
Abstract
Aims: The current study presents data from a real-world cohort of patients with refractory cardiogenic shock (CS) undergoing extracorporeal life support (ECLS) with the aims of reporting clinical experience, objectifying complications as well as survival, and identifying predictors of mortality.
Methods and results: Eighty-three patients with refractory CS underwent percutaneous ECLS implantation performed by interventional cardiologists. Follow-up was performed at hospital discharge as well as at 18 months (IQR 15-36). Good clinical outcome was defined as survival with a Cerebral Performance Category (CPC) of 1-2. Median age was 61 years (IQR 50-72) and cardiopulmonary resuscitation prior to ECLS implantation was performed in 46 patients (55.4%). Aetiologies of CS were acute myocardial infarction (63.9%), acute deterioration of ischaemic cardiomyopathy (6.0%) or non-ischaemic acute heart failure (16.9%), valvular heart disease (9.6%), and interventional complications (3.6%). Although initial ECLS weaning was successful in 47 patients (56.6%), in-hospital mortality was 68.7%. Of all 83 patients initially undergoing ECLS implantation, only 15 patients (18.1%) were alive at follow-up, 13 (15.7%) with a CPC of 1-2. Age was identified as an independent predictor of mortality (p=0.04).
Conclusions: Despite ECLS support, the long-term prognosis of patients with CS refractory to standard treatment remains poor.
Abbreviations
CPC: Cerebral Performance Category
CS: cardiogenic shock
ECLS: extracorporeal life support
IQR: interquartile range
PCI: percutaneous coronary intervention
SAPS II: Simplified Acute Physiology Score II
STEMI: ST-elevation myocardial infarction
Introduction
Mortality due to cardiogenic shock (CS) remains high despite advanced treatment strategies1-3. In the setting of refractory CS, implantation of active assist devices may be the only option to achieve haemodynamic stability. As a consequence, current guidelines recommend that active support devices such as extracorporeal life support (ECLS) may be considered in selected patients4-6.
The concept of ECLS was first described in the 1970s7. Despite major advances in technology since it was first developed, ECLS remains invasive, resource intensive, and can be associated with serious complications8-12. These include life-threatening episodes of haemorrhage, thromboembolic events, infections, limb ischaemia or secondary consequences of prolonged immobilisation. The potential life-saving benefits of ECLS must therefore be weighed against its inherent risks. Data on prognosis in patients treated with ECLS are scarce and limited by small sample sizes as well as a relatively short duration of follow-up13-16. Further, as ECLS implantation has traditionally been performed by cardiothoracic surgeons, the majority of data derive from cohorts of patients with postoperative CS which is a different entity from CS in the absence of prior surgery17-19. Although evidence is lacking, ECLS use with percutaneous insertion in patients with refractory CS has risen considerably in recent years20,21.
The current analysis presents data from a large real-world cohort of patients with refractory CS undergoing ECLS with the aim of reporting clinical experience, objectifying complications as well as survival, and identifying predictors of mortality.
Methods
PATIENTS
Data of patients undergoing ECLS due to refractory CS at one tertiary care centre were prospectively entered into an electronic hospital registry. Additional data required for this single-centre analysis were retrospectively evaluated. Patients undergoing ECLS implantation due to non-cardiac causes or postoperative CS were excluded.
The diagnosis of CS was made on established criteria, including i) systolic blood pressure <90 mmHg for >30 minutes or vasopressors required to achieve a blood pressure ≥90 mmHg; ii) pulmonary congestion or elevated left ventricular filling pressures; iii) signs of impaired organ perfusion with at least one of the following criteria: a) altered mental status; b) cold, clammy skin; c) oliguria; d) increased serum lactate. Patients included in the current analysis underwent ECLS due to refractory CS defined as critical circulatory failure resulting in organ hypoperfusion unresponsive to conventional therapy with minimal chance of survival without ECLS. Thus, patients had an increasing demand of inotrope and vasopressor doses at increasing levels of serum lactate prior to ECLS implantation. The likelihood of death in the absence of ECLS was deemed to be extremely high. Possible outcomes had to be: i) ECLS weaning to recovery (bridge to recovery); ii) heart transplantation (bridge to transplantation); or iii) implantation of a permanent left ventricular assist device (bridge to bridge).
