Abstract
Background: Cardiogenic shock (CGS) occurs in 10% of patients presenting with acute myocardial infarction (MI), with in-hospital mortality rates of 40-50% despite revascularisation.
Aims: The EURO SHOCK trial aimed to determine if early use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) could improve outcomes in patients with persistent CGS following primary percutaneous coronary intervention (PPCI).
Methods: This multicentre, pan-European trial randomised patients with persistent CGS 30 minutes after PPCI of the culprit lesion to receive either VA-ECMO or continue with standard therapy. The primary outcome measure was 30-day all-cause mortality in an intention-to-treat analysis. Secondary endpoints included 12-month all-cause mortality and 12-month composite of all-cause mortality or rehospitalisation due to heart failure.
Results: Due to the impact of the COVID-19 pandemic, the trial was stopped before completion of recruitment, after randomisation of 35 patients (standard therapy n=18, VA-ECMO n=17). Thirty-day all-cause mortality occurred in 43.8% of patients randomised to VA-ECMO and in 61.1% of patients randomised to standard therapy (hazard ratio [HR] 0.56, 95% confidence interval [CI]: 0.21-1.45; p=0.22). One-year all-cause mortality was 51.8% in the VA-ECMO group and 81.5% in the standard therapy arm (HR 0.52, 95% CI: 0.21-1.26; p=0.14). Vascular and bleeding complications occurred more often in the VA-ECMO arm (21.4% vs 0% and 35.7% vs 5.6%, respectively).
Conclusions: Due to the limited number of patients recruited to the trial, no definite conclusions could be drawn from the available data. Our study demonstrates the feasibility of randomising patients with CGS complicating acute MI but also illustrates the challenges. We hope these data will inspire and inform the design of future large-scale trials.
Introduction
Cardiogenic shock (CGS) remains an important cause of morbidity and mortality as a complication of acute myocardial infarction (MI). It occurs in approximately 10% of cases following MI12. Although the seminal SHOCK trial demonstrated significant improvement in mortality following revascularisation in such patients3, the rates of 30-day mortality remain at about 50% with no change over the past decade in retrospective analyses2 or randomised trials45.
Mechanical circulatory support (MCS) devices provide haemodynamic support in patients presenting with CGS. This immediate and temporary support may preserve organ perfusion in patients presenting with CGS, thus allowing time for revascularisation and reperfusion to enable sufficient recovery of cardiac function for restoration of haemodynamic stability.
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a short-term mechanical circulatory support device that offers additional oxygen delivery when the cardiac output is insufficient to supply cellular demands due to left and/or right ventricle failure. This offers physiological flow rates that can potentially support both the left and right ventricle. Although some historical studies suggest minimal benefit of this, most of these used VA-ECMO in refractory CGS, when the spiral of decline caused by the shock state may have become irreversible.
The EURO SHOCK trial aimed to assess whether early use of VA-ECMO in patients with CGS complicating acute MI and persisting post-primary percutaneous coronary intervention (PCI) could result in reductions in 30-day and 12-month mortality.
Methods
The study rationale, design and sample size calculation have been reported previously6. The trial was conducted in accordance with the Declaration of Helsinki and approved by the ethics committees of participating centres. The CONSORT checklist is provided in Supplementary Appendix 1. ClinicalTrials.gov: NCT03813134.
TRIAL PARTICIPANTS
A total of 15 centres from 6 countries participated in the trial. A list of the centres and the number of patients screened and recruited at each centre are provided in Supplementary Table 1.
Patients presenting with CGS due to myocardial infarction and who had had attempted/successful primary PCI (PPCI) of the culprit lesion were enrolled if there was persistent CGS 30 mins after the procedure. The definition of CGS and the inclusion criteria have been published previously6. In essence, it is defined by the presence of systolic blood pressure (SBP) <90 mmHg or maintained above 90 mmHg with the addition of vasopressor or inotropic support, with evidence of hypoperfusion. All patients had a bedside echocardiogram within 30 mins post-PCI to exclude the presence of a structural complication as the cause of CGS (e.g., ventricular septal rupture, ischaemic mitral regurgitation, left ventricular free-wall rupture). The inclusion and exclusion criteria are outlined in Supplementary Table 2.
