CASE SUMMARY
BACKGROUND: A 75-year-old male presented with an acute coronary syndrome.
INVESTIGATION: cardiac MRI, coronary angiogram, CT scan, ECG, echocardiogram, laboratory investigations, physical examination.
DIAGNOSIS: Severe three-vessel disease with critical unprotected left main stenosis. Severely depressed left ventricular function with non-viable anterior wall. Occluded right coronary artery. Severe peripheral vascular disease.
TREATMENT: Dual antiplatelet treatment, statins, heparin, planned coronary intervention.
KEYWORDS: acute coronary syndrome, left main angioplasty, three-vessel disease, left ventricular dysfunction
PRESENTATION OF THE CASE
A 75-year-old active male with no known cardiac history presented to the emergency department with a three-week history of worsening exertional chest pain. His risk factors for coronary disease included previous cigarette smoking, arterial hypertension and dyslipidaemia, and his medications included amlodipine, valsartan and atorvastatin. He was asymptomatic on admission and haemodynamically stable. The initial electrocardiogram (ECG) showed anterior Q waves, and blood tests were remarkable for elevated high-sensitivity troponin T (427 ng/L) and mild renal impairment (glomerular filtration rate 51 ml/min/m2). He had a clear chest X-ray. He was loaded orally with aspirin (300 mg), ticagrelor (180 mg) and commenced on intravenous heparin. An echocardiogram showed a severely impaired left ventricular ejection fraction (LVEF) of 20% with anterior and apical wall akinesis, and mild mitral regurgitation.
A coronary angiogram was performed via the right radial artery using 5 Fr catheters. The diagnostic images revealed a 70% calcified distal left main (LM) stenosis extending to the proximal left anterior descending (LAD) and circumflex (LCx) arteries with stenoses of 70% and 90%, respectively. Of note, a 90º angle between the left main and the left circumflex was present. The mid LAD segment harboured a 99% stenosis, presumably the culprit lesion of his prior large anterior infarct. The right coronary artery (RCA) was dominant with proximal third occlusion and was collateralised from the left coronary system (Figure 1).
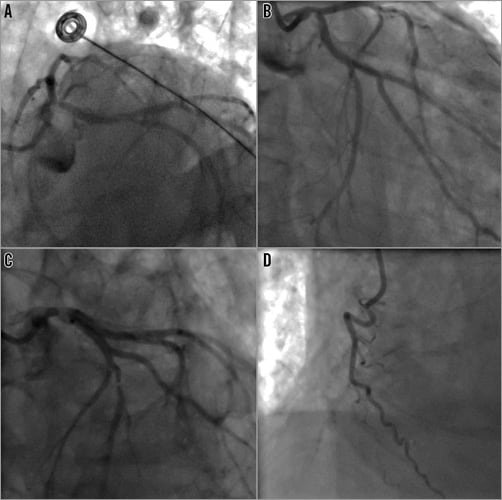
Figure 1. Coronary angiogram. A) & B) The distal left main bifurcation disease (Medina bifurcation class 1,1,1). C) The mid third left anterior descending artery stenosis. D) The occluded right coronary artery.
Immediately after the angiogram, the patient went into acute pulmonary oedema, requiring intravenous diuretics and non-invasive ventilation.
In order to investigate the viability of the anterior wall we performed cardiac magnetic resonance imaging (MRI) at 1.5 T, which demonstrated mild dilatation of the left ventricle (LV) with severely impaired systolic function and non-viable myocardium in all of the mid-distal anterior and anteroseptal walls as well as the distal inferior wall. There was viable myocardium elsewhere (Figure 2). A large mural apical thrombus was also observed. To plan the revascularisation strategy, we performed pre-contrast and arterial phase scans of the chest to the pelvis, which demonstrated a calcified 75% stenosis in the right common iliac artery with evidence of plaque ulceration and 50-75% calcified but non-ulcerated stenosis in the left common iliac (Figure 3A, Figure 3B). The external iliac and femoral arteries were diffusely diseased. The maximum common femoral artery diameter bilaterally was 4.6 mm (Figure 3C, Figure 3D). The patient was considered high-risk for surgery - logistic EuroSCORE of 20.7%.
