
Abstract
The 2017 European Bifurcation Club (EBC) meeting was held in Porto (Portugal) and allowed a multidisciplinary international faculty to review and discuss the latest data collected in the field of coronary bifurcation interventions. In particular, the topic of percutaneous coronary intervention (PCI) on left main coronary artery (LM) disease was highlighted as a contemporary priority. Herein, we summarise the key LM anatomy features, the diagnostic modalities and available data that are relevant for a patient’s procedural management. Since the clinical outcomes of patients undergoing PCI on LM disease may depend on both PCI team organisation and PCI performance, the optimal catheterisation laboratory set-up and the rationales for device and technique selection are critically reviewed. The best lesion preparation modalities, the different DES implantation technique choices and the strategies to be considered during PCI on unprotected LM for optimal PCI results are reviewed step by step.
Abbreviations
CABG: coronary artery bypass graft surgery
DES: drug-eluting stent
EBC: European Bifurcation Club
FFR: fractional flow reserve
IVUS: intravascular ultrasound
LAD: left anterior descending artery
LCX: left circumflex artery
LM: left main coronary artery
MLA: minimal lumen area
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
POT: proximal optimisation technique
Introduction
The European Bifurcation Club (EBC) was initiated in 2004 to support a continuous exchange of ideas in the field of coronary artery bifurcation interventions. The EBC hosts an annual meeting which brings together physicians, pathologists, engineers, biologists, physicists, mathematicians, epidemiologists and statisticians for multidisciplinary discussions. The 2017 meeting was held in Porto (Portugal) and comprised a series of dedicated sessions ending with a voting system to guide the consensus-building process. The topic of percutaneous coronary intervention (PCI) on left main coronary artery (LM) disease was highlighted as a contemporary clinical/scientific priority deserving an effort for an up-to-date EBC consensus and recommendations that are herein summarised.
Left main stem anatomy
The LM is the first segment of the left coronary artery. It is estimated that it usually supplies >75% of the left ventricular myocardium in cases of right dominant coronary circulation1. The LM arises from the left sinus of Valsalva, but anomalous take-off from (or above) the right sinus of Valsalva represents a relatively common anatomic variant2. Notably, the rare (estimated prevalence 0.03%) LM with an anomalous origin and “interarterial” course between the aorta and pulmonary artery is associated with the risk of sudden death2.
The LM is a large diameter artery with important variability across different individuals. Mean “reference” diameter, derived from a large intravascular ultrasound (IVUS) study3, is 5 mm, and ranges between 3.5 and 6.5 mm. After a mean length of 10.5±5.3 mm 4, the LM divides into the left anterior descending artery (LAD) and the left circumflex artery (LCX). In up to 25% of cases, a distal trifurcation exists due to the presence of an intermediate branch. Importantly, the size of the normal LM and its bifurcation into the LAD and LCX is predictable using fractal geometry and Finet’s law4. Although being variably oriented in the three dimensions, LM bifurcation geometry is often summarised according to the distal bifurcation angle (also called angle B) which is highly variable but often wide (higher than other coronary bifurcations), with a mean estimated value of 70-80° 4. Of note, when considering the side branch relevance according to the amount of myocardium supplied, the left main bifurcation is known to have its major side branch (i.e., the LCX) supplying >10% of the myocardial mass in more than 95% of cases5. Figure 1 summarises the key LM anatomical features.
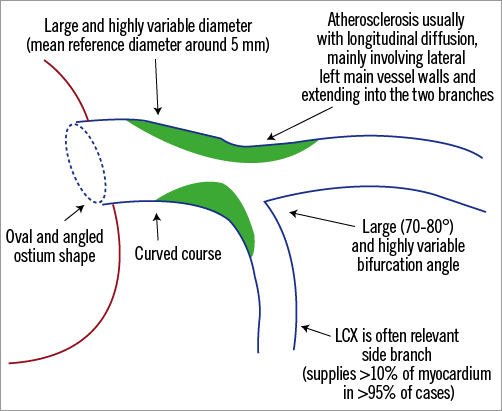
Figure 1. Schematic representation of principal left main anatomical features.
Detection of obstructive LM atherosclerotic disease is a relatively unusual occurrence in the catheterisation laboratory, accounting for approximately only 4% of all coronary angiograms, and isolated LM disease is observed in only 5-10% of these cases6.
