Abstract
Despite the rapidly evolving evidence base in modern cardiology, progress in the area of cardiogenic shock remains slow, with short-term mortality still reaching 40-50%, relatively unchanged in recent years. Despite advances with an increase in the number of clinical trials taking place in this admittedly difficult-to-study area, the evidence base on which we make day-to-day decisions in clinical practice remains relatively sparse. With only definitive evidence for early revascularisation and the relative ineffectiveness of intra-aortic balloon pumping, most aspects of patient management are based on expert consensus, rather than randomised controlled trials. This updated 2020 review will outline the management of CS mainly after acute myocardial infarction with major focus on state-of-the-art treatment based on randomised clinical trials or matched comparisons if available.
Introduction
Left or right ventricular failure subsequent to acute myocardial infarction (AMI) remains the most frequent cause of cardiogenic shock (CS), accounting for approximately 80% of cases. Mechanical complications of AMI represent less frequent causes of CS (ventricular septal rupture [4%], free wall rupture [2%], and acute severe mitral regurgitation [7%])1. Given the relatively heterogeneous nature of the causes of non-infarct-related CS in comparison to AMI-CS, such as decompensated acute on chronic heart failure, valvular heart disease, acute myocarditis, Takotsubo syndrome, and arrhythmias2, treatment of each potential underlying causative factor will not be addressed in detail, but overarching guiding principles will be elucidated.
The incidence of CS complicating AMI has historically been found to be of the order of 8% for STEMI, and 5% for NSTEMI3. Although there has been some degree of disagreement in recent registries with regard to the rate of change of this figure, and whether it is in fact increasing or decreasing, overall, the most recent data do not differ markedly4,5. Despite evidence-based therapeutic advances, foremost coronary revascularisation with subsequent survival benefit, mortality rates still remain unacceptably high at 40-50%6,7. Indeed, with an ageing population coupled with an increase in comorbidities, there are data to suggest increasing mortality rates8,9. These observations appear logical in the setting of an increased prevalence of diabetes mellitus and renal disease. In the setting of the recent COVID-19 pandemic, the picture is heterogeneous10. Admissions for AMI in general were significantly reduced in Italy, along with an increase in complications including CS-AMI11. However, in Denmark and Austria, no change in the rates and mortality for CS-AMI was noted. This may, however, reflect the severity of the COVID-19 situation in each country during the period for which data were collated12,13.
The underlying causes, pathophysiology, and treatment of AMI-CS have been reviewed previously2,14. This 2020 update will focus on evidence-based therapeutic management of AMI-CS with major emphasis on current guideline recommendations, revascularisation strategies, intensive care unit (ICU) treatment, adjunctive medication, and mechanical circulatory support (MCS) devices. The main focus is set on randomised controlled trials (RCT) or, where not available, on relevant matched comparisons. Furthermore, major research areas and gaps in evidence will be elucidated.
Definition of cardiogenic shock
The central pathophysiological hallmark of CS is critical end-organ hypoperfusion and hypoxia due to reduced cardiac output as a consequence of primary cardiac disorders2. The diagnosis of CS can be made in routine practice on the basis of clinical criteria reflective of persistent hypoperfusion without adequate response to volume replacement as manifested by cold extremities, oliguria, or altered mental status in combination with biochemical manifestations of inadequate tissue perfusion such as elevated arterial lactate.
Most accepted definitions of CS include specific blood pressure parameters, generally a systolic blood pressure <90 mmHg for ≥30 minutes, or mechanical/pressor support required to maintain values above this. It should be noted, however, that in certain cases compensatory mechanisms may preserve blood pressure through vasoconstriction, while at the same time tissue perfusion and oxygenation may be significantly decreased, the so-called “normotensive CS”14. Urine output <30 ml/hr and arterial lactate >2.0 mmol/l satisfy the criteria for clinical and biochemical manifestations of inadequate tissue perfusion.
Although not mandatory in clinical practice, objective haemodynamic parameters such as reduced cardiac index and increased pulmonary capillary wedge pressure are helpful for diagnosis confirmation, and are essential in terms of more clearly defining the status of the right heart and systemic vasculature, particularly in those patients who do not respond in the expected manner to initial therapy. The pulmonary artery pulsatility index (PAPI) may have a particular role for right ventricular function assessment here15.
Prognosis assessment by biomarkers and scores
Understanding of the complexity of CS has evolved over the last decades and in recent years additional insights have been gained in the understanding of CS severity and phenotyping such as left, right or biventricular predominance. In general terms, there is a profound depression of myocardial contractility resulting in a potentially deleterious downward spiral of reduced cardiac index, low blood pressure, hypoperfusion and maladaptive cycles of ischaemia, inflammation, initial vasoconstriction and later vasodilation, culminating in various degrees of multiorgan failure with subsequent death if untreated.
Although not specific to CS, arterial lactate has been shown to be strongly predictive of mortality. Evidence for lactate in CS had been relatively sparse. A recent sub-analysis of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial and registry attempted to clarify the prognostic value for mortality of lactate clearance versus single measurements at admission and after 8 hours in AMI-CS16. The 8-hour value was a significantly better predictive parameter than baseline or lactate clearance. Lactate at 8 hours ≥3.1 mmol/l and clearance <-3.45%/hr remained independently predictive for time to death. Another new score (CLIP score) based on biomarkers has recently been introduced. Based on the CULPRIT-SHOCK biomarker substudy, the four most relevant prognostic biomarkers are cystatin C, lactate, interleukin-6 and NT-proBNP17. The CLIP score outperformed clinical CS scores and is objective without requirement for input of subjective parameters. Multiple other novel biomarkers - involving metabolomics, proteomics, genomics and transcriptomics - in addition to lactate and creatinine measuring the degree of inflammation, renal function, and liver involvement, respectively, have been shown to be associated with impaired prognosis (Table 1).
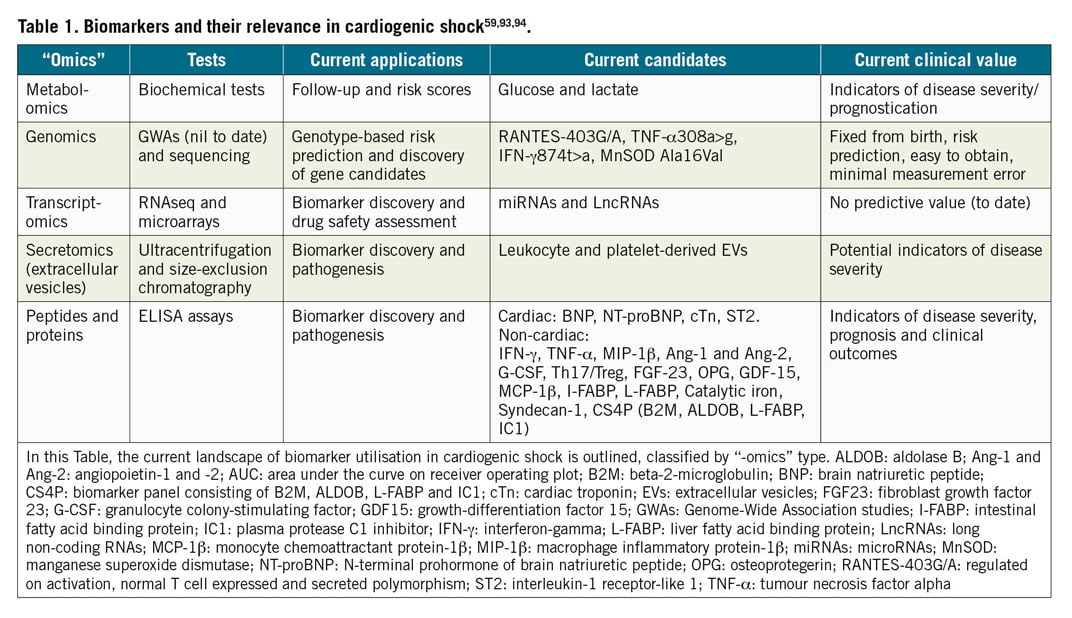
Clinical and biological factors used for prognosis assessment have been summarised in multiple scores in the I) pre-shock, II) full CS, and III) venoarterial extracorporeal membrane oxygenation (VA-ECMO) settings previously18.
