CASE SUMMARY
BACKGROUND: A 49-year-old male presented with acute ST-elevation myocardial infarction (STEMI) within 38 minutes of symptom onset via the emergency medical services (EMS) to the emergency department (ED). His ED course was complicated by cardiogenic shock and he was subsequently referred for emergent coronary angiography.
INVESTIGATION: Physical examination, ECG, laboratory investigation, diagnostic angiography and invasive haemodynamic assessment.
DIAGNOSIS: Multivessel coronary artery disease presenting with STEMI complicated by complete heart block, and cardiogenic shock.
MANAGEMENT: The patient received haemodynamic support with IV fluids, vasopressors, transvenous pacemaker and intra-aortic balloon counterpulsation. He remained in refractory cardiogenic shock.
KEYWORDS: acute myocardial infarction, cardiogenic shock, emergency coronary artery bypass graft (CABG), intra-aortic balloon pump
PRESENTATION OF THE CASE
A 49-year-old male with known coronary artery disease presented at the emergency department (ED) 38 minutes after calling emergency medical services (EMS) due to severe chest pain with radiation to the jaw and profuse diaphoresis which began shortly after a verbal altercation with a student in the high school where he worked as a principal. ECG by EMS demonstrated no ST-elevation, but due to ongoing chest pain he was routed to our hospital.
On arrival to the ED, the patient was noted to be unwell with ongoing chest pain and diaphoresis. Blood pressure was 119/77 mmHg, heart rate 68/min, respiratory rate 20/min and O2 saturation 97% on ambient air. He had known coronary artery disease with stents to the right coronary and left anterior descending arteries eight years previously, hypertension, type 2 diabetes mellitus and morbid obesity. He was a lifelong non-smoker.
Initial ECG performed in the ED was non-diagnostic (Figure 1). The patient received 0.4 mg sublingual nitroglycerine for ongoing chest pain, but subsequently became somnolent and hypotensive (80/60 mmHg). Repeat ECG was performed (Figure 2). Initial troponin I was 0.01 ng/mL, and serum chemistry values were within reference ranges. Clopidogrel 600 mg po, aspirin 325 mg po and 5,000 units of intravenous unfractionated heparin were administered, and the patient was transferred to the cardiac catheterisation lab for further management.
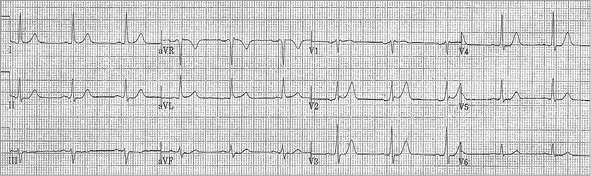
Figure 1. Initial emergency department ECG.
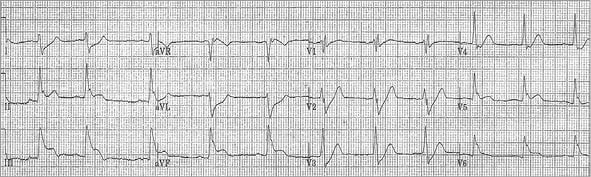
Figure 2. ECG after sublingual nitroglycerine with subsequent hypotension and somnolence.
On arrival at the catheterisation lab the patient was found to be in second degree type II heart block which subsequently degenerated to complete heart block with persistent hypotension (BP 70/30 mmHg). The right femoral artery and right femoral vein were accessed and a transvenous pacemaker was placed in the right ventricle. Despite pacemaker capture and adequate pacing, the patient remained hypotensive. An intra-aortic balloon pump was subsequently placed in the descending aorta with 1:1 counterpulsation without improvement (BP 56/40 mmHg). Intra-arterial boluses of norepinephrine and epinephrine were also administered, but the patient remained in cardiogenic shock. In order to have an accurate appraisal of the patient’s condition and to allow effective action, the decision was made to proceed with coronary angiography. Coronary angiograms are shown in Figure 3 and Moving image 1 (right coronary artery), and Figure 4 and Moving image 2, Moving image 3 and Moving image 4 (left coronary artery), demonstrating proximal occlusion of all three major epicardial coronary arteries.