TRIAL PROCEDURES
Patients were randomised to receive VA-ECMO as soon as possible − within 6 hours of randomisation − or continue standard therapy in a 1:1 fashion. Intra-aortic balloon pump (IABP) use was permitted as a means of left ventricular unloading in patients receiving VA-ECMO therapy. Randomisation was carried out using a web-based randomisation system stratified by out-of-hospital cardiac arrest (OHCA). Where possible, informed consent was obtained from the patients. If the patients were not able to provide informed consent, then a process of initial consent was employed followed by confirmation of informed consent by the patient if they regained consciousness or the capacity to provide consent. If confirmation of consent was not possible, the patients remained in the study. The trial flow diagram is shown in Supplementary Figure 1.
The use of mechanical support devices in the control therapy group was discouraged, although the use of IABP was still permitted in this group. However, if physicians felt this was in a patient’s interest to manage clinical deterioration, it was permitted but acknowledged as a protocol violation. In the VA-ECMO group, IABP was the only permitted means of left ventricular (LV) unloading.
TRIAL ENDPOINTS
The primary endpoint was 30-day all-cause mortality. Secondary endpoints included in-hospital major bleeding complications (BARC type 3-5); cerebrovascular events; vascular complications, as defined by the Valve Academic Research Consortium-2 (VARC-2) criteria7; 12-month all-cause mortality; and the composite endpoint of 12-month mortality and readmission with heart failure. Quality-of-life outcomes at 30 days were assessed using the EQ-5D-3L questionnaire and the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
All endpoint-related events were independently adjudicated by the clinical events committee (Supplementary Appendix 2).
SAMPLE SIZE
The study aimed to recruit 428 patients to demonstrate a 27.5% reduction in the primary endpoint with 80% power and α=0.05. This was based on an anticipated 30-day mortality of 50% in the standard therapy group. Details of the sample size calculation have been published previously6.
STATISTICAL ANALYSIS
The primary analysis was performed according to the intention-to-treat principle. A prespecified secondary as-treated analysis was also performed according to whether the patients received early VA-ECMO or not.
Categorical variables are expressed as percentages and compared using Pearson’s chi-squared or Fisher’s exact tests where applicable. Continuous variables are expressed as mean±standard deviation or median with interquartile range (IQR) and compared using a t-test or Mann-Whitney U test, respectively.
The time to occurrence of the primary endpoint was analysed using the Kaplan-Meier method and compared using a log-rank test. The hazard ratio (HR) with a 95% confidence interval (CI) was obtained from a Cox proportional hazards model stratified for OHCA. Event rates of secondary endpoints, not including mortality, were determined from cumulative incidence functions, taking mortality as a competing risk into account. The comparison between groups was performed using Gray’s test, stratified by OHCA and using mortality as a competing risk. An HR with a 95% CI was obtained from a Fine and Gray model with the baseline hazard stratified by OHCA and taking mortality as a competing risk.
The analysis was performed using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute).
Results
TRIAL FLOWCHART
An overview of screening and recruitment to the trial is shown in Figure 1.
From January 2020 to January 2022, a total of 333 patients were screened at the recruiting centres. Of them, 35 patients (13.25%) were recruited to the trial (Supplementary Table 1): 18 patients were randomised to standard therapy and 17 patients to early VA-ECMO.
The main reasons for screening failure included out-of-hospital cardiac arrest without return of spontaneous circulation (ROSC) or bystander cardiopulmonary resuscitation (CPR) within 10 minutes (20% of patients), recovery from CGS after PCI (18%), CGS secondary to another cardiac cause and not associated with acute MI (14%) (Figure 1).
Among the patients randomised to the VA-ECMO group, 5 patients did not receive VA-ECMO therapy (complications with vascular access or difficulty with peripheral cannulation leading to abandoning the implantation of VA-ECMO n=3; patient refusal n=1; withdrawal of consent n=1). In the standard therapy arm, there was no crossover to VA-ECMO within the predefined time frame of 6 hours. However, 1 patient from this group received VA-ECMO after this 6-hour period due to clinical deterioration. This patient has been included in the standard therapy arm for the “intention-to-treat” and the “as-treated” analyses. Hence, for the as-treated population, 22 patients received standard therapy and 12 patients received VA-ECMO. Results of the as-treated set are presented in Supplementary Figure 2 and Supplementary Figure 3.