Of these, the IABP-SHOCK II score, which was developed exclusively in AMI-CS patients, incorporates the biomarkers lactate, creatinine and glucose, and has both internal and external validation19. The SCAI CS classification with the stages A (at risk), B (beginning), C (classic), D (deteriorating), and E (extremis) was developed based on clinical considerations by expert consensus for both AMI-CS and non-AMI-CS20. It has been validated in several retrospective cohorts and one prospective study (Table 2),21,22,23,24,25,26,27,28. Overall, on the basis of these data, the SCAI classification appears to fulfil its goal as a tool correlating well with prognosis. The SCAI score may have its advantage as a dynamic score evaluating the course of CS over time but is currently not well suited as a numerical immediate score for decision making in the cath lab. Furthermore, the SCAI shock classification validation is not always very objective because many variables were not available for score validation, leading to subjective classification. In addition, as for biomarkers alone, none of the scores (including SCAI and IABP-SHOCK II) have been used so far to guide CS therapy.
Management and treatment
The Central illustration provides a general overview of the necessary steps in assessment and management of the AMI-CS patient. Figure 1 provides an up-to-date summary of RCT in AMI-CS and the respective mortality indicating relative risk (RR) and 95% confidence interval (CI).
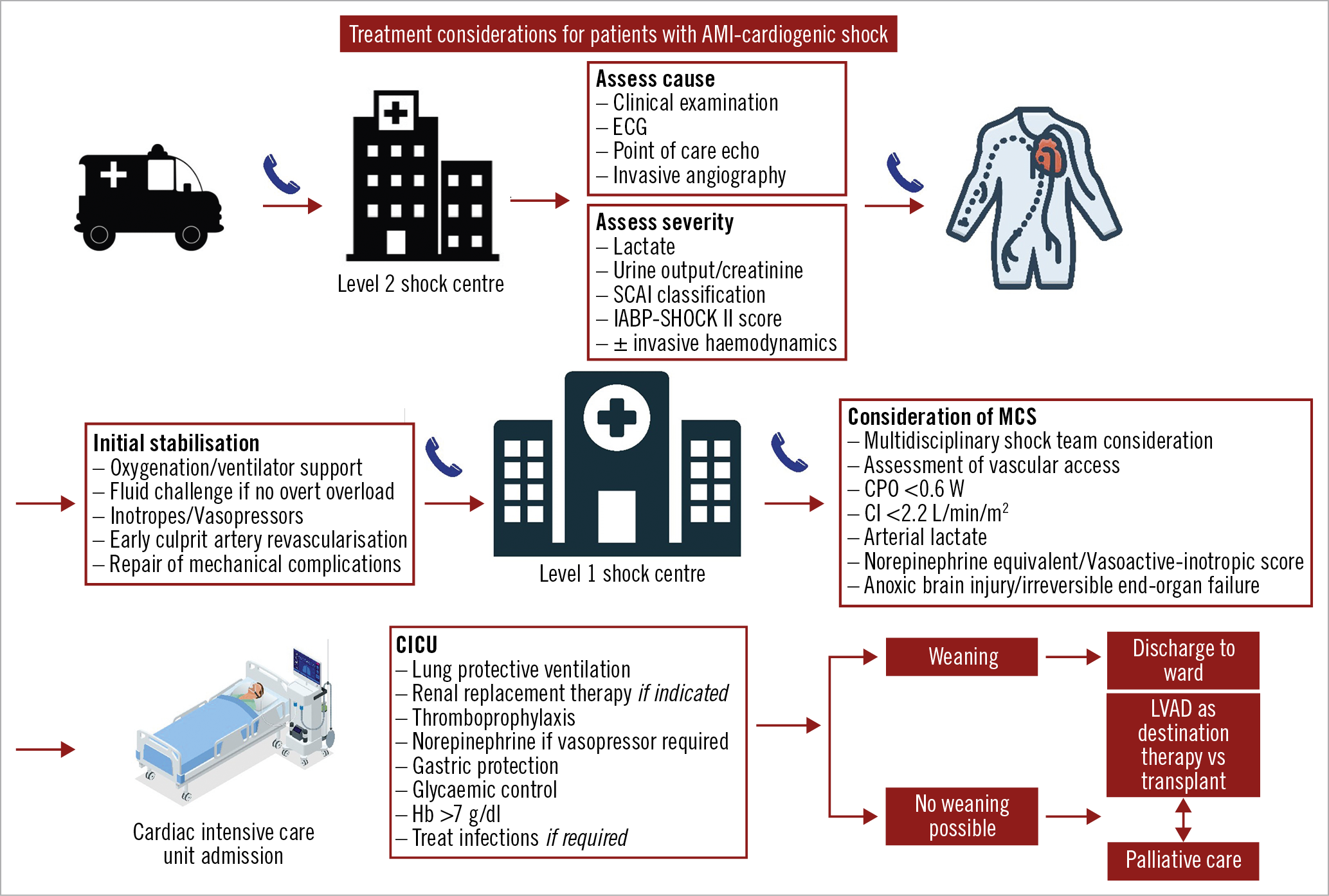
Central illustration. Treatment algorithm highlighting the key considerations in the diagnosis and management of cardiogenic shock. A level I shock centre has cardiac catheterisation and advanced MCS available 24 hrs, 7 days/week with on-site cardiothoracic surgery support, level II has PCI facilities 24/7 but is without on-site MCS. AMI: acute myocardial infarction; CI: cardiac index; CICU: cardiac intensive care unit; CPO: cardiac power output; ECG: electrocardiogram; Hb: haemoglobin; LVAD: left ventricular assist device; MCS: mechanical circulatory support; SCAI: Society for Cardiovascular Angiography and Interventions
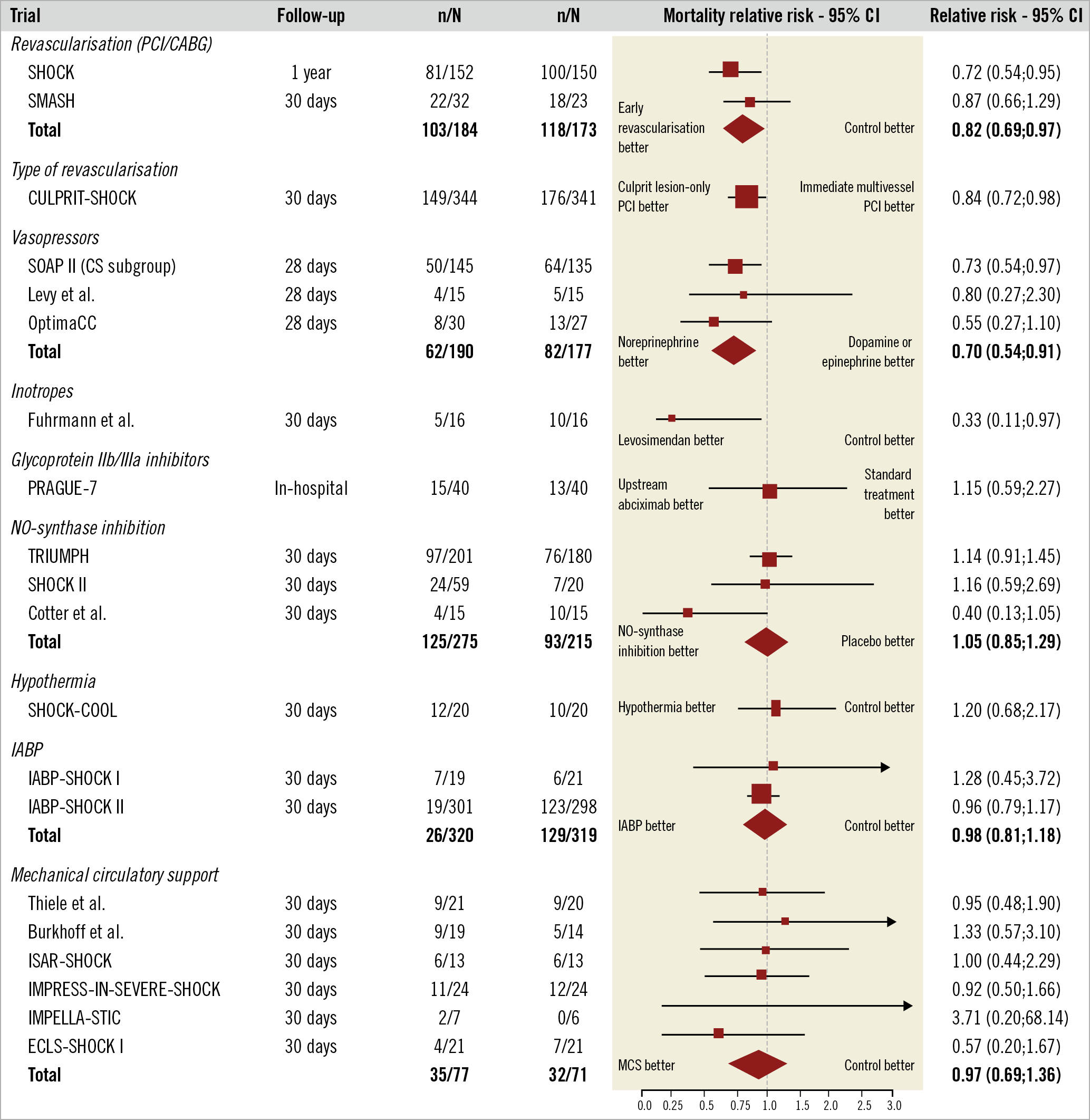
Figure 1. Current evidence from randomised clinical trials in cardiogenic shock in the PCI era. The relative risk and 95% confidence intervals (CI) are depicted for the various randomised interventions. The SOAP II trial was neutral with respect to mortality for the overall trial, thus the predefined cardiogenic shock - including various causes of cardiogenic shock - subgroup results need to be interpreted with caution. CABG: coronary artery bypass grafting; CS: cardiogenic shock; IABP: intra-aortic balloon pump; IABP-SHOCK: Intraaortic Balloon Pump in Cardiogenic Shock; SHOCK: SHould we emergently revascularise Occluded Coronaries for cardiogenic shocK; SMASH: Swiss Multicenter trial of Angioplasty for SHock; SOAP II: Sepsis Occurrence in Acutely Ill Patients II; TRIUMPH: Tilarginine Acetate Injection in a Randomized International Study in Unstable MI Patients With Cardiogenic Shock; PCI: percutaneous coronary intervention
SYSTEMS OF CARE
Where local protocol and logistical feasibility allow, patients with CS should be treated at specialised tertiary CS care centres, with the ability to start and escalate MCS, and dedicated cardiac ICU and cardiac surgery facilities on site2,29. Studies in the USA have shown that mortality rates are lower in those centres with the highest quartile of mean annual CS case volume, even when controlling for early revascularisation, and that lower-volume hospitals were less likely to offer aggressive and specific treatments for CS30. This holds true both for the case of CS-AMI and for CS due to other causes31. Furthermore, the collaboration of an expert “Shock Team” at these centres, consisting generally of an interventional cardiologist, cardiac surgeon, advanced heart failure cardiologist and cardiac intensivist, has been shown to be an independent factor in improving outcomes32. At a bare minimum, when transfer to a level one centre is not immediately logistically feasible, the patient should be brought to a centre with 24/7 PCI facilities. The definition of “immediate” will of course need to take local circumstances into account, but a recommended transfer time of <120 minutes has been proposed29.