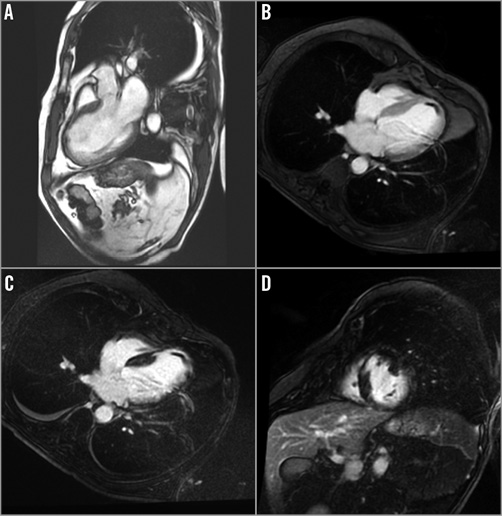
Figure 2. Cardiac magnetic resonance imaging. A) Thin and infarcted anterior wall on b-SSFP image (LVOT view). B) Early post-contrast imaging of the 4-chamber view, where a large mural apical thrombus is demonstrated. C) Transmural apical delayed enhancement on 4-chamber view, consistent with non-viable myocardium as well as mural thrombus. D) Transmural basal anteroseptal wall delayed enhancement on short-axis view, consistent with non-viable myocardium.
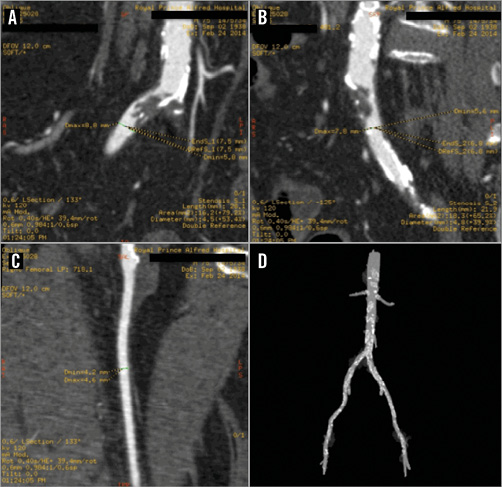
Figure 3. CT angiogram of the iliofemoral vessels. A) The right common iliac artery harbours a severe, complex (probably ulcerated) stenosis. B) The left common iliac presents a 50-75% narrowing. C) The maximal common femoral artery diameter was 4.6 mm. D) The 3D reconstruction shows calcification spread throughout the aorta and iliofemoral vessels.
What would be the best revascularisation strategy for this patient?
How would I treat?
THE INVITED EXPERTS’ OPINION
This is a complex clinical scenario in which surgical revascularisation should be considered. However, this patient is not diabetic, presents acutely with myocardial infarction, and is haemodynamically unstable with poor LV function and a non-viable LAD territory. We would therefore favour percutaneous revascularisation.
Current evidence suggests that complete revascularisation improves prognosis1. In this case, the stenosis in the distal left main (LM) into the circumflex (Cx) is likely to be the culprit. Potential targets for revascularisation are therefore the distal LM/Cx and the chronic occlusion (CTO) of the right coronary artery (RCA).
We would stage revascularisation, focusing initially on the culprit lesion. In view of the LV impairment, an LV support device should be considered. Longer-term follow-up of elective periprocedural intra-aortic balloon pump (IABP) counterpulsation in high-risk PCI suggests reduced medium-term mortality2. An Impella® LV support device (Abiomed, Inc., Danvers, MA, USA) might be even more effective but the presence of LV thrombus and iliac disease precludes its use. We would insert an IABP on the left to support the procedure. A femoral venous sheath would be placed at the same time.
A 7.5 Fr sheathless AL-1 guide from the right radial artery would give good support and allow a >1.75 mm burr, should rotational atherectomy become necessary. We would use a supportive guidewire in the Cx with a “workhorse” wire in the LAD and adopt a “provisional T”, single-stent strategy (as effective as a two-stent strategy in LM bifurcations). The LAD supplies non-viable myocardium, so would be treated as the “side branch” with the Cx as the main vessel. IVUS would assess the calcification and accurately size the vessel. It has been shown to improve outcomes. In the absence of concentric calcification, we would predilate the LM/Cx lesion and deploy a single drug-eluting stent from LM-Cx. If heavy calcification were shown on IVUS, rotational atherectomy would be cautiously performed. The “jailed” LAD would be rewired and the stent struts dilated for a “kissing” balloon inflation. This would allow future access to the LAD for a potential retrograde attempt at PCI to the RCA CTO. We would aim not to stent the LAD ostium, but would perform a “TAP” stenting if there was a suboptimal angiographic result.