Contraindications for ECLS implantation were severe comorbidities such as uncontrollable haemorrhage, irreversible brain damage, severe trauma or known terminal malignancies. The decision regarding ECLS implantation was taken by a team of cardiologists trained in intensive care medicine and interventional cardiology.
Detailed sets of clinical and functional parameters including the Simplified Acute Physiology Score II (SAPS II) were repeatedly assessed22. Further, all patients underwent continuous control of routine laboratory parameters, including serum lactate. Left ventricular ejection fraction was assessed by transthoracic echocardiography. Details on patients’ previous medical diagnoses such as history of symptomatic heart failure, chronic renal insufficiency (≥stage 3) or peripheral artery disease were based on the information provided by the treating physician or hospital charts in accordance with guideline definitions4,23,24.
PROCEDURE
ECLS implantation was performed in the catheterisation laboratory by experienced interventional cardiologists. The procedure was carried out regardless of working hours or days of the week (24 hours/7 days). All patients underwent coronary angiography prior to or at the time of ECLS implantation. Additional procedures such as percutaneous coronary intervention (PCI) or balloon valvuloplasty were performed according to standard clinical practice and guideline recommendations. ECLS implantation was performed with the assistance of perfusionists. All patients underwent ECLS implantation via a percutaneous femoro-femoral arteriovenous approach. Percutaneous cannulation using an ~18 Fr arterial cannula and an ~22 Fr venous cannula was performed using the Seldinger technique. To allow distal limb perfusion an ~6 Fr antegrade percutaneously introduced sheath was obligatory. Unfractionated heparin (70 U/kg body weight) was administered and the pump blood flow was initially set at 3 to 4 L/min.
INTENSIVE CARE TREATMENT
All therapeutic measures such as fluid management, renal replacement therapy, use of antibiotics or administration of additional medication were performed according to standard clinical practice and guideline recommendations25. While on ECLS, patients underwent heparinisation with an activated clotting time of 160-180 sec. In case of mechanical ventilation, lung protective ventilation was maintained. Although weaning of inotropes and vasopressors was targeted, additional dobutamine, norepinephrine, or epinephrine was administered if necessary for the shortest possible duration at the lowest possible dose to achieve a mean arterial blood pressure >60 mmHg. Transfusions of packed red blood cells, platelets or fresh frozen plasma were restricted to the presence of clinically relevant bleeding problems.
Physicians working at the intensive care unit were all specifically trained on the ECLS technique and management.
OUTCOME DEFINITIONS
The primary outcome was long-term mortality. Secondary outcomes included i) in-hospital mortality, and ii) long-term survival with good functional outcome. Good functional outcome was defined as a score of 1-2 based on the Cerebral Performance Category (CPC) scale. Clinical follow-up was conducted prospectively via a structured telephone questionnaire contacting the patient, the relatives or the treating physician. All events were verified by hospital charts, direct contact with the treating physician or contact with the local government registration. Data on the cause of death prior to discharge and occurrence of local and systemic complications were assessed using in-hospital documentation.
STATISTICAL ANALYSIS
Each categorical variable is expressed as number and percentage of patients. Continuous data are reported as medians with corresponding interquartile range (IQR). Two-group comparisons for survivors versus non-survivors were performed with chi-square tests for categorical variables, Student’s t-tests for normally distributed continuous variables and Wilcoxon rank-sum tests for non-normally distributed continuous variables. For outcome analysis, Kaplan-Meier curves with log-rank comparison were computed. To identify predictors of long-term mortality, univariable and multivariable Cox regression analyses were performed. All variables with a p-value <0.1 in univariable analysis were entered into a stepwise multivariable model. All statistical tests were performed with SPSS software, Version 19.0 (IBM Corp., Armonk, NY, USA). All probability values were two-tailed with α<0.05, and confidence intervals (CI) were calculated to the 95th percentile.
Results
BASELINE CHARACTERISTICS
From January 2008 until October 2012, 83 patients underwent ECLS implantation due to refractory CS. As displayed in Table 1, the median age was 61 years (IQR 50-72, range 23-84). At the day of ECLS implantation, the SAPS II score was 53 (IQR 43-61) at a maximum level of serum lactate of 6.7 mmol/l (2.5-11.1). Prior to the procedure, 46 patients (55.4%) had to undergo cardiopulmonary resuscitation.