LM disease is historically categorised into “ostial”, “mid-shaft” or “distal” (Figure 2) on the basis of angiography. Yet, atherosclerosis within the LM is usually diffuse, with frequent involvement of the bifurcation3. When atheroma develops within the vessel course, the LM usually undergoes positive remodelling, a phenomenon which may preserve lumen dimensions in some patients. Conversely, LM ostium (as well as LAD and LCX ostia) stenosis may be the consequence of localised negative vessel remodelling (sometimes without a large amount of plaque accumulation). When the LM bifurcation is diseased, atherosclerotic plaques are commonly more pronounced at the lateral walls and extend into the divisional branches3 (Figure 1). As a consequence, plaques confined to either the LAD or LCX ostium are unusual at IVUS examinations3.
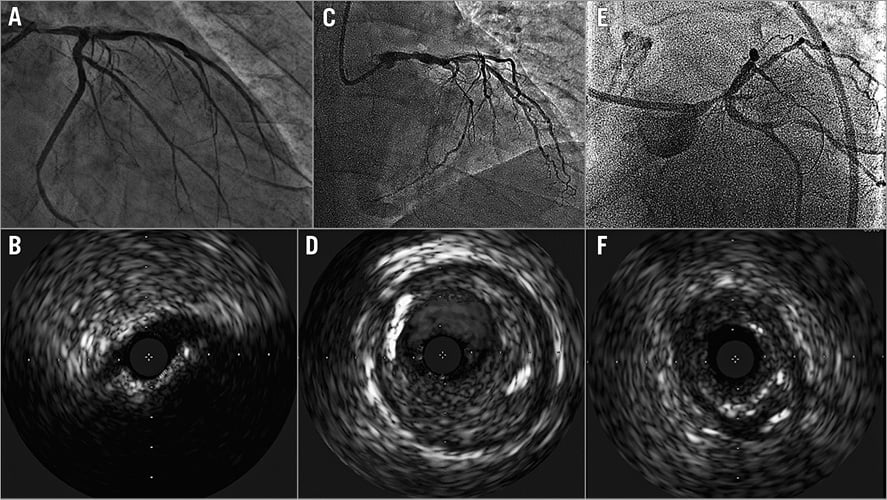
Figure 2. Examples of angiographic and IVUS findings in patients with “ostial” (A & B), “mid-shaft” (C & D) and “distal” (E & F) left main coronary lesions.
SUMMARY STATEMENT/RECOMMENDATIONS
– The LM has unique anatomic features which should be taken into account during clinical practice.
When to treat the left main?
According to the European guidelines, myocardial revascularisation is indicated for patients with LM angiographic stenosis >50% and documentation of myocardial ischaemia7. However, in clinical practice, evidence of myocardial ischaemia may be equivocal and LM disease is sometimes difficult to assess with coronary angiography (lack of appropriate angiographic views, possible absence of undiseased reference segment, interaction with the intubating catheter, etc.). Adjunctive devices (intravascular imaging/pressure wires) may help to understand the severity of LM disease.
INTRAVASCULAR IMAGING ASSESSMENT
There have been several important IVUS studies assessing LM disease severity using different parameters8. The traditional minimal lumen area (MLA) cut-off value of 6 mm2 is usually regarded as the most robust IVUS-derived threshold8. A large multicentre, prospective study supported the feasibility of treatment deferral in patients with angiographically intermediate LM lesions and an MLA >6 mm2 9. Nevertheless, three different studies investigated the possible relation between IVUS MLA and fractional flow reserve (FFR), reporting inconsistent results8. A possible important determinant for the differences in MLA estimation across studies seems to be patient population ethnicity, since significantly smaller MLA cut-offs were found in Asians vs. white North Americans8.
When planning IVUS use to evaluate the ostial lesions of the LAD and LCX, it is important to know that dedicated pullback is required to measure MLA accurately in the branch of interest.
Data regarding the possible use of optical coherence tomography (OCT) as an alternative to IVUS in LM disease assessment are limited. OCT may offer a promising high-resolution imaging of the LM lumen in the distal segment10, while proximal/ostial LM disease assessment is often technically difficult. Of note, due to the different image-generation processes, IVUS treatment thresholds cannot be applied for OCT practice.