To complete revascularisation we would consider PCI to CTO-RCA as a staged procedure, depending on the clinical course.
The patient should be considered for prophylactic ICD implantation and aspirin, clopidogrel and warfarin for three to six months, followed by clopidogrel and warfarin thereafter.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
We would definitely like to present the case of this patient at our Heart Team meeting. Although the surgical revascularisation is associated with extremely high risk, its advantage is that it gives a chance to repair mitral regurgitation if needed.
Percutaneous treatment would be started with left common iliac artery stenting with a balloon-expandable stent. Despite the fact that there are no clear data on coronary flow improvement by IABP, the second step would be counterpulsation insertion via the left femoral artery in order to decrease afterload, left ventricular wall stress and oxygen demand, and probably also to increase flow beyond the stenosis. In this case, the usage of a typical left ventricular assist device with the proximal end in the LV is prohibited due to large thrombus inside the LV.
We would choose the radial approach with a 6 Fr extra back-up guiding catheter, wiring both LAD and Cx. For the LCx a 300 cm-long, extra-support wire would be used in order to allow a fast change for a rotablation wire in case the lesion was non-dilatable.
The culotte technique with two DES for LM stenting should be used. The first step would be predilatation of the ostial stenosis in the LCx. If the lesion turns out not to be dilatable, it should be prepared with a 1.5 mm burr and then non-compliant balloons with relatively low pressures. The following step would be stent implantation into the LM/LCx, preferably using a new-generation DES with or without polymer with relatively large cells. The entire length of the LM should be covered. After rewiring of the LAD and opening the stent struts with smaller balloons, a second DES covering the distal LM/proximal LAD should be placed. The procedure should be finished with final kissing balloon dilatation and LM optimisation (POT) with a larger balloon post IVUS assessment. Depending on the amount of contrast used, the lesion in the mid Cx could be treated in the same session or as a separate procedure with a DES. Mid-LAD lesion angioplasty can be considered in a separate procedure only if there are collaterals to the distal part of the RCA (anterior wall non-viable). Mitral regurgitation should be assessed a few weeks after the procedure and, if needed, considered for a MitraClip® (Abbott Vascular, Santa Clara, CA, USA) procedure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Given his poor ventricular function, non-viable anterior wall, and high logistic EuroSCORE, coronary artery bypass grafting was deemed to be too high risk. The patient therefore underwent percutaneous coronary angioplasty of the distal LM under general anaesthesia with haemodynamic support using a primed veno-arterial extracorporeal membrane oxygenator (VA ECMO). Due to high plaque burden in the common iliac arteries and small diameter common femoral arteries, the arterial 16 Fr circuit was connected to the right axillary artery by surgical cutdown and