The most common aetiology of CS was acute myocardial infarction (n=53, 63.9%) with ST-elevation myocardial infarction (STEMI) in 42 patients (50.6%) and non-ST-elevation myocardial infarction in 11 (13.3%). Ventricular septal defect (VSD) was present in four patients (4.8%) with STEMI. Acute deterioration of chronic ischaemic cardiomyopathy was the cause of CS in five patients (6.0%). In patients with non-ischaemic acute heart failure (n=14, 16.9%), two patients (2.4%) had acute fulminant myocarditis confirmed by histology, and one patient (1.2%) displayed the typical pattern of Takotsubo cardiomyopathy. Other diagnoses were hypertrophic obstructive cardiomyopathy, arrhythmogenic right ventricular dysplasia, as well as refractory ventricular tachycardia due to intoxication attempting suicide (yew tree) and prolonged hypothermia (each n=1, 1.2%). Dilated cardiomyopathy was present in the remaining seven patients (8.4%) with non-ischaemic acute heart failure. Valvular heart disease as the primary diagnosis was present in eight patients (9.6%; aortic stenosis: n=5, 6.0%; aortic insufficiency: n=1, 1.2%; mitral stenosis: n=1, 1.2%; mitral regurgitation n=1, 1.2%). Procedural complications leading to ECLS implantation were associated with transfemoral aortic valve replacement in two patients (2.4%) and PCI in one (1.2%).
PROCEDURE, TREATMENT, AND COMPLICATIONS
In the majority of patients, ECLS implantation was performed within 24 hours after development of haemodynamic instability (n=52, 63.7%/range 1-8 days). The implantation was exclusively performed in the catheterisation laboratory and was successful in all patients. ECLS support lasted in median six days (IQR 4-8, range 1-54).
Revascularisation was performed in all patients with acute myocardial infarction, with the exception of one case with known VSD as a mechanical complication of STEMI. PCI was performed in the majority of cases (n=49, 92.5%), and three patients underwent coronary artery bypass grafting (3.8%). An additional PCI under mechanical support was performed in eight patients (12.7%). Of the 53 patients with acute myocardial infarction, 19 (35.9%) were treated with an intra-aortic balloon pump prior to ECLS. These were removed at ECLS implantation, and no other mechanical support systems (e.g., Impella CP®; Abiomed Europe, Aachen, Germany) were inserted. Balloon valvuloplasty was performed in five patients with aortic (n=4) or mitral stenosis (n=1) following ECLS implantation. A permanent left ventricular assist device was implanted in one patient (1.2%) after ECLS explantation, and three patients underwent heart transplantation on mechanical support (3.6%).
Overall, nine patients (10.8%) were spontaneously breathing, whereas 74 patients (89.2%) had to be mechanically ventilated. Prior to ECLS implantation, 13 patients (15.7%) were on renal replacement therapy. During ECLS, only two patients (2.4%) could be weaned from renal replacement therapy, whereas an additional 22 (overall n=33, 39.8%) experienced severe renal failure despite mechanical support. Antibiotic therapy had to be initiated in the vast majority of patients (n=73, 88.0%) with a septic shock constellation in 13 patients (15.7%). Access-site complications occurred in almost one third of patients (31.3%, bleeding: n=11, 13.3%; ischaemia: n=6, 7.2%; infection: n=9, 10.8%). Clinically relevant signs of haemolysis were not observed. Despite a relatively strict transfusion regimen, the majority of patients (n=67, 80.7%) required either erythrocyte concentrates (median seven packs/patient, IQR 2-13), thrombocyte replacement (median one pack/patient, IQR 0-2) or fresh frozen plasma (median two packs/patient, IQR 2-4).