PRESSURE WIRE ASSESSMENT
The use of pressure wires to obtain FFR or other pressure-gradient measures has become a standard part of interventional practice and may be considered for LM disease. A series of technical aspects deserves meticulous attention while using pressure wires in the LM. Importantly, equalisation of the two (guiding catheter and wire tip) pressures should be performed with the guiding catheter disengaged. Similarly, since left coronary cannulation may cause pressure drop in the presence of LM atherosclerosis, the guiding catheter should be disengaged throughout the pressure gradient assessment. Finally, to ensure maximal hyperaemia, intravenous rather than intracoronary adenosine11 is recommended.
During FFR evaluation in the absence of isolated LM disease, physiological interdependency in the coronary tree should be taken into account, as the presence of other lesions in the LAD and LCX has a recognised impact on FFR results12. In particular, both overestimation in the presence of diffuse LAD or LCX disease and underestimation because of significant side branch disease have been reported12. For instance, when concomitant distal branch disease is found, the use of pressure wire pullback manoeuvres (under hyperaemia or at rest) may be considered12.
Within the limitations of all of these technical/anatomical factors, a series of (observational) studies tested the use of FFR in the clinical management of patients with LM disease12. Different study designs have been adopted, but the available data collected so far seem to support the safety of LM treatment deferral when FFR is >0.8. So far, other (than FFR) pressure wire parameters have no accepted thresholds to guide LM disease management.
SUMMARY STATEMENT/RECOMMENDATIONS
– When LM stenosis severity is uncertain from angiography, liberal use of adjunctive devices (intravascular imaging/pressure wire) facilitating improved severity estimation is encouraged.
– Among different intravascular imaging parameters, IVUS MLA >6 mm2 and FFR >0.80 are acceptable criteria for deferring LM revascularisation.
Results for left main PCI
Recent large multicentre registries and prospective randomised trials have consolidated our knowledge regarding the suitability of PCI to treat selected patients with unprotected LM disease.
Over the years, registry results have consistently supported the feasibility of PCI for LM patients in “real-world” study populations selected and treated in experienced centres13,14. They also highlighted that PCI for ostial/mid-shaft lesions is associated with better clinical outcomes as compared with the more complex PCI for distal bifurcation lesions13.
So far, six prospective randomised trials have compared PCI vs. coronary artery bypass graft surgery (CABG) in patients with unprotected LM disease: the main characteristics and findings observed in these trials are summarised in Table 1. For instance, only the last two of them – Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease (EXCEL)15 and Percutaneous Coronary Angioplasty Versus Coronary Artery Bypass Grafting in Treatment of Unprotected Left Main Stenosis (NOBLE) trials16 – included large cohorts of selected patients with non-complex atherosclerotic disease. Due to their similar design and simultaneous publication, the different results of these two trials generated a lot of discussion. In particular, it has been speculated that differences in primary endpoint selection, differences in periprocedural myocardial infarction definitions and available follow-up lengths might have played a major role. In addition, different drug-eluting stents (DES) were used (e.g., the thin-strut everolimus-eluting stent in the EXCEL trial vs. a thicker-strut stainless steel biolimus-eluting stent in about 90% of NOBLE trial patients). The possible relevance of this issue is suggested by a striking difference in the definite stent thrombosis rate observed in the two trials (0.7% in EXCEL and 3% in NOBLE).
