Abstract
Cardiogenic shock remains a serious complication of acute myocardial infarction as it is associated with very poor prognosis. Despite the historical clinical benefits of the intra-aortic balloon pump (IABP), in some patients additional mechanical cardiac support is necessary. The recent introduction of the percutaneous Impella® 2.5 mechanical circulatory support system (Abiomed Inc., Danvers, MA, USA) represents a major advancement and has been used in these circumstances. Nevertheless, the data supporting the use of this technology alone, after, or in combination with the IABP in patients with cardiogenic shock is limited and the clinical benefits remain unproven. We herein provide an updated comprehensive overview of the literature supporting the use of the Impella 2.5 system compared to the use of IABP in patients with cardiogenic shock. We also discuss the potential role for combination therapy for a patient with refractory shock. We describe a case in which an IABP was used as a bail-out strategy to provide additional haemodynamic support in a patient with refractory cardiogenic shock after the Impella 2.5 system was in place. In selected cases of refractory cardiogenic shock, the use of combined therapy with both the the Impella 2.5 and IABP can provide enhanced circulatory support and could be considered an option to maintain haemodynamic support in these patients.
Introduction
Cardiogenic shock in patients with acute coronary syndrome (ACS) carries a poor prognosis1-4. The optimal strategy to provide haemodynamic support in this setting involves not only prompt coronary revascularisation and use of intravenous vasopressors but also the utilisation of mechanical circulatory support therapies.
The mechanical intra-aortic balloon pump (IABP) has been historically used in these circumstances5 to decrease left ventricular workload, augment mean arterial blood pressure, and enhance coronary blood flow6-9. Recently, other forms of left ventricular assist devices (LVAD) have been developed and approved including the percutaneous Impella 2.5 partial circulatory support system (Abiomed Inc., Danvers, MA, USA) for high-risk percutaneous coronary interventions (PCI) and patients with progressive haemodynamic collapse10-16. However, the data supporting the blanket use of this technology alone, after or in combination with the IABP in patients with cardiogenic shock is limited and remains to be proven.
We herein provide an updated comprehensive review of the literature describing the use of the novel Impella 2.5 catheter system in patients with cardiogenic shock, including comparative benefits to the IABP, and the potential value of combination therapy in a patient with refractory shock.
Percutaneous mechanical support systems: IABP and Impella 2.5
The clinical and haemodynamic value of the percutaneous Impella 2.5 and IABP system has been tested and validated in both animals and humans. However, particular emphasis has been given to the individual benefits of each technology, with only limited data comparing their actual benefits against each other or in combination in patients with cardiogenic shock. Although other percutaneous mechanical assistance devices have been introduced and tested, such as the TandemHeart® Pump (Cardiac Assist, Inc., Pittsburgh, PA, USA), and the previously used Hemopump® system (Medtronic, Inc., Minneapolis, MN, USA), their overall use has been less favourably accepted in practice because they are not user-friendly and have inherent design limitations. Thus, they are not included in the scope of this review.
IABP
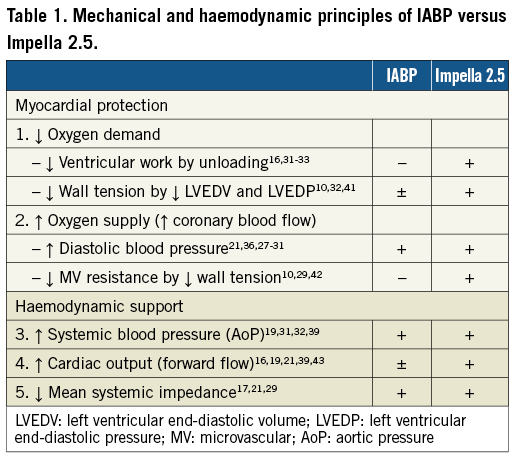
The IABP was introduced in clinical practice in the early 1970s and since then multiple studies have been conducted to evaluate its haemodynamic and clinical impact in cardiogenic shock. The physiological principal includes sequential balloon counterpulsation resulting in the augmentation of diastolic and mean aortic blood pressure, and enhanced coronary perfusion17-20. Additional haemodynamic benefits include decrease in mean systemic impedance21, increase in mean systemic pressure through diastolic augmentation, and reductions in left ventricular wall stress resulting in a decrease in myocardial oxygen consumption17 (Table 1).