IN-HOSPITAL OUTCOME
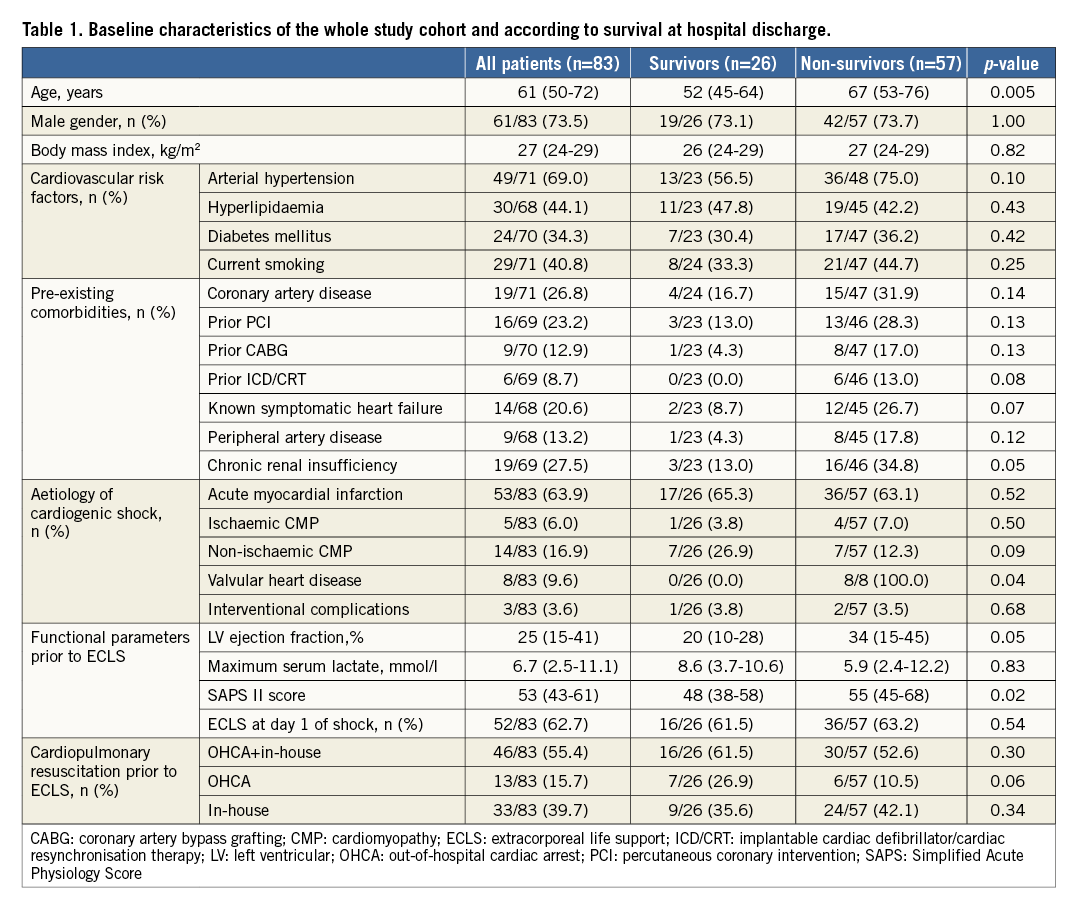
ECLS could be weaned in more than half of the cohort (n=47, 56.6%). Thus, 36 patients (43.4%) could not be stabilised despite mechanical support (Figure 1). Following an initially successful ECLS explantation, an additional 21 patients died prior to hospital discharge (overall in-hospital mortality: 68.7%, n=57). Causes of death were multi-organ failure due to prolonged or recurrent CS in the vast majority (n=41, 71.9%) (Table 2), whereas nine patients died due to septic shock (15.8%), and two patients (3.5%) experienced a major stroke. Two patients died due to anoxic brain injury (3.5%). Finally, fatal haemorrhagic shock occurred in three patients (5.3%, haematothorax: n=1, 1.8%; dislocation of the ECLS cannulas: n=2, 3.5%). After a median hospital stay of 20 days (IQR 13-37), 26 patients (31.3%) could be discharged to undergo rehabilitation.
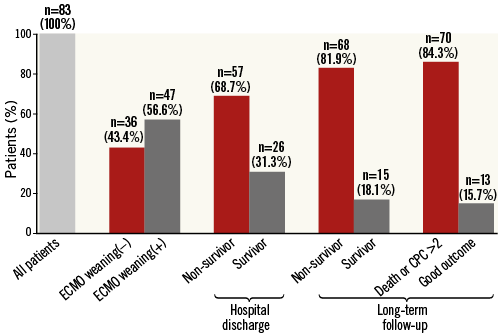
Figure 1. Overview of outcome.