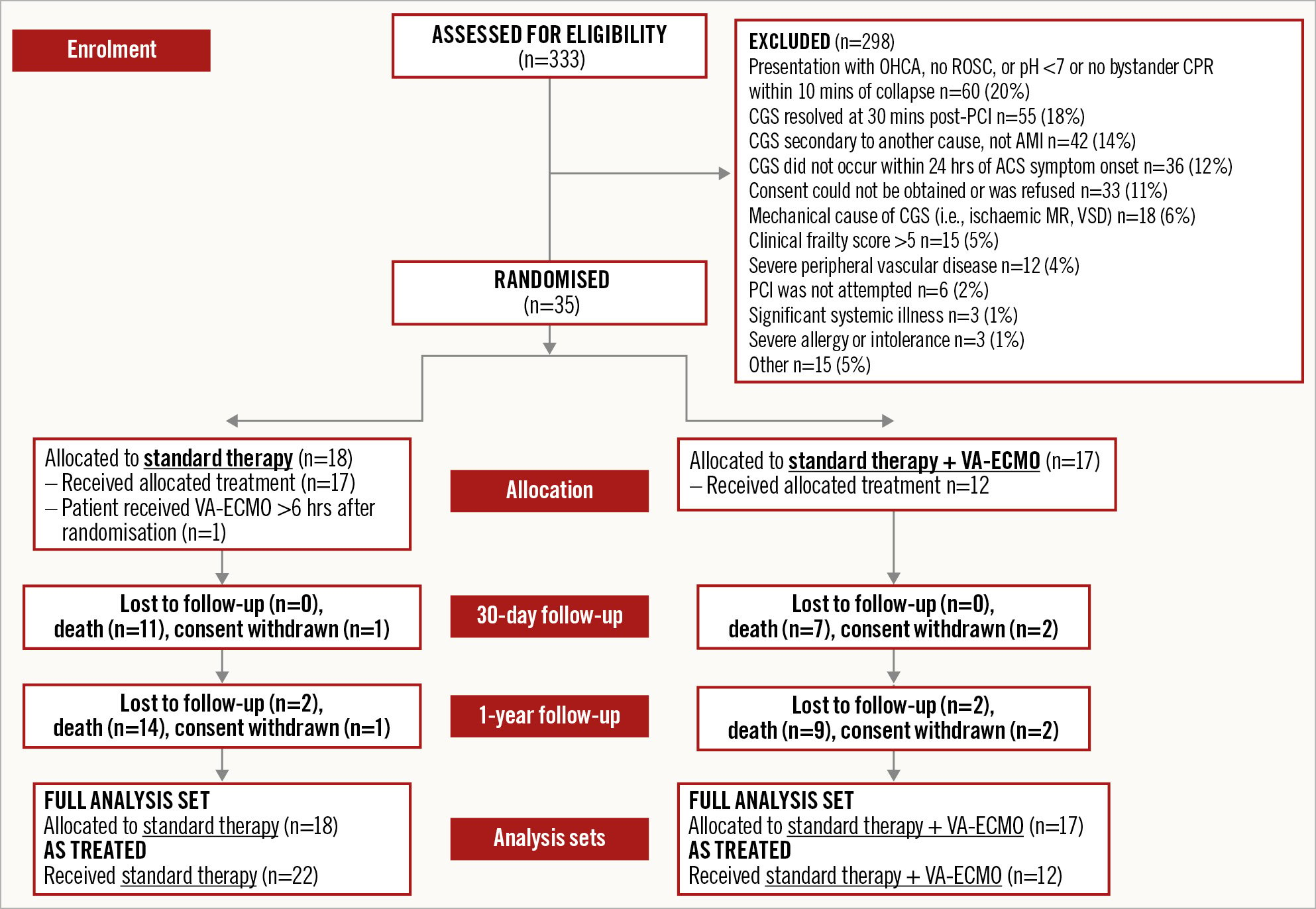
Figure 1. Consort diagram for the EURO SHOCK trial recruitment. ACS: acute coronary syndrome; AMI: acute myocardial infarction; CGS: cardiogenic shock; CPR: cardiopulmonary resuscitation; MR: mitral regurgitation; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; ROSC: return of spontaneous circulation; VA-ECMO: venoarterial extracorporeal membrane oxygenation; VSD: ventricular septal defect
BASELINE CHARACTERISTICS
The baseline characteristics of the recruited patients are outlined in Table 1. These were similar between the 2 treatment groups.