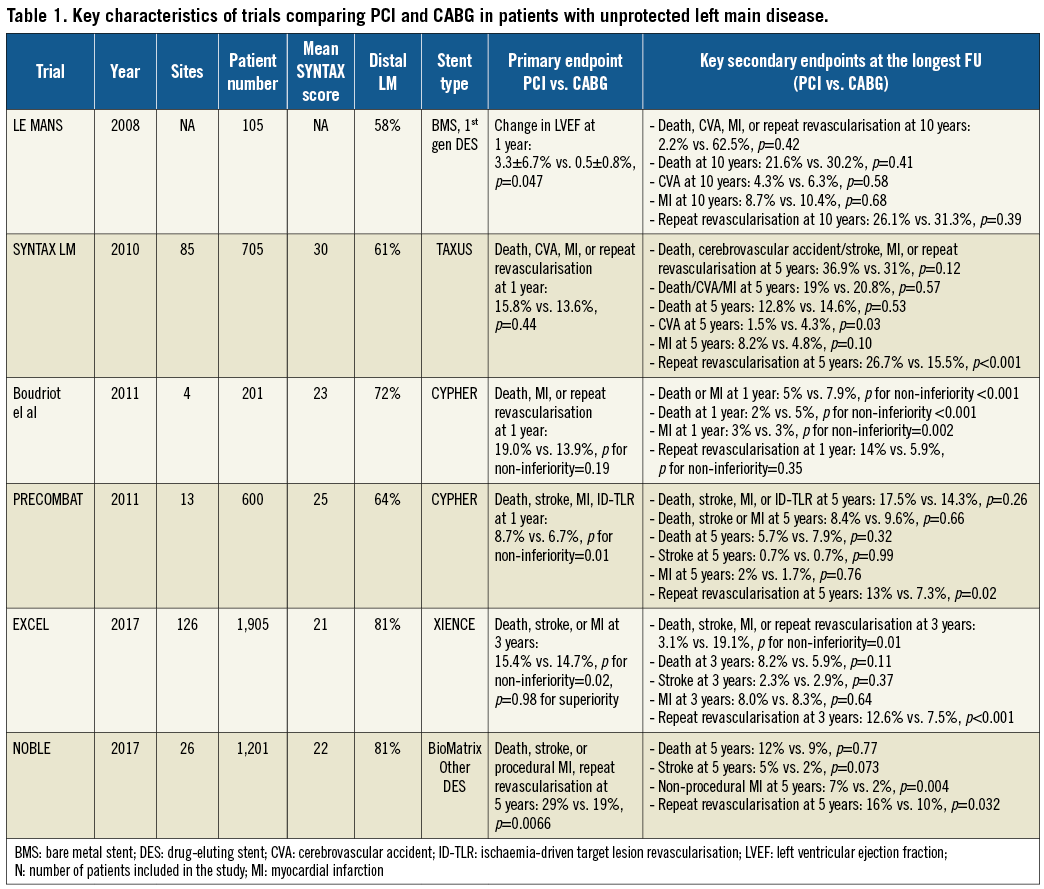
Recently, a meta-analysis including all the available trials has been carried out (also collecting key missing data in order to enable subgroup analyses)17. Long-term cardiac death differed in relation to angiographic complexity: it tended to be lower with PCI among patients with low SYNTAX scores and higher in patients with high SYNTAX scores17.
SUMMARY STATEMENT/RECOMMENDATIONS
– Registries, trials and meta-analyses suggest that PCI (performed in experienced centres) represents a valuable option for myocardial revascularisation in selected patients with unprotected LM disease.
– PCI results are influenced by LM disease pattern (bifurcation involvement) and overall coronary atherosclerotic burden (other diseased vessels, SYNTAX score).
Patient selection and cath lab set-up for left main PCI
European guidelines highlight the critical role of the multidisciplinary Heart Team in the treatment decision for stable or stabilised patients with unprotected LM disease in whom revascularisation is being planned electively or semi-electively7. In the emergency setting, on the other hand, myocardial revascularisation must be performed as soon as possible, and CABG is usually not a viable option. Notably, randomised trial data comparing PCI and CABG for the treatment of LM disease focused on selected subgroups of patients and were mainly generated from PCI centres with on-site cardiac surgery. In the absence of any data to the contrary, non-surgical centres, with appropriately trained and experienced staff, may be considered able to perform PCI as effectively as PCI centres with on-site surgery. However, to qualify for elective PCI on LM, PCI centres both with and without on-site surgery should be organised in order to ensure a correct decision-making process (Heart Team consultation), an experienced PCI team and appropriate technical equipment.
PCI TEAM
PCI on unprotected LM should always be regarded as a challenging procedure where technical problems may easily affect clinical outcomes. The operator’s experience may translate into safer decisions during both procedure planning and performance. In keeping with such a hypothesis, a retrospective Chinese study showed that patients treated by high-volume operators had better outcomes as compared with other colleagues18.
Furthermore, it is reasonable that the experience and proficiency of the whole catheterisation laboratory team (nurses, technicians, and anaesthesiologists) may play a pivotal role in successfully managing LM patients experiencing complicated PCI courses.
TECHNICAL EQUIPMENT
Cath labs where PCI on unprotected LM is electively scheduled should offer all the technical facilities that are required to evaluate (intravascular imaging, pressure wires) and appropriately treat LM atherosclerotic disease.