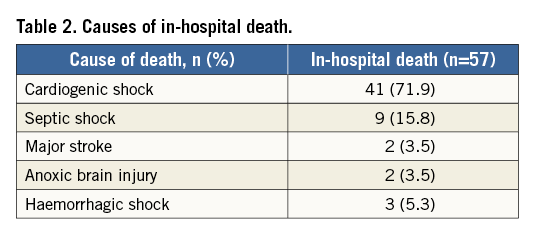
No patients undergoing ECLS due to CS in the setting of valvular heart disease survived. Further, all 14 patients above the age of 75 years died prior to hospital discharge (Figure 2A).
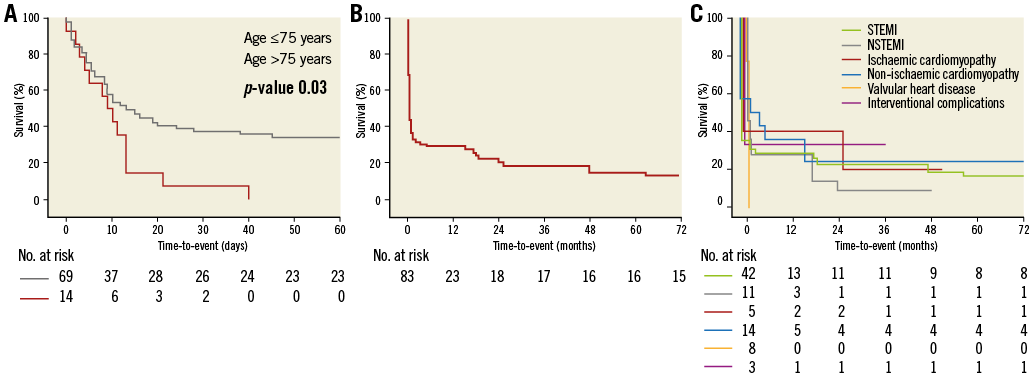
Figure 2. Survival following ECLS. A) Kaplan-Meier curve displaying short-term mortality according to age >75 versus ≤75 years. B) Survival curve displaying long-term mortality. C) Survival curves displaying long-term mortality according to the aetiology of cardiogenic shock.
LONG-TERM OUTCOME AND PREDICTORS OF ADVERSE CLINICAL OUTCOME
Follow-up was completed in all patients alive at hospital discharge and was performed at 18 months (IQR 15-36). Of the 26 patients surviving the initial hospital stay, 11 died within the follow-up period (Figure 2B) and thus long-term mortality was 81.9% (n=68). Of the 15 survivors, two had a CPC score of >2. Thus, of the 83 patients treated by ECLS, 13 survived with good functional outcome (15.7%). Survival curves according to the aetiology of CS are shown in Figure 2C.