The median age was 67 years (range: 38-83 yrs) in the standard therapy group and 68 years (range: 45-76 yrs) in the VA-ECMO group.
The extent of myocardial infarction and left ventricular dysfunction was similar between the 2 groups. The peak troponin values were also similar between the 2 groups: median peak troponin standard therapy: 1,780 ng/L (IQR 321-125,000); median peak troponin VA-ECMO group: 1,608 ng/L (IQR 536-7,490). The admission and peak lactate levels were numerically higher in the standard therapy group, although the subsequent peak lactate levels during the intensive therapy unit admission and pH levels were similar between groups. The degree of shock in both groups was similar as reflected by comparable scores with the Simplified Acute Physiology Score (SAPS II), Acute Physiology and Chronic Health Evaluation II (APACHE II), sequential organ failure assessment (SOFA) and vasoactive-inotropic score (VIS) (Table 1).
The estimated left ventricular ejection fraction (LVEF) on admission was also comparable between the 2 groups and classed as severely impaired in both groups (standard therapy median LVEF: 25% [IQR 15-35%]; VA-ECMO median LVEF: 20% [IQR 10-35%]).
Both groups had comparable times from the onset of CGS to the initial angiogram (median time 3 hours [IQR 2-5 hrs] in the standard therapy group and median time 2 hours [IQR 1-4 hrs] in the VA-ECMO group), as well as the times from the onset of CGS to primary PCI (median time 4 hrs [IQR 2-6 hrs] vs 2 hrs [IQR 1-4 hrs], respectively) (Supplementary Table 3). Angiographic and procedural characteristics and the success of primary PCI was also well matched between the 2 groups.
The median time from CGS onset to VA-ECMO was 4.8 hours (IQR 3.7-6.5 hrs). The median time from first medical contact (FMC) to VA-ECMO insertion was 4.4 hours (IQR 4.2-8.8 hrs). The timings from presentation to randomisation and implementation of the randomised strategy are summarised in Supplementary Table 3.
Table 1. Baseline characteristics
| Baseline characteristic | Standard therapy (n=18) | VA-ECMO (n=17) |
|---|---|---|
| Gender | ||
| Male | 16/18 | 13/16 |
| Female | 2/18 | 3/16 |
| Age, yrs | 65±12 | 66±9 |
| 67 (56-77) | 68 (60-73) | |
| 38-83 | 45-76 | |
| Race | ||
| Southeast Asian | 1/18 | 0/15 |
| Caucasian | 17/18 | 15/15 |
| Smoking status | ||
| Current | 6/17 | 4/14 |
| Former | 7/17 | 4/14 |
| Never | 4/17 | 6/14 |
| Hypertension | 10/14 | 10/15 |
| Diabetes | 5/16 | 7/14 |
| Type I | 0/5 | 0/7 |
| Type II | 5/5 | 7/7 |
| Diabetes treatment | ||
| Oral agents | 2/5 | 4/7 |
| Insulin and oral agents | 1/5 | 2/7 |
| Diet only | 1/5 | 0/7 |
| Unknown | 1/5 | 1/7 |
| Family history of ischaemic heart disease | 1/8 | 0/4 |
| Renal disease | 4/16 | 1/13 |
| Dialysis | 0/4 | 0/1 |
| CKD stage | ||