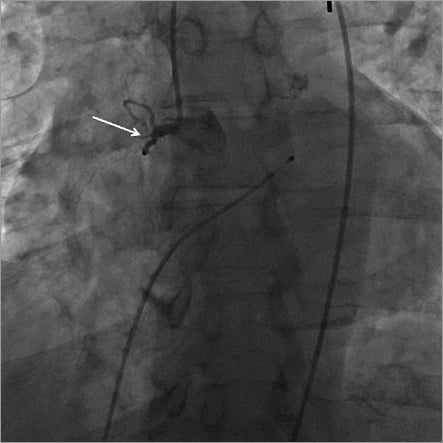
Figure 3. Right coronary artery angiogram showing proximal occlusion.
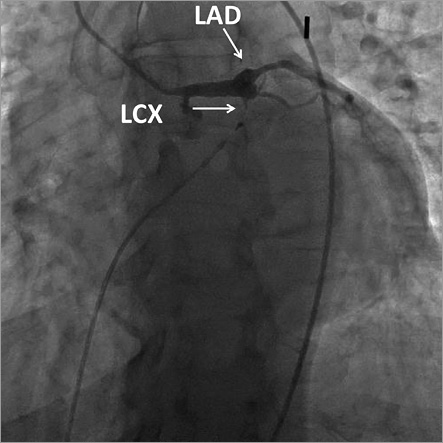
Figure 4. Left coronary artery angiogram showing proximal left anterior descending and proximal left circumflex occlusion.
The patient remained in cardiogenic shock with triple vessel occlusion despite IV fluid boluses, pressors and balloon counterpulsation. Invasive haemodynamics were obtained using a balloon-tipped PA catheter, demonstrating mean right atrial pressure of 22 mmHg and left ventricular end-diastolic pressure of 30 mmHg, consistent with biventricular failure.
How would I treat?
THE INVITED EXPERT’S OPINION
This is a very challenging obese diabetic patient with a previous history of stenting arriving at the catheterisation laboratory in profound cardiogenic shock refractory to pacing, IABP and pharmacological agents. In retrospect, the haemodynamic status deteriorated in the emergency department after nitroglycerine with the second ECG showing obvious inferoposterior STEMI and AV block. This was most likely related to the progression of RCA thrombus to complete occlusion, although the same event on dominant LCX cannot be excluded. Nevertheless, immediate bedside echocardiography in the emergency room or on arrival at the catheterisation laboratory would have been very helpful to exclude cardiac tamponade and to confirm pump failure as a mechanism of cardiogenic shock. I believe this would have had much more value than the right heart catheterisation performed in this patient later in the catheterisation laboratory.
After arrival at the catheterisation laboratory, I would, because of presumed fresh RCA occlusion, first take a JR 4 guide and quickly try to open the RCA rather than use the initial time for haemodynamic stabilisation. I recall several similar patients with very acute RCA/left main occlusion in whom such immediate recanalisation dramatically improved haemodynamic status. The operators tried to stabilise haemodynamics first but failed, which is not surprising because the heart was essentially perfused only by the ramus.
In this situation, as presented by the authors, the onset of cardiac arrest was just a matter of time, and I would immediately proceed with implantation of percutaneous femoro-femoral veno-arterial extracorporeal membrane oxygenation (VA ECMO) to stabilise haemodynamics. We do not have the Impella CP (3.5 L/min) (ABIOMED Europe GmbH, Aachen, Germany), but superior flow (up to 7 L/min) by VA ECMO would be an advantage in this very obese patient, particularly if cardiac arrest occurs during subsequent revascularisation.
The catheterisation laboratory is a perfect place for VA ECMO insertion because one can control placement of the arterial cannula tip at the aortic bifurcation and the venous cannula tip at the entry to the right atrium. Since another team simultaneously performs priming, which is easy with our portable device (CARDIOHELP; Maquet Getinge Group, Rastatt, Germany), the procedure is rather fast. I would keep IABP in place during ECMO insertion to prevent further haemodynamic deterioration.