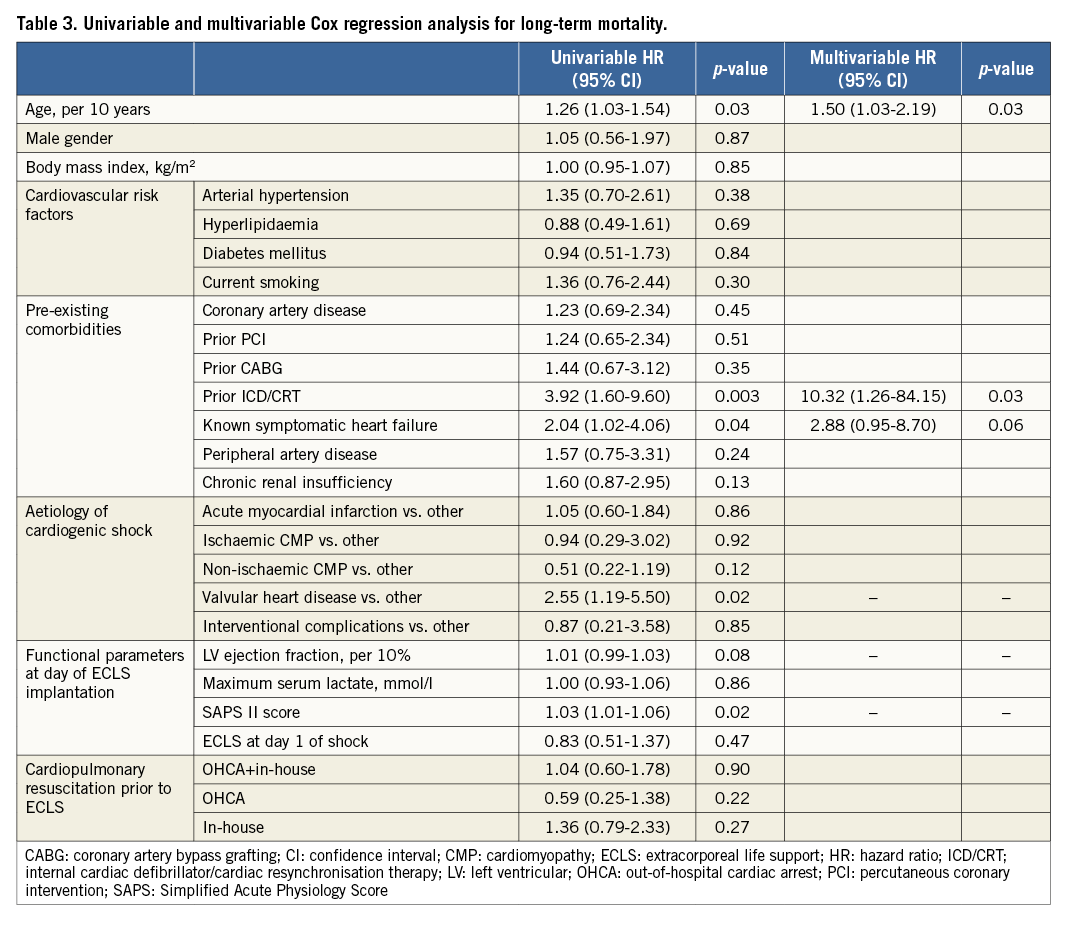
In multivariable Cox regression analysis, age, prior implantation of an internal cardiac defibrillator or cardiac resynchronisation therapy and known symptomatic heart failure were identified as independent predictors of long-term mortality (Table 3). The corresponding c-index of the model was 0.82.
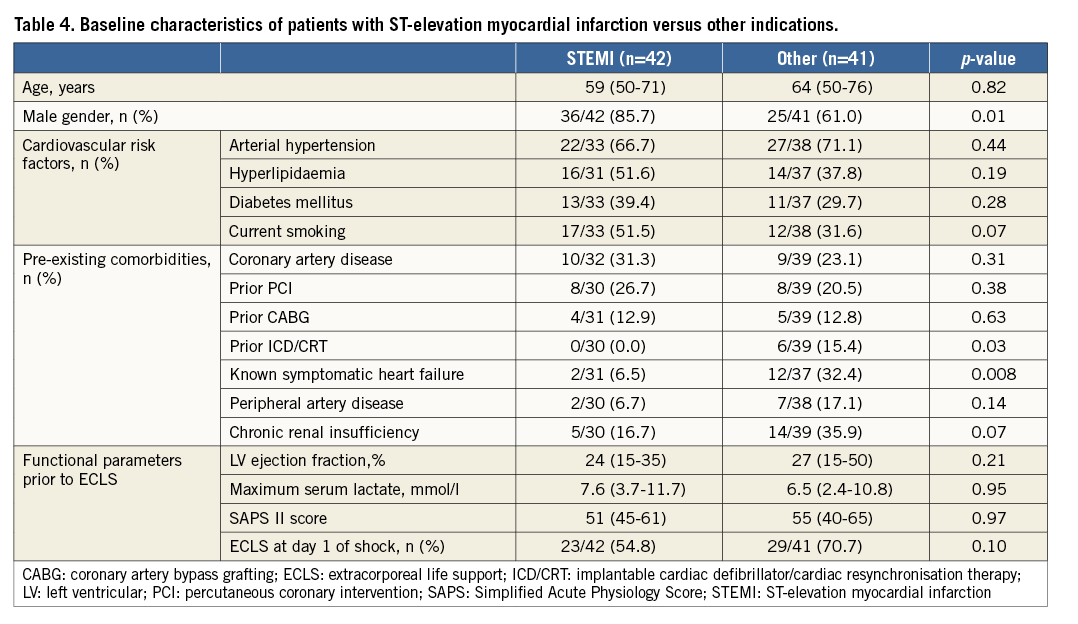
SUB-ANALYSIS OF PATIENTS WITH ST-ELEVATION MYOCARDIAL INFARCTION
STEMI patients were more often male and had a lower rate of prior ICD/CRT implantation as well as a lower prevalence of known symptomatic heart failure compared to patients who underwent ECLS for other indications (Table 4). Multivessel coronary artery disease was present in 40.5% (n=17/42). Of these, 94.1% (n=16/17) underwent additional PCI of non-culprit lesions (n=16/42). The occurrence of bleeding did not differ between groups (p=0.25). The rate of ECLS weaning was similar (54.8% versus 58.5%, p=0.45). With respect to in-hospital mortality (66.7% versus 70.7%, p=0.44) and long-term mortality (81.0% versus 82.9%, p=0.53), clinical outcome did not differ between STEMI patients and patients on ECLS due to other indications.
Discussion
The main findings of the current study are that i) prognosis of patients in refractory CS remains poor despite ECLS, ii) the rate of local and systemic complications under ECLS is high, and iii) ECLS is a therapeutic option in this otherwise futile situation, especially in younger patients.
Despite modern treatment strategies such as early revascularisation and optimal intensive care management, the mortality due to CS still approaches rates up to 50%1-3. Medical therapy consists primarily of volume management as well as inotropic agents and vasopressors enhancing cardiac output and vascular tone. The haemodynamic benefits are counterbalanced by adverse effects such as increased myocardial oxygen demand, arrhythmogenicity, and compromise of tissue microcirculation which may translate into an increased mortality risk26,27. Mechanical circulatory support systems are an alternative to increase systemic blood flow avoiding the possible