| Stage 2A | 1/4 | 0/1 |
| Stage 3A | 3/4 | 1/1 |
| Prior cerebrovascular event | 1/16 | 0/15 |
| Non-TIA | 1/1 | - |
| Prior MI | 3/15 | 1/15 |
| STEMI | 1/3 | 0/1 |
| NSTEMI | 2/3 | 1/1 |
| Prior PCI or CABG | 6/16 | 2/15 |
| Prior PCI | 5/6 | 2/2 |
| Prior CABG | 1/6 | 0/2 |
| Prior admission for heart failure | 0/16 | 0/16 |
| Dyslipidaemia | 7/8 | 4/6 |
| Peripheral arterial disease | 2/9 | 0/7 |
| Preadmission medications | ||
| Aspirin | 4/5 | 2/4 |
| Anticoagulant | 1/5 | 0/4 |
| Statins | 3/5 | 3/4 |
| ACE inhibitor | 1/5 | 1/4 |
| AT2 blocker | 1/5 | 1/4 |
| Beta blocker | 2/5 | 2/4 |
| Diuretic | 3/5 | 0/4 |
| ARNI | 1/5 | 0/4 |
| Other | 2/5 | 1/4 |
| Lab results | ||
| Peak troponin, ng/L | 1,780 (321-125,000) | 1,608 (536-7,490) |
| Haemoglobin, g/L | 138±23 | 117±41 |
| White cell count, x109/L | 18±6 | 18±8 |
| Platelet, x109/L | 250±4 | 233±88 |
| Urea, mmol/L | 8±5 | 6±2 |
| Creatinine, mg/dL | 1.5±0.5 | 1.2±0.4 |
| eGFR, mL/min/1.73 m2 | 51±16 | 53±22 |
| Admission lactate, mmol/L | 8.2±4.6 | 5.9±3.7 |
| Peak lactate, mmol/L | 10.2±3.7 | 8.1±4.8 |
| pH | 7.22 (7.07-7.34) | 7.18 (7.12-7.26) |
| CRP, mg/L | 20±48 | 28±43 |
| NT-proBNP, ng/L | 4,133±7,799 | 5,442±8,726 |
| BMI, kg/m2 | 28±4 | 27±5 |
| Blood pressure - admission | ||
| Systolic BP, mmHg | 107±38 | 90±23 |
| 95 (81-125) | 82 (75-105) | |
| Diastolic BP, mmHg | 68±27 | 55±15 |
| 58 (52-80) | 55 (46-60) | |
| Intubated | 12/16 | 8/12 |
| Highest VIS score | 75 (18-312) | 67 (5-102) |
| Highest SAPS II 0-48 hr | 52 (47-67) | 61 (51-83) |
| Highest APACHE II 0-48 hr | 21 (19-28) | 32 (9-37) |
| Highest SOFA 0-48 hr | 10 (7-12) | 9 (4-12) |
| Killip class | ||
| Class II | 0/17 | 1/13 |
| Class III | 1/17 | 0/13 |
| Class IV | 16/17 | 12/13 |
| Neurological assessment | ||
| Conscious and alert with good cerebral performance | 5/12 | 4/9 |
| Conscious with moderate cerebral impairment | 2/12 | 0/9 |
| Comatose | 5/12 | 5/9 |
| Glasgow Coma Scale | 9±6 | 8±6 |
| 8 (3-15) | 3 (3-15) | |
| 3-15 | 3-15 | |
| Location of MI | ||
| Anterior | 7/16 | 3/14 |
| Inferior | 2/16 | 5/14 |
| Lateral | 0/16 | 3/14 |
| Anterolateral | 6/16 | 3/14 |
| Inferolateral | 1/16 | 0/14 |
| Echocardiographic data | ||
| Echo performed (post-randomisation) | 12/17 | 11/13 |
| LVEF, % | 30±24 | 23±12 |
| 25 (15-35) | 20 (10-35) | |
| Mitral regurgitation | 5/12 | 0/11 |
| LV thrombus | 0/12 | 0/11 |
| Angiographic data | ||
| Single vessel disease | 12/18 | 8/14 |
| Multivessel disease | 6/18 | 6/14 |
| Diseased vessels (DS >50%) | ||
| 1VD | 5/18 | 7/16 |
| 2VD | 1/18 | 3/16 |
| 3VD | 5/18 | 3/16 |
| LMS isolated | 4/18 | 0/16 |
| LMS + 1VD | 0/18 | 1/16 |
| LMS + 2VD | 1/18 | 1/16 |
| LMS + 3VD | 6/18 | 1/16 |
| IRA lesion location | ||
| Prox RCA | 4/18 | 2/16 |
| Mid RCA | 0/18 | 3/16 |