the femoral 20 Fr circuit to the right femoral vein (Figure 4). Continuous support at 2.5-3.0 l/min was provided throughout the procedure. An 8 Fr sheath was inserted in the left femoral artery and an 8 Fr Judkins left coronary guide was used to engage the LM. A strategy of provisional bifurcation stenting from the LM into the LCx was planned. The LAD was easily wired but, due to the extreme angle of the LCx origin and its severe stenosis, wiring this vessel proved to be very difficult. Finally, a Fielder wire (Asahi Intecc, Aichi, Japan) using a pullback wiring technique was successful. The anatomical characteristics of the lesion did not allow a FineCross™ microcatheter (Terumo, Tokyo, Japan) to cross the lesion; therefore, a 1.5×8 mm Apex Flex (Boston Scientific, Marlborough, MA, USA) balloon was used. An anchor-balloon technique was used (2.5×12 mm Emerge™; Boston Scientific) in the LAD to facilitate balloon placement into the left circumflex. After several inflations with the small balloon, the FineCross catheter could be advanced and the Fielder wire was exchanged for a Grand Slam wire (Asahi Intecc). Thereafter, several predilatations with sequentially larger balloons were performed, to prepare the lesion adequately (Figure 5A). A kissing balloon inflation using a 3.0×15 mm Emerge balloon in the left circumflex and a 2.5×15 mm Emerge balloon in the LAD was also performed. Of note, after this inflation and subsequent balloon inflations in the LM, the arterial pressure trace became flat, with no pulsatility, and maintenance of arterial pressure was transiently completely dependent on VA ECMO support (Figure 6). Subsequently, a 3.0×18 mm Resolute Integrity™ drug-eluting stent (Medtronic, Minneapolis, MN, USA) was advanced, requiring the balloon anchor technique once again for positioning (Figure 5B, Moving image 1). After deployment, the stent was post-dilated with a 3.5×15 mm non-compliant Sprinter® balloon (Medtronic) to 20 atm, and final kissing balloon inflation with the 3.5 mm balloon in the left circumflex and a 2.5 mm balloon in the LAD was performed, achieving a good final angiographic result (Figure 7). There was normal flow in the LAD, and the residual ostial stenosis was less than 50% with no evidence of dissection. Thus, a single-stent strategy was employed (Moving image 2).
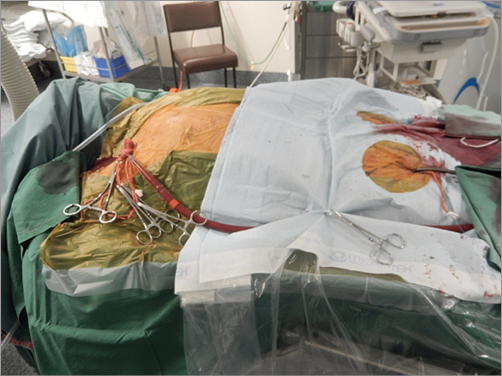
Figure 4. Veno-arterial extracorporeal membrane oxygenator (VA ECMO) connections. Due to peripheral vascular disease, the 16 Fr arterial conduit was connected to the right axillary artery and the femoral conduit to the right femoral vein.
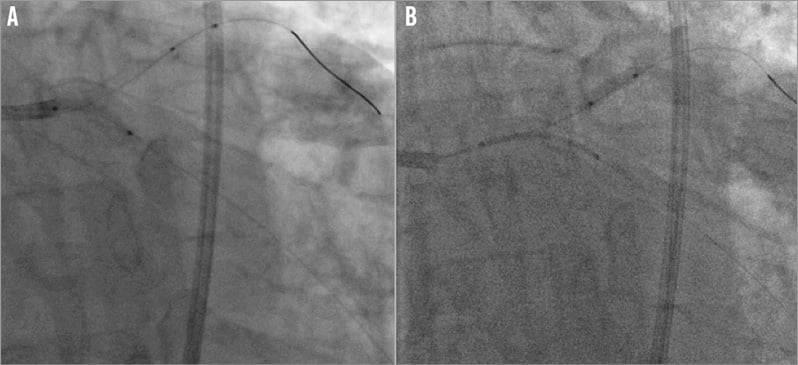
Figure 5. Angioplasty of the distal left main. A) Lesion preparation with up to a 3.0 mm balloon. B) Anchor-balloon technique to track a 3.0×18 mm Resolute Integrity stent into the left circumflex artery. The balloon was inflated in the mid third left anterior descending artery whilst the stent was pushed through the left circumflex ostium (Moving image 1).
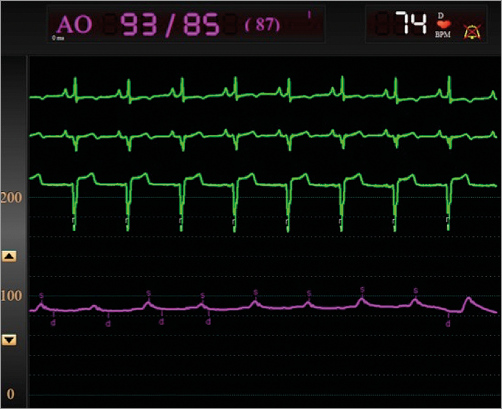
Figure 6. Flat pressure tracing after kissing balloon inflation in the left main. There was no pulsatility on the tracing and the VA ECMO supported the arterial pressure, reflecting the transient loss of cardiac output.