REVASCULARISATION
The key issues surrounding revascularisation for CS-AMI are summarised in this section with important interim developments addressed. Although nowadays the results of the SHOCK trial33, which compared early revascularisation with initial medical stabilisation, may be viewed in a different light in that it failed to meet its primary 30-day endpoint of decreased mortality in the early revascularisation group compared to the medically managed group, the long-term results showing reduced mortality at 6 months, 1 year and 6 years34, as well as the evidence borne out in subsequent registries, appear to justify the Class IB recommendation for early revascularisation35,36,37. In summary, as in the case for AMI without CS, early revascularisation is key; multiple registries have shown a delay in revascularisation in the setting of cardiogenic shock to be associated with poorer outcomes38,39.
The vast majority (between 70 and 80%)18 of patients who present with AMI-CS have multivessel coronary disease. Until the results of the randomised, multicentre Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial7, there was a dearth of evidence to guide decision making. However, both the 30-day and one-year results of this trial clarify that there is significant net clinical benefit to culprit-only revascularisation, driven principally by a difference in mortality. This was consistent across all subgroups40. Specifically, the rate of death and renal replacement therapy, as a composite endpoint, in the culprit lesion-only PCI group was 45.9%, compared to 55.4% in the multivessel PCI group (RR 0.83, 95% CI: 0.71-0.96; p=0.01) including a significant mortality reduction. Importantly, the majority of surviving patients in CULPRIT-SHOCK underwent staged protocol-recommended revascularisation during follow-up in the initial culprit lesion-only PCI group. Thus, the preferred revascularisation strategy is culprit lesion PCI with subsequent staged revascularisation after clinical stabilisation similar to the ST-elevation myocardial infarction (STEMI) setting without CS.
Current guidelines recommend early revascularisation by PCI or coronary artery bypass grafting (CABG) depending on coronary anatomy and amenability to PCI35,36,37. Based on evidence from four observational reports, comparing PCI versus CABG, the type of revascularisation did not influence the outcome in AMI-CS, and thus there is little evidence to recommend one strategy in preference to the other41. A trial of culprit lesion-only PCI with staged revascularisation versus immediate CABG in patients with multivessel disease and CS may clarify matters and is currently in development in the USA and in Germany.
ACCESS SITE
Current guidelines recommend radial access as default strategy in non-shock STEMI36 or non-ST-elevation acute coronary syndromes (NSTE-ACS)37, and also stable coronary artery disease35, on the basis of a survival benefit and a lower risk of vascular complications. In CS, the benefit does not have the same breadth of evidence, but that which is available appears to favour radial access. Radial access was associated with a reduction in all-cause mortality in a meta-analysis of observational data analysing 8,131 patients with AMI-CS42. Recent data from the CULPRIT-SHOCK trial confirmed the benefit of the radial approach in CS with lower mortality at 30-day follow-up (37.3% vs 53.2%, adjusted odds ratio [aOR] 0.57, 95% CI: 0.34-0.96)43. Where the femoral approach is chosen, use of ultrasound-guided access may reduce bleeding complications44.