Of note, the need for haemodynamic support devices is anticipated to be higher in the setting of elective PCI conducted on LM patients and their elective use represents a valuable option. In some circumstances, rapid and effective deployment of haemodynamic support devices can be life-saving during complications of LM interventions. Accordingly, it is important that these are immediately available and can be inserted and activated quickly.
SUMMARY STATEMENT/RECOMMENDATIONS
– Non-emergent PCI in patients with unprotected LM should be performed by an experienced and appropriately equipped PCI team.
Setting up for left main stenting
LEFT MAIN WIRING
In patients where LM plaque is recognised to be confined to the ostium or mid-shaft, the use of just one wire is acceptable. In such cases, it is advisable to take special care of distal wire positioning (i.e., selection of a suitable distal segment of the main branch, usually the LAD) in order to have the possibility to engage/disengage the guiding catheter liberally during the PCI.
In all other cases, it seems advisable to wire both LM distal branches before first balloon dilation. A notable exception is represented by calcific LM bifurcation lesions where rotablation is planned. Systematic placement of a wire in the side branch(es), i.e., “jailed wire” technique19, increases bifurcation PCI safety by:
– favourably modifying the bifurcation angle (facilitating side branch access throughout the procedure19);
– helping to maintain side branch patency after main vessel predilation and stenting19,20;
– serving as a marker to locate a branch ostium in case of its occlusion19;
– offering the potential to re-access the side branch by advancing a small balloon according to the balloon rescue technique21 (see previous EBC consensus report11).
In the majority of LM bifurcations, workhorse wires may be chosen to wire the branches. When possible, the most challenging branch should be wired first. The second wire is then inserted in order to try to limit wire wrap risk (no rotation manoeuvres, wire kept separate outside the patient). To facilitate wiring, operators should pay attention to the guidewire tip shape22. When wiring fails (Figure 3), the first step is to modify the wire tip shape. Soft-tip wires with increased torquability or lubricity may represent reasonable secondary options. In case of persistent branch wiring failure, possible valuable options are (single, dual lumen or deflectable) microcatheters. Finally, main vessel debulking (by either rotablation or laser) can be considered in order to modify bifurcation plaque shape favourably. Due to the risk of plaque shift potentially occluding the side branch ostium, main vessel balloon predilation should be considered as the very last option, and undersized, non-compliant balloons are advisable (Figure 3).
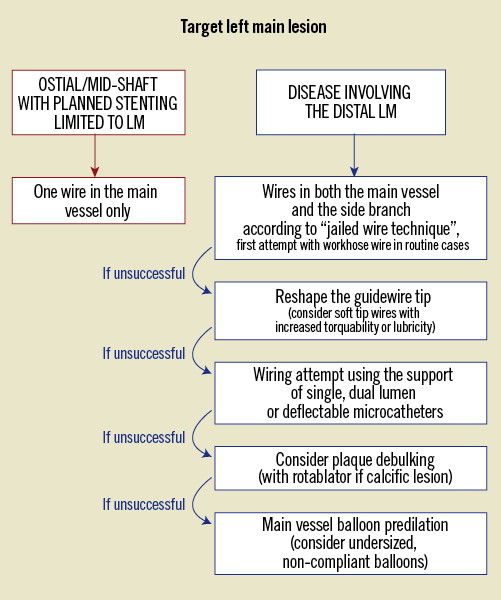
Figure 3. Wiring technique escalation for difficult branch access in left main PCI.
LEFT MAIN “PREPARATION”
The lesion preparation technique depends on the vessel anatomy and the operator’s experience. When fibrocalcific plaques are noticed, rotablation and non-compliant/scoring balloons are usually selected before stent implantation.
Side branch predilation remains controversial as there are recognised potential disadvantages which include the risk of dissection and the possible triggering of restenosis. Accordingly, routine side branch predilation is not recommended. However, in the presence of side branch severe ostial stenosis or a very calcified lesion or difficult SB access, predilation (with undersized and/or non-compliant balloons) is strongly advised.
SUMMARY STATEMENT/RECOMMENDATIONS
– Guidewire placement in both branches is recommended for all non-ostial/mid-shaft LM PCI.
– Optimal main vessel preparation is always recommended before LM stent implantation.
– Optimal side branch preparation is recommended in side branches with severe ostial stenosis or very calcified lesions or difficult access.