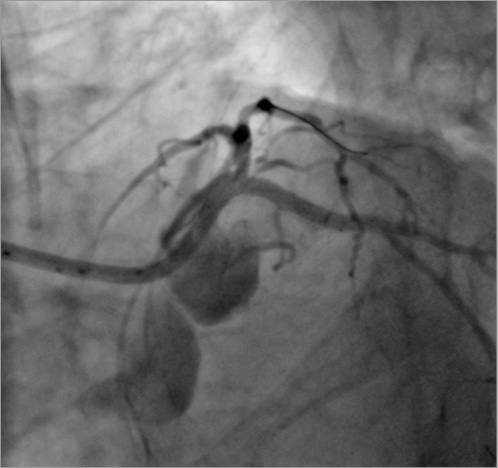
Figure 7. Final angiographic result. Good expansion of the stent and preservation of the left anterior descending artery ostium are observed. Lack of contrast clearance in the aortic root due to loss of systolic function and complete VA ECMO support at that moment (Moving image 2).
Although our initial intention was to assess the result with IVUS, the patient’s haemodynamic instability and transient complete dependence on ECMO support post stenting made us reconsider this strategy and rely on the angiographic images to finalise the procedure.
ECMO support was maintained for one hour after the procedure. After securing haemodynamic stability, the ECMO cannulas were disconnected and the arterial and venous accesses were surgically closed. Following this, the patient was extubated. The patient was kept on inotropic support for the next 12 hours. There were no post-procedure complications and the patient was discharged 72 hours post intervention.
Discussion
Complex PCI in patients with severely depressed left ventricular systolic function are associated with very high rates of short-term mortality, even with the use of partial circulatory assist devices, such as the Impella 2.5 (Abiomed) or intra-aortic balloon pump3. In this case, there were several issues complicating the procedure:
– marked left ventricular systolic dysfunction
– chronically occluded dominant right coronary artery, being backfilled by the left system
– complex anatomical characteristics of the lesion (distal LM, calcification, wide LM-circumflex angle)
– peripheral vascular disease.
As expected, the angioplasty was a complex and prolonged procedure. As the anterior wall distal to a very tight stenosis in the mid third of the LAD was not viable, the decision to stent from the LM into the LCx and provisionally stent the other branch was made. The patient did not tolerate balloon inflations in the LM, and repeatedly lost ventricular ejection after these manoeuvres, which otherwise would have led to a cardiac arrest without the VA ECMO support.
Our group have previously described the use of VA ECMO for complex cardiac interventions4. Moreover, VA ECMO has been successfully used in patients with cardiogenic shock and acute myocardial infarction as a bridge to revascularisation5. Besides VA ECMO, there are several circulatory support devices available that could be used for complex high-risk PCIs6. The intra-aortic balloon pump only modestly enhances the cardiac output and requires some residual left ventricular function to be effective7. Additionally, a real benefit in patients with severe cardiac impairment is debatable8. The TandemHeart™ (CardiacAssist, Inc., Pittsburgh, PA, USA) is an effective, although complex, support device: it requires a transseptal puncture and at least a 15 Fr arterial sheath. It is contraindicated, therefore, in patients with severe peripheral vascular disease9. The Impella LP2.5 system allows for partial haemodynamic support, slightly higher than IABP, but has a 12 Fr device shaft and is contraindicated in patients with intraventricular thrombus10, as in the present case. We chose VA ECMO because it can be connected to different accesses (the patient’s femoral arteries were too small to accommodate the 15 Fr cannula), can provide transient total circulatory support and is relatively simple to use in an experienced centre. In the present case, the device provided continuous (and transiently complete) support throughout the procedure and could be withdrawn shortly after the intervention, allowing for rapid recovery. Finally, this case underscores how careful pre-procedural planning of complex interventions in high-risk patients can lead to successful results.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Anchor-balloon technique in the left anterior descending artery to position the stent into the distal left main – proximal left circumflex.
Moving image 2. Final angiographic result.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.

