
The rise of modern interventional cardiology was ushered in by the revolutionary performance of the first coronary angioplasty procedure by Andreas Gruentzig over 40 years ago. Since then, interventional cardiology has experienced several additional revolutions, including the introduction of primary angioplasty for acute myocardial infarction, the advent of intracoronary stent technology, and the most recent rise of structural heart intervention. Long gone, however, are the days of percutaneous coronary intervention (PCI) on focal 80% lesions such as the one first treated by Gruentzig. In fact, it seems that the proportion of these types of lesion decreases year after year, to the point where it is often hard to remember the last time a patient with a chronic coronary syndrome and a focal type A stenosis presented to the cardiac cath lab for PCI. Instead, what the modern day interventionalist is increasingly faced with are cases of either increasing clinical acuity or advanced patient and lesion complexity (or both)1. It is remarkable that, despite dramatically increasing clinical/anatomic complexity, the range of adjunctive tools and devices at our disposal is now so advanced that we are able to tackle these types of cases routinely in the cath lab.
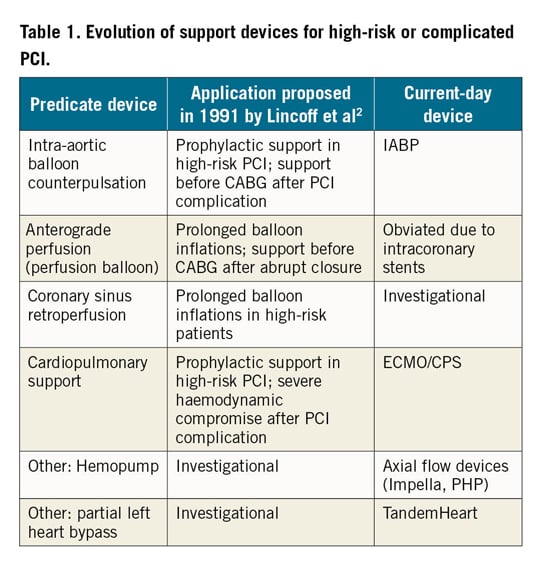
One such category includes percutaneously implanted devices for haemodynamic support. Initially used with a temporising role following cases of abrupt closure during PCI, these devices have undergone iterative developmental changes over the years. Reflection on a seminal review by Lincoff et al in 19912 demonstrates that many of the devices proposed and conceived of at that time are the same devices (albeit refined) at our disposal today (Table 1). The Impella family of transaxial support devices (Abiomed, Danvers, MA, USA) is one such type of device that is implanted using large-bore arterial access, directly unloading the left ventricle and ejecting blood into the aorta. Depending upon the specific device used, haemodynamic support of ~4 L/min can be provided with the typical percutaneous insertion of an Impella in the cath lab. Given these device characteristics, the two most common indications for the use of the left-sided Impella devices are for cardiogenic shock and for high-risk PCI. For a variety of reasons, the use of this device has undergone significant growth over the past several years. The manuscript by Chieffo and colleagues details this growth among a number of Italian sites within the IMP-IT registry3.
Notably, while the use of the device increased significantly over time within this observational registry, the absolute numbers of devices used at the 17 included sites within the study period remained small on an absolute scale, with a maximal usage of ~70 total devices for cardiogenic shock and ~45 devices for high-risk PCI in 2017. There was a high level of clinical acuity and procedural complexity of patients treated with this device, across both clinical presentations, cardiogenic shock and high-risk PCI. This is reassuring, and indicative of the relatively recent availability of the device in Italy. On the other hand, examination of the timing of device utilisation demonstrates that the majority of patients with cardiogenic shock had the device (perhaps suboptimally) implanted after PCI; even in high-risk PCI, the device was implanted after PCI in a substantial proportion of patients.
Among shock patients in IMP-IT with a median duration of Impella support of 72 hours, the majority were INTERMACS level I, with severely depressed ejection fraction, and overall in-hospital mortality was 47%. Among high-risk PCI patients, the majority had severe multivessel coronary artery disease with a mean left ventricular ejection fraction of 31%. Unlike unstable cardiogenic shock patients, the outcomes in the high-risk (and presumably non-emergent) PCI cohort were better, with an in-hospital mortality rate of 5.7%. Beyond mortality outcomes, the study investigators should also be commended for the collection and especially clinical adjudication of additionally relevant adverse events within the registry, including device-related complications such as bleeding events, vascular complications, and haemolysis. In these analyses, the overall rate of device-related complications was 37.1% in cardiogenic shock, with a 15.7% incidence of life-threatening or severe bleeding adjudicated by the relatively insensitive GUSTO bleeding scale. For high-risk PCI, the overall rate of device-related complications was lower at 10.7%, with a 5.1% rate of life-threatening or severe bleeding, despite a duration of Impella support of only a median of 1.5 hours.
In spite of the exemplary efforts of the study investigators to characterise the usage and outcomes of Impella within this registry, one of the common limitations of all observational analyses of devices such as Impella relates to how to benchmark or “anchor” these observed rates of adverse events in relation to a putative control group (either without haemodynamic support or with a different device). Rendering within-study and cross-study comparisons even more challenging is that there are probably differing thresholds for device usage at the physician and site level, as well as increasing availability of the device over time combined with a learning curve for device implantation and complication management. Observational studies of device utilisation in more mature markets can at times appear to be reassuring (and sometimes attributed to a learning curve with the device), but some of the improved outcomes may in fact be reflective of indication expansion to a different cohort of patients such as those with better ejection fraction (Popma JJ. PROTECT III First Look: High-Risk PCI Outcomes in 800 Impella-Supported Patients. Presented at TCT, San Francisco, CA, USA, 27 September, 2019). This is the fundamental problem with observational study designs – and not just isolated to this specific device. As much as we might wish to draw stronger conclusions from observational data, it is hard to be definitive about anything other than the descriptive nature of device uptake as well as the practice patterns and gross rates of adverse events (some of which may be attributable in part to the underlying patient substrate in addition to the device itself).
For all larger bore haemodynamic support devices with a significant rate of device-related complications, careful patient selection and meticulous attention to implantation/management/removal technique are critical elements in mitigating adverse events. While many of these devices have certainly provided additional options for some of our sickest patients, it is additionally imperative to identify which populations of patients are most ideally suited for them. It is not enough simply to assume the efficacy of devices that clearly have offsetting benefits and risks. Hand in hand with rising overall usage patterns comes a clinical responsibility to identify which patients are actually “protected” with these devices, and which patients may on the contrary derive lesser benefits from them while at the same time being exposed to potential harm. Unfortunately, observational data cannot provide the definitive answer for these types of questions. In some cases, it can be incredibly challenging to differentiate between outcomes directly attributable to a specific device, and outcomes related to inherent characteristics of the patients for whom that device is chosen. Increasingly, it appears that we are learning this lesson the hard way4,5, particularly in the absence of robust randomised trial evidence of device benefit. Where we go next from here is at present uncertain, but we should take careful note of how other revolutionary practices such as primary PCI for acute myocardial infarction, the introduction of coronary stent technology, and the rise of structural heart intervention came to fruition only through the conduct of serial randomised clinical trials. It behoves us to consider strongly how these precedents were established if we believe that applications for percutaneous haemodynamic support represent one of the next major revolutions within our field.
Conflict of interest statement
The author declares institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, and ReCor Medical.