Multiple studies have validated the clinical benefit of IABP in patients with acute coronary syndrome, cardiogenic shock, and high-risk PCI22-27. Based on this data, the 2004 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on ST elevation myocardial infarction (MI) recommended the use of IABP as a stabilising measure in patients with cardiogenic shock refractory to pharmacological therapy, including those with acute mitral regurgitation due to papillary muscle dysfunction/rupture, acute ventricular septal rupture, recurrent angina, poor left ventricular function, or a large area of myocardium at risk, who are being considered for prompt revascularisation. Such recommendations did not change in the 2007 focused update28.
Impella 2.5
The novel percutaneous Impella 2.5 catheter system (Abiomed Inc., Danvers, MA, USA) was introduced and cleared by the U.S. Food and Drug Administration (FDA) in 2008 under a 510(k) for use as a partial circulatory support device11-14. This small transvalvular catheter-mounted microaxial rotary pump actively pumps up to 2.5 litres of blood per minute from the left ventricle into the ascending aorta. Demonstrable haemodynamic results are multiple and interdependent, and include: reductions in left ventricular workload, wall tension, left ventricular end-diastolic pressure and coronary microvascular resistance, enhanced coronary perfusion, and increase in aortic antegrade blood flow, diastolic arterial pressure and mean systemic pressure16,29-31 (Table 1). These benefits were initially quantified and reported in acute animal models. Meyns et al32 showed in an animal model that the Impella 2.5 system could reduce myocardial oxygen consumption during ischaemia, enhance reperfusion and reduce infarct size proportionally to the degree of left ventricular unloading (Figure 1). Interestingly, in a calf model Reesink et al33 demonstrated that the Impella 2.5 was superior to the IABP and resulted in more effective ventricular unloading and circulatory support. Similar comparisons have been made to evaluate the benefit of the Impella 2.5 over open chest cardiac massage in ischaemic cardiac arrest34. In this animal study, the percutaneous device improved myocardial perfusion, maintained cerebral perfusion and systemic circulation with similar rates of successful defibrillation vs. cardiac massage.
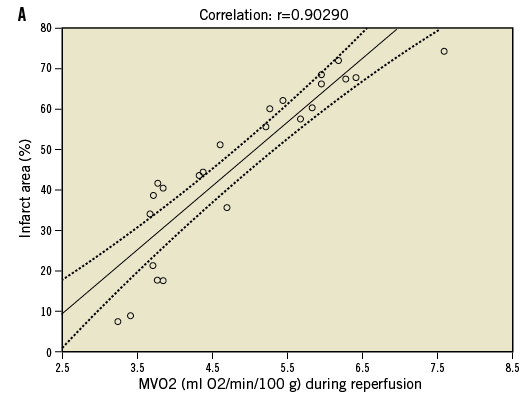
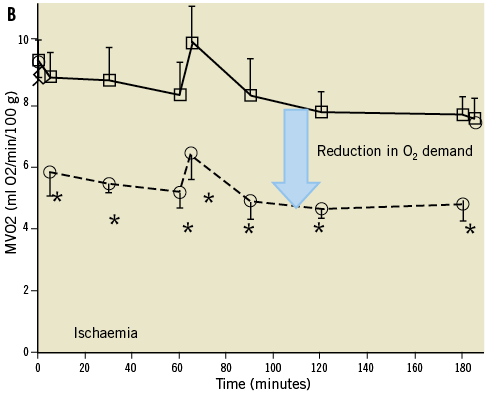
Figure 1. Effects of left ventricular unloading during myocardial ischaemia. Panel A demonstrates strong positive correlation between infarct size and oxygen demand. Panel B shows the beneficial effects of left ventricle unloading on oxygen demand and infarct size during myocardial ischaemia. Adapted with permission from Meyns B et al32
Although similar benefits have been demonstrated in humans, the majority of the Impella 2.5 data involves patients undergoing high-risk PCI. In this specific group of patients, the Impella 2.5 system was proven safe and feasible, and was associated with mean increase in aortic pressure, hyperaemic coronary flow velocities, coronary perfusion, and global improvement in cardiac output11,12,30-32,35,36. In the results of the first U.S. multicentre registry study “USpella Registry” the Impella 2.5 was associated with significant increase in left ventricular ejection fraction (5 %), a 97 percent 30-day survival rate37 and an improvement in New York Heart Association (NYHA) functional class in 52% of patients41. Moreover, lower rates of major cardiovascular events were observed when compared to standard high-risk PCI without haemodynamic support. These benefits appear to be sustained during one-year follow-up and associated with improved left ventricle (LV) ejection function35. The results of these and other clinical trials explain the increasing use and acceptance of this technology in a selected group of high-risk patients undergoing PCI12,13.