| Acute marginal | 1/18 | 0/16 |
| LMS | 4/18 | 2/16 |
| Prox LAD | 7/18 | 5/16 |
| Mid LAD | 1/18 | 2/16 |
| Prox LCx | 1/18 | 1/16 |
| Mid LCx | 0/18 | 1/16 |
| Previous CABG | 1/18 | 0/16 |
| Attempted PCI | 16/18 | 14/16 |
| Stent implanted | 15/18 | 15/16 |
| DES | 15/15 | 15/15 |
| TIMI flow pre-PCI | ||
| TIMI 0 | 11/18 | 10/16 |
| TIMI 1 | 3/18 | 1/16 |
| TIMI 2 | 1/18 | 3/16 |
| TIMI 3 | 3/18 | 2/16 |
| TIMI flow post-PCI | ||
| TIMI 0 | 0/18 | 3/16 |
| TIMI 1 | 1/18 | 0/16 |
| TIMI 2 | 2/18 | 1/16 |
| TIMI 3 | 15/18 | 12/16 |
| IABP inserted | 8/18 | 3/16 |
| Pre-PCI | 2/8 | 1/3 |
| Post-PCI | 5/8 | 2/3 |
| Post-randomisation | 1/8 | 0/3 |
| Any NIRA lesions | 11/18 | 7/16 |
| Total number NIRA lesions | 39 | 23 |
| PCI to NIRA lesions | 4/39 | 1/23 |
| Anticoagulant regimen | ||
| Heparin | 18/18 | 16/16 |
| Bivalirudin | 0/18 | 0/16 |
| Antiplatelet regimen | ||
| Aspirin | 16/18 | 14/16 |
| Clopidogrel | 8/18 | 3/16 |
| Prasugrel | 6/18 | 7/16 |
| Ticagrelor | 4/18 | 5/16 |
| Cangrelor | 1/18 | 2/16 |
| GP IIb/IIIa inhibitor | 3/18 | 0/16 |
| Out-of-hospital cardiac arrest | ||
| No. of patients | 8/18 | 9/17 |
| Time to ROSC, min | 19±18 | 27±20 |
| 13 (8-31) | 16 (15-50) | |
| 5-46 | 15-50 | |
| Cardiac arrest rhythm | ||
| PEA | 1/6 | 0/3 |
| VF | 5/6 | 3/3 |
| Data are expressed as n/N, mean±standard deviation, median (IQR) or range. ACE: angiotensin-converting enzyme; AT2: angiotensin II receptor type 2; ARNI: angiotensin receptor/neprilysin inhibitor; BMI: body mass index; BP: blood pressure; CABG: coronary artery bypass graft; CKD: chronic kidney disease; CRP: C-reactive protein; DES: drug-eluting stent; DS: diameter stenosis; eGFR: estimated glomerular filtration rate; IABP: intra-aortic balloon pump; IQR: interquartile range; IRA: infarct-related artery; LAD: left anterior descending; LCx: left circumflex; LMS: left main stem; LV: left ventricle; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NIRA: non-infarct-related artery; NSTEMI: non-ST-segment elevation myocardial infarction; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; PEA: pulseless electrical activity; RCA: right coronary artery; ROSC: return of spontaneous circulation; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischaemic attack; TIMI: Thrombolysis in Myocardial Infarction; VD: vessel disease; VF: ventricular fibrillation | ||
PRIMARY OUTCOME MEASURE: 30-DAY ALL-CAUSE MORTALITY
The primary outcome of 30-day all-cause mortality occurred in 43.8% (7/17) of patients randomised to the VA-ECMO group and in 61.1% (11/18) of patients randomised to standard therapy (HR 0.56, 95% CI: 0.21-1.45; p=0.22) (Figure 2).
The primary outcome was also numerically lower in the as-treated analysis (HR 0.40, 95% CI: 0.13-1.26; p=0.105) (Supplementary Figure 2).