Left main stent sizing
The selection of a stent well matched with the LM anatomy is recognised as a critical step for successful PCI. Accordingly, the individual patient’s LM anatomy definition by intravascular imaging is strongly advised whenever angiography is unclear.
Two different scenarios determine stent diameter sizing selection in LM PCI: either a stent selected to cover the LM only or a stent selected to cover the LM bifurcation.
The first situation is encountered in LM ostium/mid-shaft PCI where the stent should be selected according to the best match with the treated LM size. DES from different manufactures are available with different diameter ranges, but only a few manufacturers provide large diameter stents whose nominal range falls within the typical LM size (4.5-5 mm) (Table 2). Bench-top tests have confirmed the ability of different DES platforms with a nominal size of 3-4 mm to maintain structural integrity when expanded to higher diameters23,24. However, major concerns exist regarding specific expansion limits, suboptimal plaque coverage (due to reduced metal-artery ratio) and the preservation of drug-elution kinetics.
The second scenario is the more common situation where the LM coverage is achieved with the same stent which is implanted in either the LAD or the LCX. In this situation, the stent size is selected according to the distal vessel size11, so that 3-4 mm DES are usually considered. Then, appropriate stent expansion in the LM is recommended according to the proximal optimisation technique (POT), using appropriately sized balloon post-dilation11. As previously stated, the feasibility of DES overexpansion has been documented in bench tests, but knowledge of the specific cut-off diameters of DES should guide selection of the most suitable stent for the individual patient’s anatomy. Table 2 shows the maximal stent expansion of some contemporary DES according to the manufacturers’ instructions for use.
Of note, the LM is associated with a specific risk of longitudinal stent deformation25 due to interference with the guiding catheter, the occurrence of stent malapposition (before correction with POT) and the possible need for advancing devices through the implanted stent (to treat distal lesions or to perform intravascular imaging). Accordingly, the resistance of the stent to longitudinal compression is a measurable parameter26 which may be taken into consideration during DES selection in specific LM interventions.
SUMMARY STATEMENT/RECOMMENDATIONS
– Knowledge of DES platform characteristics is pivotal during LM stenting procedures.
– DES should be selected and post-dilated (POT) in order to reach adequate matching with the individual patient’s anatomy.
Left main bifurcation stenting technique selection
NON-COMPLEX LEFT MAIN BIFURCATIONS
When the left main stem plaque involves one branch only (such as Medina 1,1,0 or 1,0,1), the stent strategy should aim to cover with a single stent from the most relevant and diseased vessel (usually the LAD, in selected cases the LCX) back into the main stem according to the provisional strategy11. Then, POT is recommended to be performed systematically. The selected stent should have sufficient length (8-9 mm) in the LM to accommodate an appropriately sized balloon needed for the POT post-dilation11. This, coupled with the diffuse atherosclerosis pattern in the LM, implies a stent selection length allowing covering up the LM ostium in many cases. Of note, since LM ostium coverage is associated with higher risk of longitudinal stent deformation, maximal attention to prevent this complication is required. In particular, fast correction of malapposition by POT and maximal attention to catheter interference with protruding struts are mandatory.
Whether it is necessary to perform stent side-cell re-crossing and eventual kissing balloon inflation after POT is currently unknown. Side branch ostium angiographic stenosis after MV stenting may be the result of “carina shift”, a phenomenon that may not imply functional perturbation. Expert consensus11 suggests that kissing balloon inflation should be undertaken if a suboptimal result in the side branch ostium is clearly recognised or the possibility exists for downstream PCI in the future. Therefore, in younger patients with a large subtended myocardial mass or having the LAD selected as the secondary branch (stent implanted into LM-LCX), rewiring and kissing can be considered.
COMPLEX LEFT MAIN BIFURCATIONS
When both vessels are significantly diseased, the choice of stent technique is greater and the chances of needing to stent both LM branches are higher. The EBC consensus is that the vast majority of true bifurcation anatomies can be approached using a stepwise provisional technique which includes the potential to end with double stenting if needed11. Accordingly, if the two branches have comparable importance, the more diseased vessel should initially be stented from the branch back into the LM. The use of a side branch rescue balloon technique21 may increase the overall safety of this approach11. After side branch patency confirmation, POT should be undertaken systematically. The less diseased vessel should then be rewired and a kissing balloon inflation performed, using balloons that are sized 1:1 to the branch vessel diameters. Following this sequence, an acceptable result on both branches may often be achieved. When this is not the case, a second stent can be implanted using a different strategy. Expert consensus suggests that T/TAP or culotte techniques are adequate techniques for bail-out side branch stenting11. Whenever a second stent is implanted, the performance of high-pressure kissing inflation is mandatory and may benefit from a “sequential” strategy where the two balloons are firstly individually dilated to high pressures (to ensure ostial LAD and LCX expansion), followed by a simultaneous balloon inflation at lower pressures (to avoid MV overstretch). Since kissing balloon inflation is known to be associated with proximal stent segment oval shape deformation, repeat balloon post-dilation (called “re-POT”) is advisable whenever long kissing balloon overlap in the LM occurs.