cardiotoxicity and long-term morbidity of medical therapy8-10. Intra-aortic balloon counterpulsation as the most widely used mechanical support system failed to show haemodynamic and clinical benefit in patients with infarct-related CS when tested in a randomised fashion2,28. ECLS provides a more potent haemodynamic support (ECLS: >4.5 l/min; intra-aortic balloon counterpulsation: 0.5-1.0 l/min). This theoretically creates the opportunity to reduce the high mortality rates currently associated with conventionally managed CS. However, especially in patients without prior cardiac surgery, data on the safety and efficacy of ECLS in refractory CS are scarce, led to inconsistent results, and are limited due to small sample sizes as well as relatively short follow-up duration13-16.
A previous retrospective analysis by Sheu et al reporting data on 30-day survival in 46 patients with STEMI in profound shock demonstrated a mortality of 39.1%13. The rate of peripheral complications associated with ECLS was 26.1%, and in 87.0% of patients antibiotic treatment due to sepsis had to be initiated. Combes et al reported on 55 patients with acute myocardial infarction, dilated cardiomyopathy, and fulminant myocarditis14. Mortality was 76.4% at a median follow-up of 11 months. Severe complications such as major bleeding, arterial ischaemia or wound infection occurred in 56.8%. Almost one fifth of the patients (18.5%) could only be transiently stabilised and had to undergo permanent assist device implantation or heart transplantation. In the cohort reported by Belle et al, in-hospital mortality was 51.9% in 27 patients with CS and 95.8% in 24 patients with refractory cardiac arrest15. Further, the rate of complications was again high (e.g., major bleeding: 39.2%, limb ischaemia: 17.6%, sepsis: 13.7%). Finally, Beurtheret et al recently published results of the cardiac-RESCUE program analysing data of 87 patients16. In-hospital mortality was 63.1%. Notably, no patient above the age of 62 years survived, and more than half of all patients (60%) suffered one to five complications while on ECLS support, including major stroke in 12.6%.