PERI-INTERVENTIONAL ANTIPLATELET AND ANTITHROMBOTIC MEDICATIONS
There is a lack of RCT-derived evidence for antiplatelet and antithrombotic medication specifically in the setting of CS, and thus all recommendations are derived from more general AMI trials. Caveats to be aware of include the presence of impaired enteral resorption in CS for oral antiplatelets, often potentiated by the co-administration of opioids. The ongoing Dual Antiplatelet Therapy for Shock patients with Acute Myocardial Infarction (DAPT-SHOCK-AMI) trial (ClinicalTrials.gov: NCT03551964), assessing intravenous cangrelor versus crushed oral antiplatelets, may shed light on this matter. The benefit of routine upstream use of glycoprotein IIb/IIIa inhibitors has not been shown to be superior to standard treatment (Figure 1),45. These considerations are discussed further in the recent ESC position paper on antithrombotic therapy in patients with ACS complicated by CS or out-of-hospital cardiac arrest (OHCA)46.
INTENSIVE CARE UNIT TREATMENT
FLUIDS, VASOPRESSORS, INOTROPES
Due to the complexity of most CS presentations, these patients are best treated in specialised ICUs, allowing close monitoring of volume status, vasopressor and inotropic support, and the prophylaxis and treatment of multiorgan dysfunction syndrome (MODS)2,29. Fluid administration in CS is based mainly on pathophysiological considerations and, according to current guidelines, a fluid challenge as first-line therapy should be considered unless there are signs of overt fluid overload (class 1C recommendation). Despite the frequency with which inotropes and vasopressors are administered in patients in CS (approximately 90%)6, it should be remembered these drugs increase myocardial oxygen consumption and vasoconstriction, and may impair microcirculation and increase afterload. Thus, as a general rule they should be administered at the lowest possible dose for the shortest possible duration.
In the SOAP II trial47, involving 1,679 shock patients, 280 of whom had CS, norepinephrine appeared favourable compared to dopamine given the propensity of dopamine to cause arrhythmia in the overall cohort. Furthermore, when only the CS subgroup was considered, this group was noted to have a lower mortality with norepinephrine. As shown in the OPTIMA-CC trial in AMI-CS, norepinephrine also appears favourable over epinephrine in terms of minimising both metabolic changes (including lactic acidosis) and heart rate, without having an appreciable difference in effect on cardiac index48. Indeed, there was in fact more refractory CS with epinephrine than norepinephrine (37% vs 7%; p=0.008), leading to the trial being terminated early. Thus, norepinephrine appears to be the vasoconstrictor of choice in refractory CS, reflected in ESC recommendations, albeit with a class IIb B recommendation. There are no data on vasopressin in the CS setting.
The target mean arterial pressure (MAP) is not well defined in CS. Previously, in contrast to the recommendations for septic shock, consensus cautioned aiming for a MAP >65 mmHg as it had been shown to be potentially associated with more side effects49. However, a recent combined analysis from two RCT assessing two different MAP goals in patients after OHCA showed lower indirect infarct size as measured by cardiac troponin in the subgroup of AMI-CS patients randomised to a target MAP of 85-100 mmHg50. This is currently only hypothesis-generating and requires a large-scale RCT to prove the optimal MAP in CS.
Inotropes, e.g., dobutamine, may be given simultaneously with norepinephrine in an attempt to improve cardiac contractility (class IIb, level of evidence C)51. Despite pathophysiological considerations potentially favouring the mechanism of action of inodilators such as levosimendan or phosphodiesterase inhibitors, their current evidence base is very limited. Proof of concept was suggested in a very small trial of 32 CS patients with lower mortality in levosimendan in comparison to enoximone (Figure 1),52. However, to date, these findings have not been replicated in larger-scale studies53,54,55,56.
GENERAL INTENSIVE CARE MEASURES
Although there is no specific evidence base in CS, ICU treatment should follow general best practice guidelines, with regard to lung protective ventilation (6 ml/kg predicted body weight tidal volume), thromboprophylaxis, stress-ulcer prophylaxis, glycaemic control, and nutritional supplementation.
Indications for renal replacement therapy do not differ from those in the general ICU setting (uraemia, refractory volume overload, metabolic acidosis [pH <7.2] and refractory hyperkalaemia [>6.0 mmol/l]). There is also now conclusive evidence from two large RCT showing no benefit of earlier initiation of renal replacement therapy57,58.
Elevated liver transaminases, as a surrogate for liver hypoperfusion, have been shown to be associated with increased mortality, and so care should be taken to stabilise haemodynamics in this setting59.
BLEEDING IN CARDIOGENIC SHOCK
Moderate/severe bleeding is common in CS, ranging from 20-90% depending on the definition used, and is also influenced by concomitant use of MCS60. A recent pre-specified post hoc analysis of the CULPRIT-SHOCK trial revealed that a total of 21.5% of patients with AMI-CS experienced at least one bleeding event up to 30 days61. Most frequent were Bleeding Academic Research Consortium (BARC) category 3a bleeding events (33%); 5.4% of bleeding events were fatal. Treatment with active MCS by VA-ECMO or Impella® (Abiomed, Danvers, MA, USA) emerged as the major risk factor for bleeding, as was noted in a 2014 meta-analysis62. Next to the requirement for significantly larger access sheaths, the risk of bleeding may be increased both by consumptive coagulopathy and by acquired platelet dysfunction in the setting of high shear stress in case of MCS use. Use of glycoprotein IIb/IIIa inhibitors was also found on multivariate testing to be a statistically significant predictor of bleeding. Bleeding was associated with a significantly higher mortality at 30 days (hazard ratio [HR] 2.11, 95% CI: 1.63-2.75; p<0.0001) and, when patients with only severe bleeding events were compared to those without, the HR was even higher (HR 2.80, 95% CI: 1.94-4.05; p<0.0001).
Trials in non-CS patients with bleeding demonstrated that a restrictive transfusion regimen can improve outcome. Generally accepted ICU strategies avoid correction of haemoglobin levels >7 g/dl (>4.3 mmol/l) unless there is a clinical bleeding problem. Transfusion of stored blood serves, on top of the pre-existing systemic inflammatory state in CS, as a source of further inflammation, with alterations in normal nitric oxide biology contributing to vasoconstriction, platelet aggregation and impaired oxygen delivery.
HYPOTHERMIA
Target temperature management is part of standard of care in OHCA and should therefore be a basic element in the treatment of CS patients suffering cardiac arrest. However, there is little clinical trial evidence in the CS case itself. Theoretical assumptions suggested a possible beneficial effect of hypothermia in non-resuscitated CS patients; this was not borne out in the SHOCK-COOL trial including 40 patients. In fact, lactate clearance was found to be impaired, suggesting even harm63.
MECHANICAL COMPLICATIONS
A detailed description of mechanical complications is beyond the scope of this review and has been summarised previously64. However, given the presence of moderate or greater mitral regurgitation in 5-10% of patients with CS, of note is some recent work on percutaneous mitral valve repair (PMVr) using edge-to-edge repair as salvage therapy in patients with mitral regurgitation and refractory CS. A recent multicentre pooled patient-level systematic review looked at 141 patients at 14 institutions who presented with SCAI stage B-E CS and 3+ or 4+ mitral regurgitation - not necessarily as a consequence of AMI - and were treated with PMVr65. No periprocedural complications were reported; successful PMVr was achieved in about 89% of cases. When stratified by procedural results, successful PMVr reduced rates of in-hospital (HR 0.36, 95% CI: 0.13-0.98; p=0.04) and 90-day mortality (HR 0.36, 95% CI: 0.16-0.78; p=0.01).
