, there is a Class IIb recommendation for the elective insertion of an appropriate haemodynamic support device as an adjunct to PCI in high-risk patients7.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Left anterior oblique-cranial view, pre intervention.
Moving image 2. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Left anterior oblique-caudal view, pre intervention.
Moving image 3. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Anteroposterior cranial view.
Moving image 4. Delivery of 2.5 Impella catheter.
Moving image 5. Final angiogram. Anteroposterior cranial view of final angiogram after rotational atherectomy, balloon angioplasty and placement of three drug-eluting stents.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Left anterior oblique-cranial view, pre intervention.
Moving image 2. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Left anterior oblique-caudal view, pre intervention.
Moving image 3. Diffusely calcified, severe proximal to mid left anterior descending coronary stenosis. Anteroposterior cranial view.
Moving image 4. Delivery of 2.5 Impella catheter.
Moving image 5. Final angiogram. Anteroposterior cranial view of final angiogram after rotational atherectomy, balloon angioplasty and placement of three drug-eluting stents.