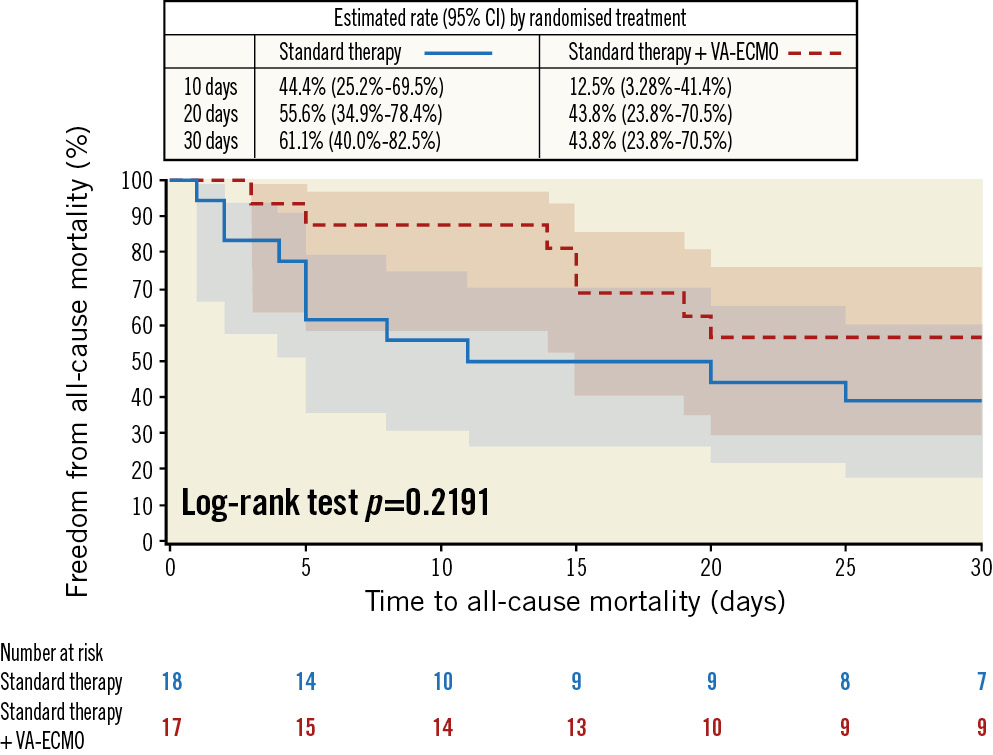
Figure 2. Primary endpoint of 30-day all-cause mortality − intention-to-treat analysis. CI: confidence interval; VA-ECMO: venoarterial extracorporeal membrane oxygenation
SECONDARY OUTCOME MEASURES
The secondary in-hospital outcomes are described in Table 2.
There were numerically lower rates of all-cause and cardiovascular death, ischaemic stroke, recurrent MI and acute kidney injury in patients randomised to VA-ECMO. In contrast, and as expected given the additional procedural nature of VA-ECMO, a numerically higher number of vascular complications and major bleeding events were observed in patients randomised to the VA-ECMO group. In terms of non-cardiovascular death, the predominant cause of death in both groups was from hypoxic brain injury (Supplementary Table 4).
There was a noticeably higher rate of failure of discharge from primary admission at 30 days with those patients randomised to standard therapy (83.3% compared with 57.1% in the VA-ECMO group). Left ventricular function assessment at 30 days is summarised in Supplementary Appendix 3.
Table 2. In-hospital outcomes − intention-to-treat analysis.
| Standard therapy | VA-ECMO + standard therapy | |
|---|---|---|
| Total number of patients | 18 | 17 |
| All-cause death | 13/18 (72) | 7/14 (50) |
| CV death | 6/18 (33) | 2/14 (14) |
| Stroke | 2/18 (11) | 0/14 (0) |
| Ischaemic stroke | 2/18 (11) | 0/14 (0) |
| Recurrent myocardial infarction | 2/18 (11) | 0/14 (0) |
| Major bleeding (BARC 3-5) | 1/18 (6) | 5/14 (36) |
| Escalation to other (non-VA-ECMO) support device for refractory shock | 1/6 (17) | 0/5 (0) |
| Escalation to VA-ECMO (crossover) | 1/18 (6) | NA |
| Any vascular complications | 0/18 (0) | 3/14 (21) |
| Acute kidney injury | 8/18 (44) | 4/14 (29) |
| Failure of discharge from primary admission | 15/18 (83) | 8/14 (57) |
| Data are N or n/N (%). Percentages are Kaplan-Meier or cumulative incidence estimates. BARC: Bleeding Academic Research Consortium; CV: cardiovascular; VA-ECMO: venoarterial extracorporeal membrane oxygenation | ||
Quality-of-life OUTCOMES AT 30 DAYS POST-DISCHARGE
There were a limited number of EQ-5D-3L questionnaires completed at 30 days in both the standard therapy (n=2) and VA-ECMO (n=4) groups. The responses are summarised in Supplementary Table 5. Among the EQ-5D-3L respondents, in the standard therapy group, there were no reported problems with mobility, self-care, or usual activities at 30 days, while half of the respondents from the VA-ECMO group reported some difficulties in these domains at 30 days. Similar responses for anxiety/depression were reported at 30 days in both groups.