MECHANICAL CIRCULATORY SUPPORT
Although the theoretical concept of MCS is appealing, in terms of reducing the dependency on inotropes/vasopressors while the heart is bridged to recovery or decision, at present there exist only limited data derived from RCT based on clinical outcomes.
INTRA-AORTIC BALLOON PUMPING
Despite decades of familiarity with the IABP, only in recent years has conclusive evidence established a lack of benefit in AMI-CS. The IABP-SHOCK II trial randomised 600 patients with AMI-CS to early revascularisation with or without IABP6. No difference in the primary study endpoint of 30-day mortality was noted, or in terms of any of the trial’s secondary endpoints. Longer-term follow-up, up to six years, clarified that these results were sustained, and that they held for the intention-to-treat as well as for the as-treated group66,67. Thus, nowadays IABP may only be considered for the mechanical complications group (III B recommendation without and IIb C recommendation in the setting of mechanical complication)35,36,51.
The neutral results of IABP-SHOCK II together with the downgrading in guidelines led to a decrease in IABP use to <30% in the USA68, <10% in Germany69, and <2% in Denmark70, respectively. The decline in IABP use was associated with an increase of active MCS including Impella/TandemHeart® (LivaNova, London, United Kingdom) and VA-ECMO in CS from approximately 1% in 2006 to 8% in 2014 in the USA68. Similarly, VA-ECMO implementation has developed towards a routine procedure with a more than eightfold increase in Germany from 2010 to 201571,72.
ACTIVE PERCUTANEOUS LEFT VENTRICULAR MCS
The mechanism of action of various MCS devices has been outlined previously18,73. New developments include the Impella ECP (expandable CP) device with the ability to achieve peak flows >3.5 L/min despite only requiring access though a 9 Fr sheath. It is unsheathed in the descending aorta and expands to approximately 18 Fr. When being removed, it is first resheathed back down to 9 Fr. Figure 2 shows the currently available devices, including a brief overview of technical features and unloading properties on the left and/or right ventricle.
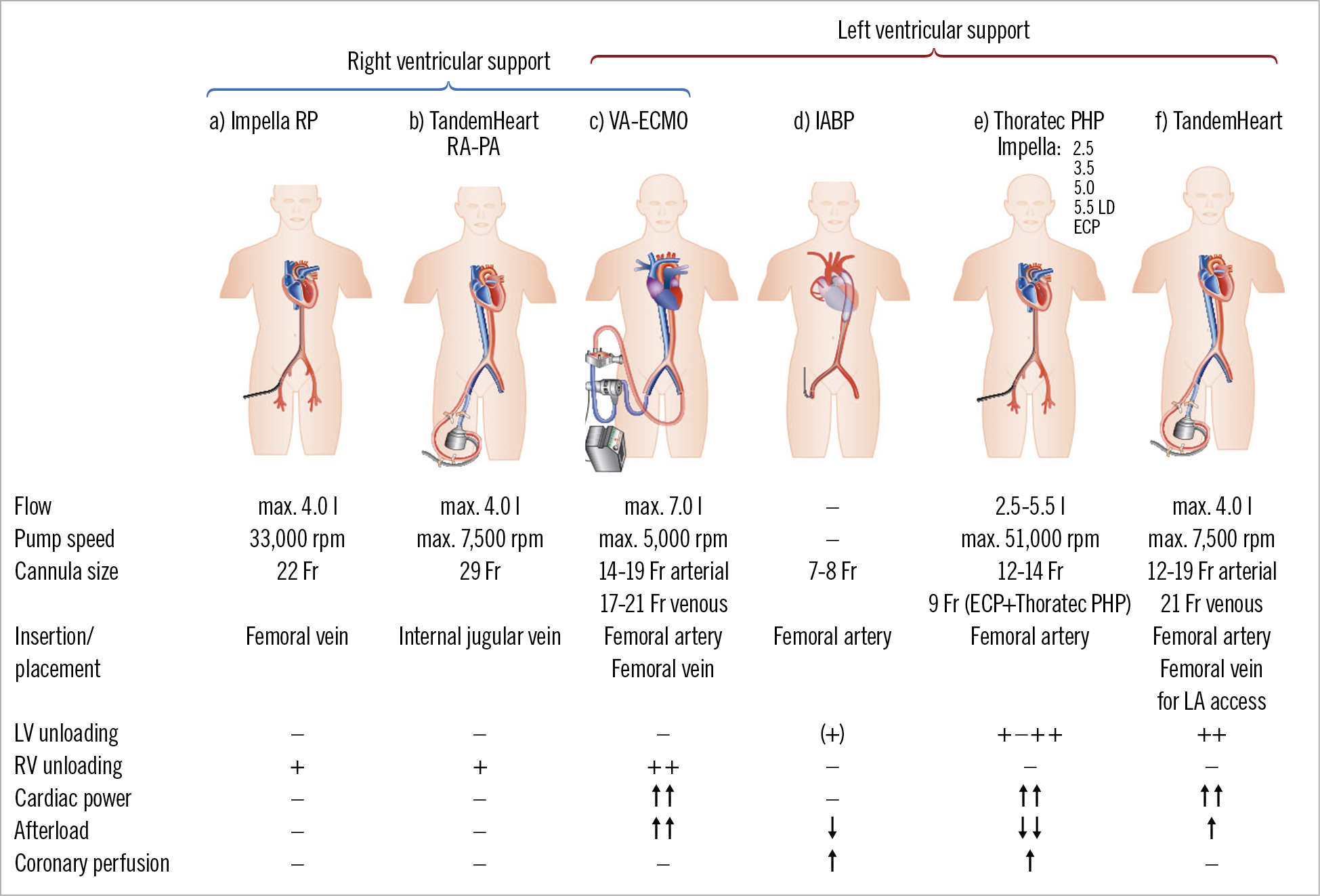
Figure 2. Schematic drawings of current percutaneous mechanical support devices for cardiogenic shock with technical features. On the left side are devices for right ventricular support and on the right side those for left ventricular support. a) Impella RP. b) TandemHeart RA-PA (right atrium – pulmonary artery). c) VA extracorporeal membrane oxygenation (ECMO). d) Intra-aortic balloon pump. e) Microaxial devices: including new development of Impella ECP and Thoratec PHP. f) TandemHeart.
Randomised data on clinical outcomes with regard to the use of percutaneous MCS devices in CS are still limited (Figure 3A). A meta-analysis of active MCS devices against control showed no difference in mortality for the 148 included patients. There were improvements in arterial lactate and MAP after device insertion. On the other hand, there were no effects on other haemodynamic parameters and, more importantly, haemodynamic effects were counterbalanced by significantly more bleeding complications60. A more recent small (n=15) trial in AMI-CS (IMPELLA-STIC) attempted to assess the potential additional benefit of Impella 5.0 LP in patients already managed with an IABP74. There was no difference in the primary endpoint, change in cardiac power index at 12 hours. Adverse events, especially major bleeding, were common in the Impella + IABP group (0% vs 71.4%).