After the prompt haemodynamic stabilisation expected by VA ECMO, I would proceed with PCI of the RCA first, because it is most likely the culprit. However, multiple culprits are also possible and even likely in this patient. If the RCA is indeed acute, recanalisation should be straightforward despite possible very late stent thrombosis as a mechanism of occlusion. If the RCA is opened, subsequent angiography may be used to evaluate the left coronary artery beyond occlusions based on right to left collaterals. I would continue with intervention on the vessel without right to left collaterals because this may indicate a second acute occlusion. In this case, the wire is expected to pass relatively easily while unsuccessful wire probing with well developed right to left collaterals would argue for a CTO. Obviously these are only preliminary thoughts on revascularisation which cannot be fully discussed at this stage when only three proximal occlusions are seen.
Conflict of interest statement
The author receives lecture fees from the Maquet Getinge Group, AstraZeneca, Ely Lilly, and Krka.
How would I treat?
THE INVITED EXPERT’S OPINION
For revascularisation of this particular case of STEMI with proximal occlusion of all major epicardial vessels, several issues need to be considered before planning the revascularisation strategy. Potential treatment options include: a) immediate coronary artery bypass graft (CABG) surgery, b) PCI of the culprit lesion only, c) PCI of the culprit lesion with staged revascularisation of the other lesions, d) immediate multivessel PCI of all lesions.
In general, fewer than 5% of patients in cardiogenic shock undergo immediate CABG1. Based on the extremely unstable situation, I would try to contact the cardiac surgeon but would immediately proceed with the preparation of PCI of the culprit lesion. In general, and also in this particular case, the Heart Team would decide –based on the time of operation room (OR) and OR team preparation for CABG– to go for PCI in this situation.
Based on the ECG and the angiogram, the left circumflex coronary artery (LCX) is most likely the culprit lesion, which would also explain the higher degree AV block. I would therefore go for aspiration thrombectomy of the LCX and implant a drug-eluting stent into the LCX.
The next step would be to consider whether to intervene also on the other lesions, i.e., the occlusion of the LAD and the RCA. I would try to intervene on the LAD which, based on the angiogram, should be possible. I would also implant a drug-eluting stent into the LAD. Based on the angiogram, I would also try to intervene on the RCA, which, however, may be more a chronic occlusion of the formerly implanted stent. I would try to recanalise this occlusion only if it can be performed with a reasonable amount of contrast dye. This would also depend on the haemodynamic stability of the patient. If recanalisation were to be successful, balloon angioplasty with subsequent drug-coated balloon angioplasty would be reasonable.
Taken all together, based on the angiogram it is not entirely clear which lesion is the culprit lesion. Therefore, all lesions should be attempted by PCI. In case there were a clearly identifiable culprit lesion, this patient would have been randomised into the currently ongoing European CULPRIT-SHOCK multicentre trial (www.clinicaltrials.gov: NCT01927549) randomising patients with cardiogenic shock complicating myocardial infarction to either immediate multivessel PCI or culprit lesion only PCI with potential subsequent staged PCI.
With respect to mechanical support, I would not have implanted an IABP, based on the results of the IABP-SHOCK II trial1,2. If the patient were to be in refractory cardiogenic shock at the end of the PCI, I would consider implanting a miniaturised extracorporeal life support system percutaneously in the catheterisation laboratory and transferring the patient to the intensive care unit afterwards.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A 49-year-old male presented to the emergency department (ED) with ongoing chest pain and evolution of an acute inferolateral ST-segment elevation myocardial infarction (STEMI) complicated by refractory cardiogenic shock despite multiple intravenous vasopressor agents and intra-aortic balloon pump (IABP) support. Emergent left heart catheterisation demonstrated 100% proximal occlusion of the left anterior descending (LAD), proximal left circumflex and proximal right coronary (RCA) arteries. Given the lack of haemodynamic improvement, despite intra-aortic balloon counterpulsation, a 12 Fr arterial sheath was inserted, and an Impella 2.5 system (Abiomed, Inc., Danvers, MA, USA) was advanced into the left ventricle for additional haemodynamic support (Figure 5). This resulted in stabilisation of the patient (SBP >90 mmHg), who was transferred to the operating room for an emergent complete revascularisation with coronary artery bypass graft (CABG) surgery while on IABP in combination with Impella for continued haemodynamic support. He received 4-vessel coronary artery bypass grafting with reverse saphenous vein graft (rSVG) to the LAD, rSVG to the posterior descending artery, rSVG to the posterolateral obtuse marginal branch, and rSVG to the first diagonal branch. First medical contact (FMC) to complete revascularisation was four hours and 24 minutes.