Cardiogenic shock
Impella 2.5
In patients with cardiogenic shock, the role of the Impella 2.5 system has been tested in only a limited number of cases and clinical trials involving non-randomised registry data (Table 2). In fact, most of the data evaluating the benefits of the Impella 2.5 system in patients with cardiogenic shock derives from the experienced gathered in high-risk PCI trials. Colombo et al15 was were some of the early authors to report the clinical benefits of the Impella 2.5 system in the setting of acute fulminant myocarditis and cardiogenic shock. During the same year, Meyns et al reported the safety, feasibility, and efficacy of the Impella 2.5 device in 16 patients with severe cardiogenic shock, of whom 11 had failed maximal inotropic support or IABP. The authors concluded that the Impella 2.5 system results in a significant decrease in pulmonary capillary wedge pressure (29±10 mmHg to 17±5 mmHg [p=0.04]), and increase in cardiac output (4.1±1.3 l/min to 5.5±1.3 [p=0.003]), mean arterial blood pressure (57.4±13 mmHg to 74.9±13 mmHg [p=0.003]), and end-organ perfusion16. In a different study, Rossiter-Thornton et al highlight the role for intraoperative Impella 2.5 use during cardiopulmonary bypass withdrawal for patients with severe haemodynamic compromise, shedding some light on the value of the Impella system in other important clinical scenarios38. Recent expanding applications support the potential benefit of the Impella in patients undergoing high-risk balloon aortic valvuloplasty39. Although there are currently no randomised control trials comparing the Impella to IABP and medical therapy in patients with cardiogenic shock, the use of the Impella 2.5, according to the USpella Registry data, during post-MI cardiogenic shock, resulted in a significant increase in cardiac power output and reduced mortality37.
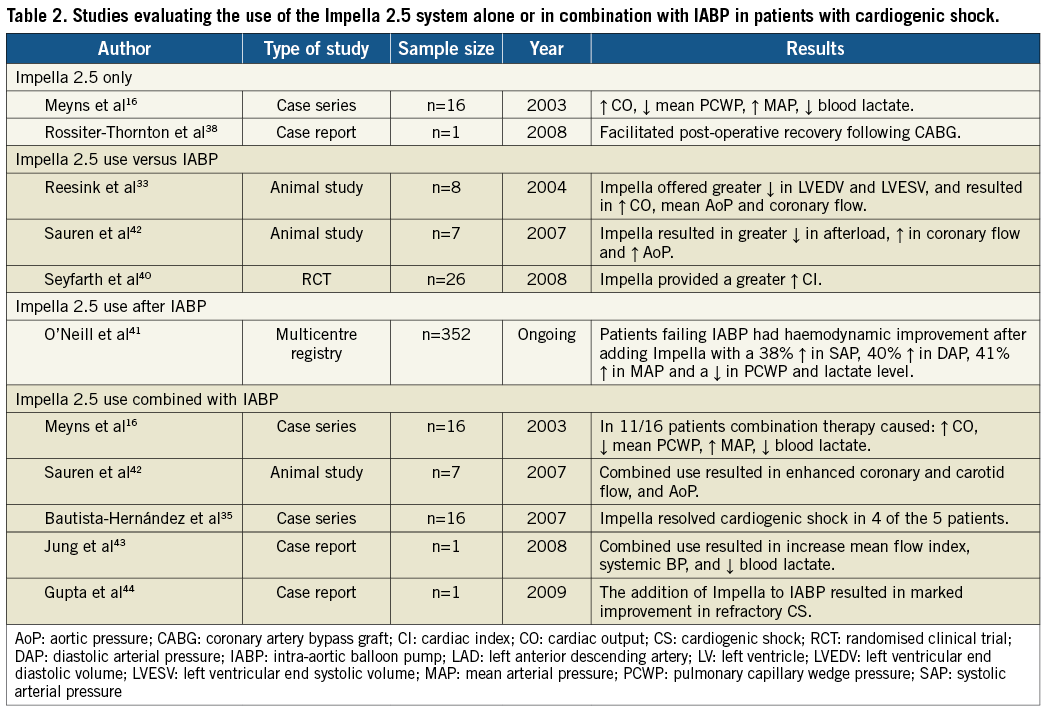
Impella 2.5 use versus IABP
Although several trials have supported the use of the IABP and the Impella alone in patients with cardiogenic shock, only a few studies have actually compared the benefits of one to the other. Seyfarth et al report their experience in 25 patients with cardiogenic shock following acute myocardial infarction and compare the Impella 2.5 system with IABP. In this study, the Impella 2.5 system provided superior haemodynamic support when compared to the IABP (Cardiac index after 30 min of support: 0.49±0.46 l/min/m2 vs. 0.11±0.31 l/min/m2, respectively [p=0.02]). However no significant differences were seen on 30-day mortality rates40. The study was however not powered for mortality.