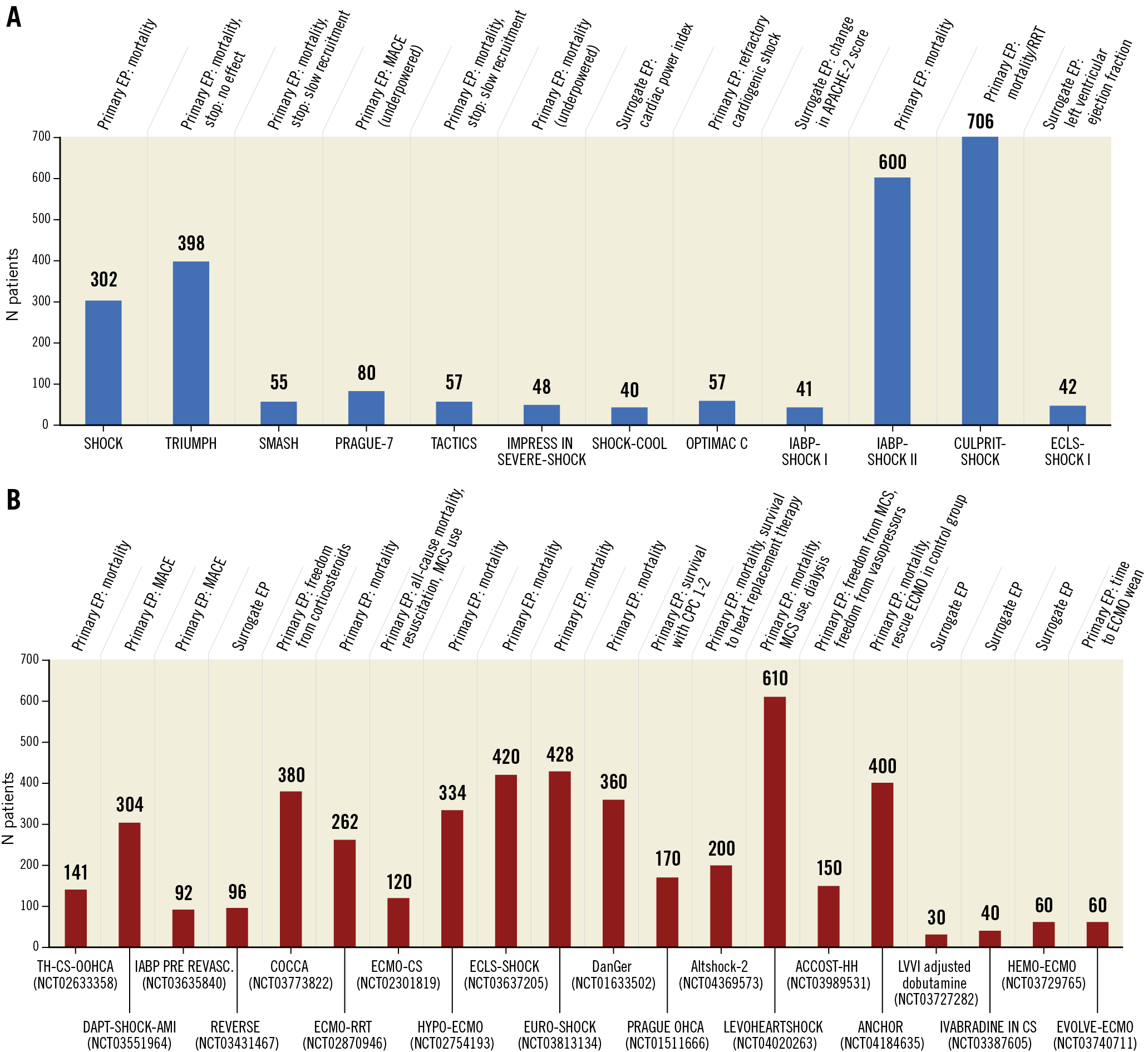
Figure 3. Enrolment data for major randomised cardiogenic shock trials. A) Number of patients included in major randomised cardiogenic shock trials including the primary endpoint (EP). Blue bars indicate finalised trials. In parentheses is the clinicaltrials.gov number if available. Data last accessed (on clinicaltrials.gov) 22 October 2020. B) Number of patients included in major randomised cardiogenic shock trials. Red bars indicate ongoing or planned randomised trials. In parentheses is the clinicaltrials.gov number if available. Data last accessed (on clinicaltrials.gov) 22 October 2020.
Acronyms and tested strategy of ongoing or planned randomised trials: ACCOST-HH: Adrecizumab vs placebo in cardiogenic shock. Altshock-2: IABP within six hours of onset of cardiogenic shock versus standard of care (no device) in cardiogenic shock. ANCHOR: VA-ECMO under echo guidance via the femoral route, with IABP in the contralateral femoral artery versus standard management of cardiogenic shock (i.e., no devices) complicating myocardial infarction. COCCA: low dose corticosteroid therapy (hydrocortisone and fludrocortisone) versus placebo in cardiogenic shock. DanGer: Impella CP versus control in cardiogenic shock complicating myocardial infarction. DAPT-SHOCK-AMI: Multicentre randomised double-blind trial comparing intravenous cangrelor and oral ticagrelor in patients with acute myocardial infarction complicated by initial cardiogenic shock and treated with primary angioplasty. ECLS-SHOCK: VA-ECMO versus control in severe cardiogenic shock complicating myocardial infarction. ECLS-SHOCK: Extracorporeal life support and revascularisation versus revascularisation alone in patients with severe infarct related cardiogenic shock. ECMO-CS: VA-ECMO versus control in cardiogenic shock complicating myocardial infarction. ECMO-RRT: VA-ECMO plus routine renal replacement therapy versus VA-ECMO and standard of care in cardiogenic shock. EURO-SHOCK: VA-ECMO versus control in cardiogenic shock complicating myocardial infarction. HEMO-ECMO: Simultaneous haemoperfusion with ECMO versus ECMO alone for cardiogenic shock. HYPO-ECMO: VA-ECMO with moderate hypothermia versus VA-ECMO with normothermia in cardiogenic shock. IABP pre Revasc: IABP pre revascularisation versus control in cardiogenic shock complicating acute myocardial infarction. LevoHeartShock: Levosimendan in addition to conventional strategy versus placebo in addition to conventional strategy in cardiogenic shock. PRAGUE OHCA: VA-ECMO versus control in refractory out-of-hospital cardiac arrest. REVERSE: VA-ECMO with Impella CP versus VA-ECMO alone in cardiogenic shock. Ivabradine in CS: Ivabradine vs placebo initiated 3 hrs after dobutamine in patients with cardiogenic shock/stage D heart failure who require dobutamine and whose HR is >100. Note that ACS patients are excluded from this study. EVOLVE-ECMO: In patients with CS on ECMO, initiation of percutaneous LA venting via septal puncture when B-lines detected on lung ultrasound, versus when refractory pulmonary oedema is detected on chest radiograph and/or inadequate LV opening detected on echocardiography. LVVI adjusted dobutamine: initiate dobutamine at 5 mcg/kg/min and adjust according to the ejection volume index versus initiate dobutamine at 5 mcg/kg/min and adjust according to the attending physician in patients with an EF documented at <40% and cardiogenic shock. Note that ACS patients are excluded from this study. TS-CS-OOHCA: Anti-inflammatory effect of therapeutic hypothermia in out of hospital cardiac arrest patients with cardiogenic shock. CPC: cerebral performance category; EP: endpoint; Heart replacement therapy: heart transplant or left ventricular assist device implantation; MACE: major adverse cardiac events; RRT: renal replacement therapy
In the setting of a lack of sufficient data from RCT, matched comparisons provide the best second option evidence. A matched-pair mortality analysis of 237 Impella-treated versus 237 IABP-treated AMI-CS patients confirmed a lack of mortality benefit with the Impella device (30-day mortality 48.5% vs 46.4%, p=0.64)75. Of note, severe or life-threatening bleeding (8.5% vs 3.0%, p<0.01) and peripheral vascular complications (9.8% vs 3.8%, p=0.01) were observed more frequently with the Impella device. A second propensity-matched analysis, involving 1,680 pairs of AMI-CS patients, added further weight to this observation, actually suggesting potential harm with the use of Impella rather than IABP76. Among the pairs, there was a significantly higher risk of in-hospital death associated with use of Impella versus IABP (absolute risk difference 10.9%, 95% CI: 7.6-14.2%; p<0.001) and a higher risk of in-hospital major bleeding (absolute risk difference 15.4%, 95% CI: 12.5-18.2%; p<0.001). These associations were consistent regardless of whether patients received a device before or after initiation of PCI.