The differing mortality rates can most likely be explained by the relatively small size of the reported cohorts with differing baseline characteristics. As demonstrated in the current analysis, age especially appears to be an independent predictor of in-hospital as well as long-term mortality. This is supported by the finding of the current analysis that all patients over the age of 75 years died prior to hospital discharge. Patients enrolled in the cardiac-RESCUE program were ~15 years younger than those of the current analysis (46 versus 61 years)16. However, as demonstrated in the SHOCK trial (mean age 66 years) and the IABP-SHOCK II trial (median age 70 years), CS frequently occurs in the elderly, and a cohort with a mean age of 46 years as presented in the cardiac-RESCUE program does not entirely reflect real-world patients1-3.
Further, the question of optimal timing of mechanical support might have been of importance. In the current analysis, all patients were in deep refractory CS with a likelihood of death deemed to be high in the absence of ECLS. Thus, one could argue that some patients were in a futile clinical situation. A potential benefit of an early use at the onset of CS could be prevention of multi-organ dysfunction syndrome. The current analysis primarily included patients undergoing ECLS at day one of CS. Further, no association of ECLS timing with mortality could be observed. In addition, the consistently high rate of complications associated with ECLS does not support the concept of early insertion and a widespread use in all CS patients irrespective of the severity of CS. Although the support provided by ECLS appears appealing as it is higher than with other potentially less invasive devices such as the Impella CP or the HeartMate PHP™ (Thoratec Corp., Pleasanton, CA, USA, currently under investigation to obtain CE approval in Europe), it may also have negative impact on outcome11. This is especially important, as approximately 60% of patients will survive without any active device, as shown in the IABP-SHOCK II trial2. Consequently, early use of mechanical support devices might lead to complications with subsequent adverse clinical outcome in patients who still had non-invasive therapeutic options. Devices with low complication rates may thus be favoured in the early stage of CS. In the current study, care was taken to minimise complications (e.g., antegrade sheath to allow limb perfusion)29. However, factors such as prolonged immobilisation which may promote systemic infection or bleeding associated with the large cannula size or derangements in coagulation and platelet function by extracorporeal circulation can hardly be modified. Further, the primary use of devices other than ECLS is supported by the fact that ECLS, in contrast to axial flow or left atrial to femoral artery devices, does not lead to left ventricular unloading. Rather, ECLS increases the afterload of the left ventricle, potentially enhancing oxygen demand and impeding myocardial protection, which may influence organ recovery30,31. Nevertheless, ECLS holds some advantages over other devices due to its ability to support right ventricular, left ventricular, or biventricular failure at very high blood flow rates, as well as the potential to support patients with concomitant lung injury by its oxygenation properties. These facts emphasise the role of ECLS as a potential bail-out strategy after failure of less invasive devices.
Limitations
Some important limitations of the current analysis need to be mentioned. First, the data are observational, and thus prone to selection bias. Second, the sample size is still too small to draw definitive conclusions. Nevertheless, the current study analyses data from one of the largest cohorts of patients without prior surgery undergoing ECLS so far published. Further, more haemodynamic parameters (e.g., heart rate) or additive laboratory data (e.g., leukocyte count) could have been included in the analysis. However, these parameters are included in the SAPS II score and thus were indirectly analysed. Inclusion of the SAPS II score instead of the individual components was performed to ensure statistical robustness by reducing the number of analysable parameters with respect to our sample size. Finally, we cannot provide data on haemodynamic indices such as cardiac index or pulmonary capillary wedge pressure. In addition, venting mechanisms and LA decompression were not assessed. The impact of these parameters and treatment procedures on outcome warrants further analyses.
Conclusion
Despite ECLS, the mortality of patients with refractory CS remains high. Next to the futile clinical state, this might also be in part explained by the high rate of local and systemic complications. Nevertheless, ECLS is a therapeutic option in high-risk patients, especially at a younger age.
| Impact on daily practice The long-term prognosis of patients with CS refractory to standard treatment remains poor, despite ECLS support. The rate of local and systemic complications associated with this mechanical support system is high. Thus, prevention as well as treatment of complications needs to be a major focus in ECLS therapy. Nevertheless, especially in younger patients, ECLS may be a therapeutic option in this otherwise futile situation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