Furthermore, randomised control trials are necessary to establish superiority between both strategies in patients with cardiogenic shock. Although some may argue in favour of the haemodynamic benefits with the Impella, the scarcity of clinical data limits the determination of one strategy over the other as first line therapy in patients with cardiogenic shock.
Impella 2.5 use after IABP
Despite the historic safety and efficacy data of IABP in cases of profound shock and low cardiac output, IABP often cannot independently support the systemic circulation. In such cases, haemodynamic support may be obtained using a more potent ventricular support device such as the Impella catheter pump.
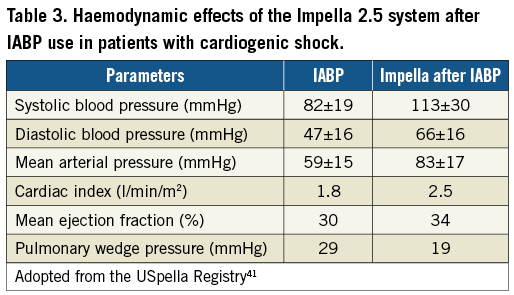
In the USpella Registry41, a total of 71 patients presented with acute myocardial infarction and cardiogenic shock. In these patients, use of the Impella increased significantly the cardiac power output and was associated with a reduction in mortality. Importantly, in a subgroup analysis of those with refractory cardiogenic shock switching from IABP to the Impella resulted in marked haemodynamic improvement, as reflected by a 38 percent increase in systolic blood pressure, 40 percent increase in diastolic blood pressure, and 41 percent increase in mean arterial pressure. Switching to the Impella improved the overall cardiac index and significantly decreased the mean wedge pressure (Table 3). Thirty-day survival rates of 96 percent were observed with the use of the Impella.
Impella 2.5 use in combination with IABP
Although one could postulate that the simultaneous use of IABP and the Impella is likely superior to either strategy alone, the safety and efficacy of this approach remains clinically untested and controversial.
Interestingly, however, in a few animal studies the combined use of IABP and the Impella provided improved haemodynamic support and further enhanced myocardial perfusion when compared to either one alone. In Sauren et al’s first report42, the combined strategy resulted in enhanced systemic, cerebral, and coronary artery blood flow and further increase in mean arterial blood pressure when compared to IABP alone. Adding the Impella to the IABP caused a significant leftward shift of the pressure-volume loop resulting in an important reduction of its area; indirectly reflecting improvement in left ventricular workload, end-diastolic pressure and myocardial oxygen demand. Each device individually contributed to the improvement seen in the myocardial oxygen “supply-demand” relationship. Importantly, the combination strategy of IABP and the Impella intensified the haemodynamic improvements beyond the benefits of either device alone (Figure 2). It is thus possible that combination therapy may in fact improve overall cardiac power output, the strongest haemodynamic correlate of mortality (Figure 3) in cardiogenic shock1.