A third recent publication assessed 4,782 patients undergoing PCI treated with MCS in the USA77. CS was present in 50%. After propensity adjustment, and accounting for clustering of patients by hospitals, Impella use compared to IABP was associated with an increased risk of death (OR 1.24, 95% CI: 1.13-1.36), bleeding (OR 1.10, 95% CI: 1.00-1.21) and stroke (OR 1.34, 95% CI: 1.18-1.53). Interestingly, patients treated by Impella in comparison to IABP were less sick. Thus, a selection bias inherent to any observational data is less likely to be the cause of higher mortality with the Impella device.
Taken together, these results suggest that very careful patient selection for Impella is warranted, particularly in terms of weighing up the haemodynamic benefits against potential device-related complications. Due to the retrospective and non-randomised nature of these studies, it is possible, even allowing for complex statistical propensity matching, that unmeasured confounding is occurring. The DanGer clinical trial (NCT01633502), currently ongoing, with the aim of recruiting 360 participants with STEMI complicated by CS and randomising them to either treatment with the Impella CP or conventional guideline-driven treatment, may shed some additional light on the role of Impella78. The trial protocol stipulates that the device should be placed prior to PCI and has a hard clinical endpoint of all-cause mortality. Patients with OHCA who remain comatose after return of spontaneous circulation (ROSC) are excluded. Over 200 patients have been included to date. Patients recruited thus far are profoundly unwell, with 100% of patients having a lactate >2.5 mmol/L and median LVEF 20%. This trial will provide valuable information on this difficult-to-treat cohort but may limit generalisability to the entire CS spectrum because usually 50% of CS patients have undergone resuscitation.
EXTRACORPOREAL MEMBRANE OXYGENATION
In initial iterations, surgical insertion of ECMO was associated with substantial complications such as lower extremity ischaemia (16.9%), compartment syndrome (10.3%), amputation (4.7%), stroke (5.9%), major bleeding (40.8%), and significant infections (30.4%)62. The development of miniaturised systems and percutaneous cannula insertion has led to a significant uptake of VA-ECMO by interventional cardiologists for CS treatment. It offers the advantages of low costs in comparison to other percutaneous MCS devices, high flow providing full circulatory support even in resuscitation situations, the ability to provide full oxygenation, and also combined support of the right and left ventricle.
Outcome data on VA-ECMO in CS remain relatively sparse. A significant mortality benefit with VA-ECMO use has been shown in a single meta-analysis, which relied only on four small observational studies79. In CS without ongoing cardiopulmonary resuscitation (CPR), VA-ECMO resulted in a 33% higher 30-day survival compared to control (95% CI: 14-52%; p<0.001; number needed to treat 3)79.
A single small RCT (n=42) has assessed the efficacy of VA-ECMO in AMI-CS in terms of left ventricular recovery at 30 days80. Left ventricular ejection fraction at 30 days was not found to be significantly different amongst surviving patients in the VA-ECMO and control groups (p=0.86). All-cause 30-day mortality was low and not different between groups, questioning the inclusion of severe AMI-CS patients (19% VA-ECMO vs 33% control, p=0.37).
Currently, further RCT to assess VA-ECMO in the setting of AMI-CS are in the early or more advanced phase of patient recruitment (Figure 3B). These trials are adequately powered and most use 30-day mortality as the primary endpoint. Among them, the ECLS-SHOCK trial (NCT03637205) currently has the highest number of enrolled patients (>160)81. It only includes high-risk CS patients after AMI with lactate >3 mmol/l and recommends VA-ECMO insertion before revascularisation. In addition, it has a dedicated protocol for ECMO venting and also an escalation strategy in the non-ECMO arm. In contrast, the EURO-SHOCK trial (approximately 10 AMI-CS patients so far) (NCT03813134) has no protocol-defined venting strategy and specifies the addition of ECMO post rather than pre PCI82. The ANCHOR trial (NCT04184635) is still in the planning phase and will combine VA-ECMO with IABP in comparison to control (n=400). A major problem of this trial is the chosen primary study endpoint of death in the ECMO group and death or rescue ECMO in the control group. The allowance of crossover in the control arm will make interpretation of trial results difficult. The ECMO-CS trial (NCT02301819) is only powered to assess a composite endpoint of death, resuscitated cardiac arrest and implantation of another mechanical circulatory device, rather than mortality directly. Until more data are available, thorough consideration must be given to identifying appropriate candidates for VA-ECMO support to avoid unnecessary use, which might consume resources and expose patients to possible complications.
A common issue related to peripheral cannula insertion is an increase in afterload which may lead to inadequate left ventricular unloading. Multiple venting manoeuvres have been described to prevent volume overload such as combining VA-ECMO with IABP, Impella, atrial septostomy, or other (Figure 4).
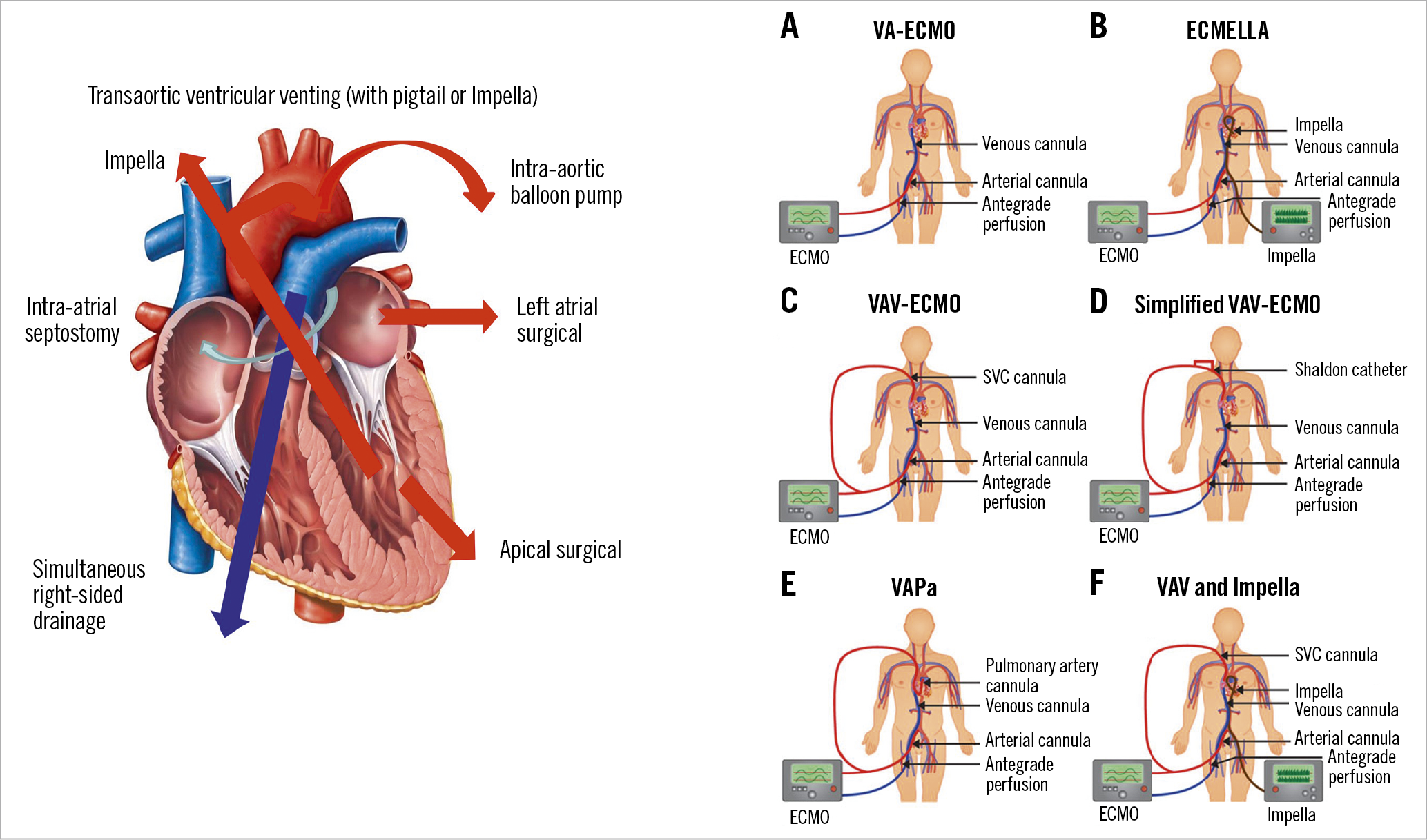
Figure 4. Left: considerations on potential surgical or percutaneous approaches to unload the left ventricle in the setting of venoarterial extracorporeal membrane oxygenation. Right: venoarterial extracorporeal membrane oxygenation and upgrades in cardiogenic shock and lung failure. A) Conventional set-up of venoarterial extracorporeal membrane oxygenation circuit consisting of venous and arterial femoral cannula with distal perfusion catheter. B) ECMELLA (venoarterial extracorporeal membrane oxygenation and Impella). C) Venoarteriovenous extracorporeal membrane oxygenation configuration with a second cannula originating as Y-configuration from the arterial extracorporeal membrane oxygenation system to jugular vein. D) Simplified venoarteriovenous-extracorporeal membrane oxygenation configuration with a bypass from distal perfusion catheter to both lumens of jugular Shaldon catheter. E) Venoarterial-pulmonary arterial extracorporeal membrane oxygenation with an additional cannula positioned in the pulmonary artery. F) Venoarteriovenous-extracorporeal membrane oxygenation configuration in combination with Impella. Adapted from Lüsebrink et al86, with permission from Oxford University Press.