Among different techniques for elective double stenting, “DK crush” has become increasingly popular since it reduces the final kissing inflation failure rate as compared with classic stent crushing. A recent trial conducted in centres experienced with this technique reported better one-year target vessel failure rates as compared with provisional stenting27. Of note, the provisional stenting protocol in this study differed from the above-mentioned “stepwise” approach since it permitted kissing balloon inflation and, eventually, further stenting only if the side branch had residual diameter stenosis >75%, dissection ≥type B, or TIMI flow grade <3 27.
Another large European randomised trial (EBC MAIN) is currently comparing the provisional approach (with a stepwise, side branch intervention protocol) versus a planned two-stent technique28.
As a final remark, the optimal treatment of patients with LM trifurcations is as yet undefined. However, as a general approach, the EBC suggests conducting PCI procedures with the aim of limiting the amount of stent metal implanted.
SUMMARY STATEMENT/RECOMMENDATIONS
– In distal LM PCI, the stent technique should depend on the individual patient’s anatomical characteristics, but POT is pivotal.
– Provisional is the recommended technique for distal LM disease not involving both branches.
– In LM disease involving both branches, the stent technique should depend on the individual patient’s anatomical characteristics and the operator’s skill, but a stepwise provisional strategy may be effective.
– The performance of high-pressure kissing balloon inflation is mandatory for double-stenting procedures.
Left main PCI result optimisation
Intracoronary imaging (IVUS, OCT) and functional (pressure wire-based) assessments are useful to optimise the LM PCI. Their use seems justified in this setting as there is a relationship between suboptimal LM PCI results and adverse clinical outcomes. For instance, IVUS was extensively used in the EXCEL and NOBLE trials15,16.
Both IVUS and OCT can identify important intraprocedural stent features, such as stent underexpansion, strut malapposition or edge dissections, thus prompting further interventions to optimise the final result.
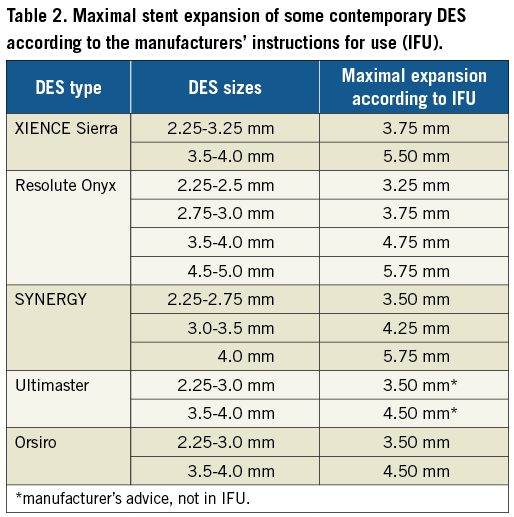
As compared with angiographic guidance, IVUS-guided LM stenting has been found to be associated with a clinically detectable benefit29,30, so that its use is strongly recommended. Past IVUS experiences allowed recognising important cut-offs of post-PCI lumen dimensions which, if achieved, may predict favourable clinical outcomes29. For OCT guidance, data regarding parameters to respect are just starting to be recognised31; however, no large study in the setting of LM is available. Automatically generated three-dimensional stent reconstructions highlight the potential for OCT as a promising tool to check important technical aspects in the course of LM bifurcation stenting such as abluminal guidewire tracking or stent side-cell rewiring identification (Figure 4).