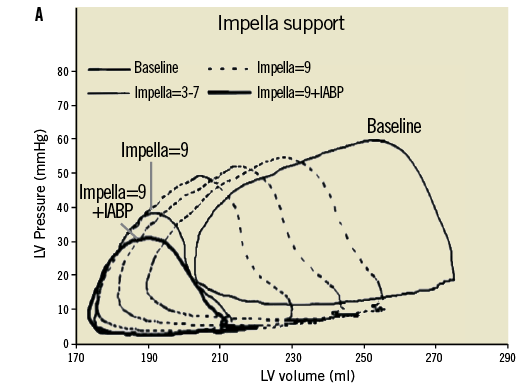
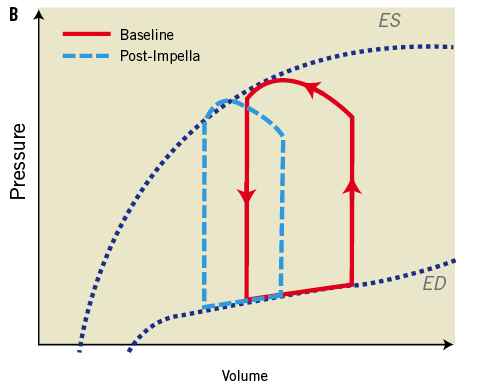
Figure 2. Effects of Impella pump on LV wall tension and ventricular workload. Panel A shows the LV pressure and volume relationship with increasing Impella support and additional effects with IABP in ischaemic myocardium15. Panel B shows improvement in pressure volume loop following the effects of Impella on LV unloading. Adapted with permission from Sauren et al15
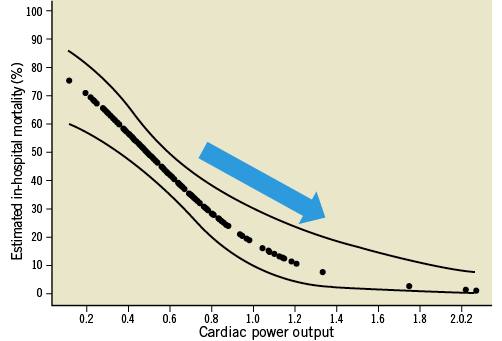
Figure 3. Improved mortality estimates with LV Impella support. Unadjusted estimates of in-hospital mortality reduction by cardiac power output in the SHOCK trial. Adapted with permission from Fincke R et al1
Importantly, according to the expert opinion of bioengineers, forward flow of the Impella catheter system may be degraded by approximately 10 percent during diastole due to IABP-induced diastolic pressure augmentation. Nevertheless, if the goal of therapy is to increase coronary flow then combination strategy may be useful, however if the goal is to enhance forward flow, an overall reduction in the Impella flow may be seen during its simultaneous use.
The clinical experience of combination therapy is represented by a few anecdotal reports. Jung et al describe a case where combination therapy improved macrocirculation and microcirculation parameters using orthogonal polarisation spectral intravital microscope in a patient with acute myocardial infarction complicated by cardiogenic shock43. Subsequently, Gupta et al report a case of profound shock due to non-ischaemic cardiomyopathy. In this report, the Impella system was successfully used in combination with the IABP for haemodynamic support44. In both cases, the authors report significant improvement in circulatory parameters. Bautista-Hernandez et al reported a series of cases where the Impella device was used in patients with cardiogenic shock following either open heart surgery or high-risk PCI despite maximum inotropic support and use of an IABP. In this case series, the use of the Impella was safe and effective; however no benefits were seen in the subset of patients with valvular heart disease35.
In this report, we describe a case of combined use of the Impella and IABP in a patient with refractory cardiogenic shock. Although limited to one case, our experience suggests that this strategy may be safe, feasible and, at times, necessary in these circumstances.
Case report
A 48-year-old man with no prior medical history presents with two-hour onset acute anterior wall myocardial infarction, resulting in acute heart failure, cardiogenic shock and severe global left ventricular systolic dysfunction with estimated ejection fraction of 10 to 15 percent. The patient was treated with full dose aspirin, intravenous heparin and BiPAP for respiratory support. Emergent cardiac catheterisation was undertaken immediately after arrival. Coronary angiography demonstrated a totally occluded proximal left main coronary artery (LMCA), with grade 2 right dominant collateral flow. Immediately following left coronary angiography, the patient developed recurrent episodes of ventricular fibrillation (VF) and asystole, requiring prolonged chest compressions, multiple shocks, temporary pacemaker placement, and airway intubation. An Impella 2.5 circulatory assist device (Abiomed Inc., Danvers, MA, USA) was promptly inserted into the left ventricle through a contralateral left femoral artery approach (Figure 4A, Figure 4B) providing flow rates of up to 2.2 litres per minute at a performance level of 8. Use of the Impella catheter pump resulted in immediate haemodynamic support and a sustained mean systemic arterial pressure of approximately 55 mmHg during refractory VF (Figure 4C).