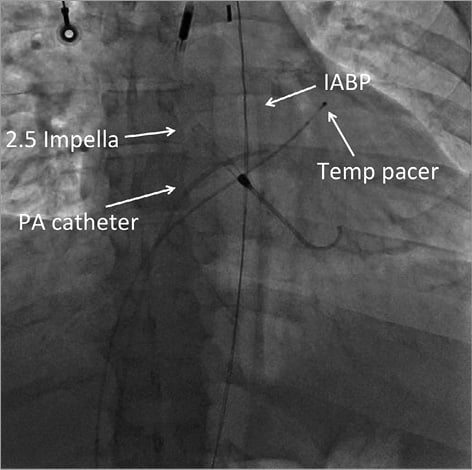
Figure 5. Hardware.
A transthoracic echocardiogram performed four weeks postoperatively revealed an ejection fraction of 15%. The patient’s subsequent course was notable for recurrent hospital admissions for acutely decompensated heart failure (HF) exacerbations, leading to a state of inotrope dependency. He was classified as American College of Cardiology (ACC), American Heart Association (AHA) Stage D, and was listed as United Network for Organ Sharing (UNOS) IA for Orthotropic Heart Transplant (OHT). The patient underwent a successful orthotropic cardiac transplantation 12 months following his initial presentation with cardiogenic shock. He has recovered and has returned to work.
Cardiogenic shock in patients with STEMI may be a consequence of extensive LV infarction or secondary to mechanical complications. Revascularisation with timely PCI or CABG is the preferred reperfusion strategy for patients with STEMI and shock due to pump failure, irrespective of the time delay. Shock and severe congestive heart failure are perhaps the only clinical scenarios in which acute revascularisation of significant stenoses in non-infarct arteries can be justified3. According to the 2013 ACCF/AHA guidelines for the management of ST-elevation myocardial infarction (STEMI), it is a Class I, LOE B recommendation to revascularise emergently with either percutaneous coronary intervention (PCI) or CABG in patients with cardiogenic shock due to pump failure after STEMI irrespective of the time delay from MI onset3. The SHOCK trial demonstrated clear benefit with emergency revascularisation (PCI or CABG) compared with immediate medical stabilisation and delayed revascularisation in patients with ST-elevation/Q-wave or new LBBB MI and cardiogenic shock4. This benefit remained statistically significant for revascularisation up to 18 hours after the onset of cardiogenic shock, and the mortality rates at six and 12 months were significantly lower in the group of patients which underwent emergency revascularisation5.
In patients with cardiogenic shock following STEMI, there is an ACCF/AHA Class IIa, LOE B recommendation for the use of IABP counterpulsation in patients who do not quickly stabilise with pharmacologic therapy3. There is a Class IIb, LOE C recommendation for the use of alternative LV assist devices for circulatory support in patients with refractory cardiogenic shock3.
The evidence behind the simultaneous use of IABP and the Impella in patients with cardiogenic shock is very limited, and there are no randomised clinical trials. However, in a few case reports, the combined use of IABP and the Impella provided improved haemodynamic support and enhanced myocardial perfusion further when compared to either one alone6-8. Adding the Impella device to the IABP led to significant improvement in left ventricular haemodynamic parameters, including workload, end-diastolic pressure, myocardial oxygen demand and cardiac power output in a patient with cardiogenic shock following STEMI6. More studies are needed to verify this hypothesis, but case reports emphasise that addition of the Impella to IABP support may improve overall cardiac power output, the strongest haemodynamic correlate of mortality9.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Left anterior oblique 19 projection. Right coronary artery angiogram demonstrating proximal occlusion with evidence of prior stenting.
Moving image 2. Left anterior oblique 21 with caudal 11 projection. Left coronary artery angiogram demonstrating proximal left anterior descending and proximal left circumflex occlusion.
Moving image 3. Right anterior oblique 33. Left coronary artery angiogram demonstrating proximal left anterior descending and proximal left circumflex occlusion.
Moving image 4. Anteroposterior projection. Hardware: 2.5 Impella, intra-aortic balloon pump, pacer wire, pulmonary artery catheter.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.