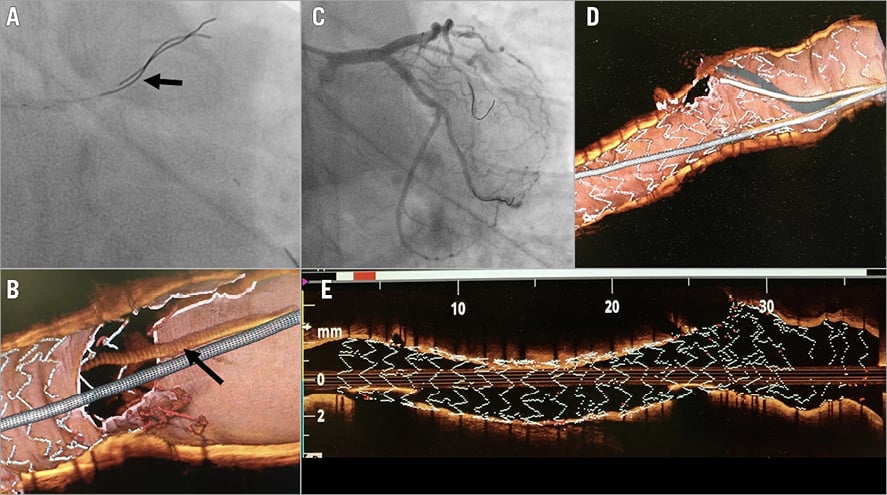
Figure 4. Examples of OCT three-dimensional reconstructions during complex left main bifurcation PCI. Panels A and B show, respectively, distal rewiring (black arrow) on angiography and 3D OCT reconstructions. Panel C shows the final result on angiography achieved with the culotte technique. Panels D and E show the optimal result as assessed by 3D OCT reconstructions.
In provisional procedures where the stent has been implanted from the LM to the LAD, LCX ostium stenosis severity is difficult to estimate. FFR has been reported to help in determining the need for further interventions32.
Improved processing (by advanced computational modelling) of intravascular imaging and pressure data has the potential to provide further data regarding LM PCI optimisation33.
SUMMARY STATEMENT/RECOMMENDATIONS
– Intracoronary imaging and functional assessment may improve the decision-making process in the course of LM PCI.
– The use of intracoronary imaging during LM PCI is recommended whenever unexpected difficulties are encountered or the achievement of an optimal result is uncertain.
Patient follow-up after successful LM stenting
After successful LM stenting, the risk of the occurrence of clinical events is tangible. Unfortunately, the optimal dual antiplatelet therapy regimen and the optimal follow-up modality are unknown, representing an important piece of missing knowledge. As a general approach, the patients successfully treated by PCI on the LM should be treated by antiplatelet therapy selected according to the setting of clinical presentation, the overall risk of thrombotic events and the bleeding risk34.
During follow-up, LM PCI patients are a high-risk subset for adverse events and an invasive management is justified in the case of symptom recurrence or documented ischaemia. Furthermore, according to European guidelines7, in asymptomatic patients who previously underwent successful LM stent implantation, late (3-12 months) control angiography may be considered (Class IIB, level of evidence C), whereas non-invasive follow-up may be considered >2 years after PCI (Class IIB, level of evidence C).
Coronary CT angiography is an emergent technique allowing assessment of the patency of LM stents35, especially in selected patients where the risk of stent-related artefacts is considered low (large stent implanted, no stent overlaps, etc.) (Figure 5).
Figure 5. Example of coronary computed tomography angiography to assess late patency after successful PCI on unprotected LM. Panels A and B show the post-PCI results achieved after the provisional strategy (crossover stenting, POT, kissing balloon inflation, re-POT). Panels C and D show the stent patency docu-mentation obtained by angio-CT scanning at long-term follow-up.
SUMMARY STATEMENT/RECOMMENDATIONS
– After successful PCI on the LM, due to the absence of specific data, the antiplatelet therapy regimen should be prescribed according to the clinical presentation.
– Intensive follow-up strategies can be considered for patients who have undergone successful LM PCI, especially when complex procedures with double-stent techniques have been used.
Conclusion
PCI on unprotected LM represents an evolving field where both PCI team organisation and PCI performance may play a pivotal role. The present EBC consensus paper summarises an updated experts’ view on contemporary best practices for LM PCI.
Conflict of interest statement
All authors have read and agree to the manuscript as written and have reported that they have no conflicts of interest relevant to the contents of this paper to disclose.
The references can be found in the online version of this article.
Supplementary data
To read the full content of this article, please download the PDF.