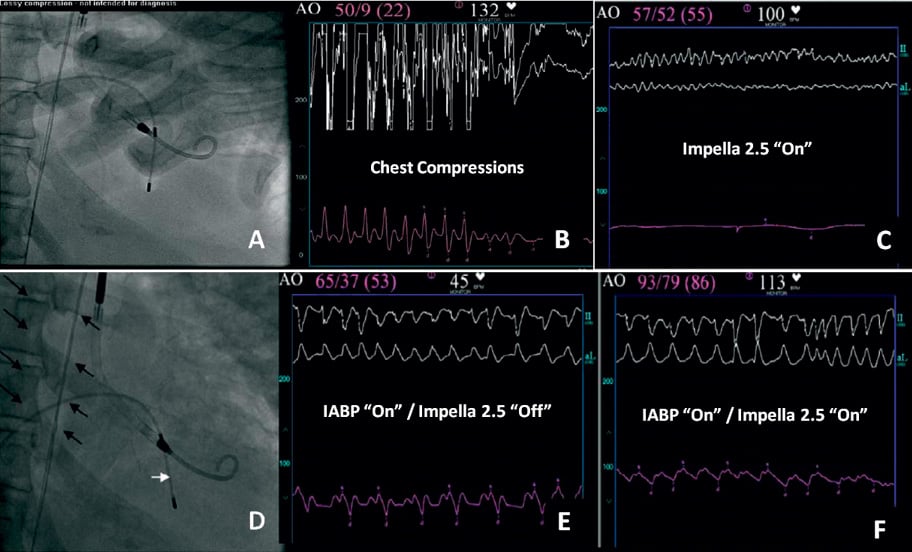
Figure 4. Panel A: Impella 2.5 catheter across the aortic valve into the left ventricle during cardiac arrest and chest compressions. Panel B: Pressure waveform obtained during manual compression (Impella in “Off” position); mean arterial pressure 22 mmHg. Panel C: Pressure wave form obtained with the Impella 2.5 during ventricular fibrillation; notice the linear continuous waveform with mean pressure of 55 mmHg. Panel D: Insertion of IABP (black arrows) in addition to Impella to improve haemodynamic support and coronary perfusion following successful revascularisation; temporary pacer wire (white arrow). Panel E: Haemodynamic support obtained with IABP only (Impella catheter in “Off” position); mean aortic pressure 53 mmHg. Panel F: Haemodynamic support during simultaneous use of IABP and Impella 2.5; notice improvement in mean aortic pressure of 86 mmHg.
Percutaneous revascularisation of the LMCA was simultaneously undertaken. Throughout the procedure the patient developed repeated episodes of pulseless ventricular tachycardia (VT) and VF, during which satisfactory haemodynamic support was provided with the Impella system. Despite successful coronary revascularisation with a left main bare metal stent, continuous use of the Impella 2.5 system, and a maximum intravenous dose of neosynephrine and norepinephrine bitartrate, refractory shock persisted. An IABP was inserted through the contralateral right common femoral artery to provide enhanced circulatory support, decrease left ventricular workload and improve diastolic systemic perfusion pressure, as shown in Figure 4D, Figure 4E and Figure 4F. The simultaneous use of the IABP and Impella catheter was maintained safely for 72 hours and resulted in no apparent complications or device malfunction. Haemodynamic stability was clinically acknowledged during combination therapy and represented by the decreasing dose requirements of intravenous pressors. Echocardiographic examination 48 hours from date of intervention revealed correct Impella catheter placement yet no improvement in left ventricular systolic function. Unfortunately, despite enormous efforts and continued improvement of haemodynamic measurements, the patient’s hospital course was complicated by persistent multi-organ failure and sepsis resulting in death four days later.
Conclusions
Although a substantial and increasing number of data exists to support the use of IABP or the Impella system in patients with cardiogenic shock, additional clinical studies will be necessary to further understand the efficacy and safety of these strategies when used in combination. This review summarises the available experience that has been gained to date with the novel Impella mechanical LV support pump in patients with cardiogenic shock, providing insight to its potential clinical role when compared to or combined with conventional IABP. This case report is one of the very few to describe the safety and feasibility of the combined use of the Impella 2.5 and IABP in refractory cardiogenic shock. Although it is intuitive to believe that the simultaneous use of both the Impella and IABP results in enhanced haemodynamic support during refractory shock, it is also possible that their combined use may in fact be counterproductive. Ultimately, further studies will be necessary to evaluate the potential applicability of this and other approaches.
Acknowledgements
The authors would like to acknowledge the editorial contributions of Dr. Daniel Raess (Abiomed, Inc., Danvers, MA, USA).
Conflict of interest statement
The authors have no conflicts of interest to declare.