A recently published multicentre cohort study assessed whether venting in patients with VA-ECMO was associated with lower mortality83. Patients (n=225) with severe CS treated with VA-ECMO and Impella unloading (ECMELLA) were propensity matched with 225 patients treated with VA-ECMO without Impella. Left ventricular unloading was associated with lower 30-day mortality (HR 0.79, 95% CI: 0.63-0.98; p=0.03) without differences in various subgroups. However, complications were noted to occur more frequently in the venting cohort, specifically severe bleeding (HR 2.87, 95% CI: 1.92-4.35; p<0.01) and access site-related ischaemia (HR 1.96, 95% CI: 1.22-3.20; p<0.01).
This study is in agreement with previous meta-analyses which have also shown a mortality benefit with unloading VA-ECMO. Russo et al identified 17 observational studies which included 3,997 patients receiving a concomitant LV unloading strategy while on VA-ECMO (IABP 91.7%, percutaneous ventricular assist device 5.5%, pulmonary vein or transseptal left atrial cannulation 2.8%)84. Mortality was 60% in the total cohort. The risk ratio (RR) for mortality was lower in those with venting than in those without (RR 0.79, 95% CI: 0.72-0.87; p<0.00001). There was no interaction between the specific unloading modalities and mortality. Kowalewski et al conducted a similar meta-analysis, including 7,581 patients from 62 observational studies85. An unloading strategy was associated with a lower mortality risk (RR 0.88, 95% CI: 0.82-0.93; p<0.0001) and higher probability of VA-ECMO weaning (RR 1.35, 95% CI: 1.21-1.51; p<0.00001). A recent review article summarises all aspects of unloading in VA-ECMO use86.
In the special situation of cardiac arrest with ongoing CPR, VA-ECMO use (ECMO cardiopulmonary resuscitation [eCPR]) was associated with an absolute 13% increase of 30-day survival compared to control (95% CI: 6-20%; p<0.001; number needed to treat 7.7) in the above-mentioned meta-analysis79. At present, the Prague out-of-Hospital Cardiac Arrest trial (ClinicalTrials.gov: NCT01511666), assessing eCPR in refractory OHCA, is ongoing, and powered to assess superiority with regard to mortality (with good neurological outcomes) at six months.
GENERAL REFLECTIONS ON MECHANICAL CIRCULATORY SUPPORT
Appropriate patient selection for MCS remains a key consideration. Based on IABP-SHOCK II, approximately 50-60% of CS patients survive without any device8. In these patients, device utilisation will have no impact on survival and, in the worst case scenario, may lead to complications associated with device insertion, up to and including death. Among the 40-50% not surviving, there may also be futile situations for patients with severe CS or those with anoxic brain injury, where even the best available device will be unable to change the ultimate clinical outcome. Thus, we conclude that it may be estimated that there is a key kernel of only approximately 25% of CS patients who are, in fact, appropriate candidates for MCS. Those factors best identifying the members of this group remain to be fully elucidated.
Appropriate patient selection is also influenced by the balance between efficacy, institutional experience, and device-related complications. Particularly in terms of the data on Impella, the key determinant of overall clinical benefit seems to be the balance of increased haemodynamic support versus the risk of bleeding complications. The availability of the Impella ECP may alter this dynamic, given the smaller sheath size needed to facilitate its entry. However, this remains to be seen and needs to be borne out in RCT.
Current guidelines recommend considering the use of percutaneous MCS in selected patients depending on age, comorbidities, and neurological function in particular, in refractory CS without any preference for device selection (IIa C recommendation)35,36,51. It is important to note that the recommendation for the use of MCS only in the setting of “refractory shock” is based on limited available evidence where it is possible that the initiation of MCS at this point may, at least in some cases, be too late. The optimal timing of initiation of MCS remains a matter of debate, and has often been left to the discretion of the operator in randomised trials. Results from registry studies are conflicting, with some showing a benefit87,88,89,90,91, whereas other larger analyses have even shown harm with pre-revascularisation insertion of Impella76.
In terms of the role for complete revascularisation versus culprit-only PCI specifically in the setting of MCS-supported revascularisation, registry data suggest - in contrast to the large-scale randomised CULPRIT-SHOCK trial - no significant differences in survival and rates of acute kidney injury. However, if anything, this is only hypothesis-generating based on the non-randomised evidence92. Overall, it appears that, as in non-CS STEMI, rapid diagnosis and initiation of treatment are critical, but formal RCT are required to clarify the findings to date, particularly for the case of Impella in light of the disparate data outlined above.
Conclusion
In general, RCT are difficult to perform in the CS setting and, to date, only a small number of trials have managed to recruit the required number of patients to assess key clinical outcomes. Furthermore, despite the proliferation of primary PCI networks and advances in antiplatelet and antithrombotic pharmacology, the 30-day mortality rate of CS has changed little. There is hope on the horizon, however, with more clinical trials than ever actively recruiting (Figure 3B), with the aim of clarifying the role of pharmacology and MCS. Especially with regard to Impella (DanGer), levosimendan (LevoHeartShock) and VA-ECMO (ECLS-SHOCK, EURO-SHOCK, ANCHOR), it is hoped that we will soon have more data to clarify the best management approach and that these data may lead to an improvement in short- and long-term outcomes.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