Similarly, there were limited responses from the Minnesota Living with Heart failure Questionnaire (2 responses at 30-day clinic in the standard therapy group and 3 responses in the VA-ECMO group). Lower scores were reported for both the physical (standard therapy median: 38 [IQR 28-47]; VA-ECMO median: 13 [IQR 8-34]) and emotional (standard therapy median: 20 [IQR 10-30]; VA-ECMO median: 6 [IQR 5-13]) components of the questionnaire. The total median scores were 86 (IQR 58-114) in the standard therapy group and 33 (IQR 21-73) in the VA-ECMO group.
IN-HOSPITAL SERIOUS ADVERSE EVENTS
There was a total of 11 patients with a serious adverse event (SAE) during admission (31.43%: 5 in the standard therapy group and 6 in the VA-ECMO group). The reported SAEs are summarised in Supplementary Table 6, with similar types and numbers demonstrated between the randomised groups.
ONE-YEAR OUTCOME DATA
All-cause mortality at 12 months was numerically lower in the VA-ECMO group; this occurred in 51.8% (8/17) of patients randomised to the VA-ECMO group and in 81.5% (14/18) patients randomised to standard therapy (HR 0.52, 95% CI: 0.21-1.26; p=0.14) (Figure 3).
Twelve-month all-cause mortality was also numerically lower in the as-treated analysis (Supplementary Figure 3).
The 12-month composite endpoint of all-cause mortality and readmission with heart failure was also numerically lower in the VA-ECMO group: VA-ECMO group: 59.8% (9/17); standard therapy group: 79.2% (14/18) (HR 0.57, 95% CI: 0.24-1.34; p=0.19).
The rates of 12-month readmission for heart failure were similar between the treatment arms: VA-ECMO group: 8.0% (1/17); standard therapy group: 6.9% (1/18) (HR 1.19, 95% CI: 0.11-13.22; p=0.89). LVEF assessed at 12 months is summarised in Supplementary Appendix 3.
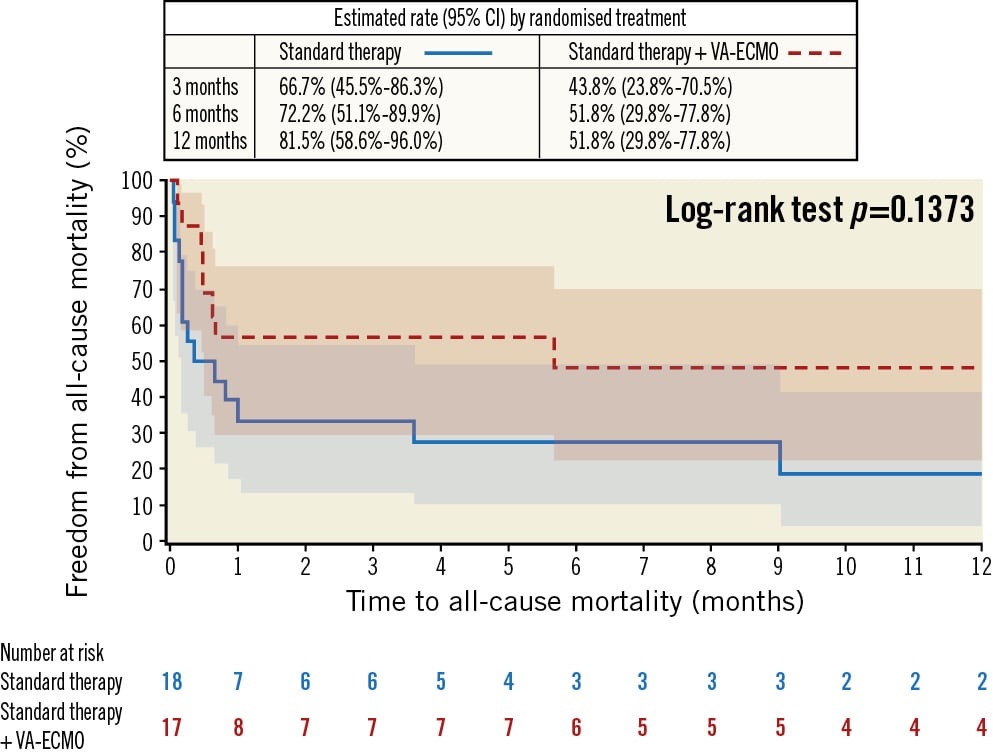
Figure 3. 12-month all-cause mortality − intention-to-treat analysis. CI: confidence interval; VA-ECMO: venoarterial extracorporeal membrane oxygenation
Discussion
The results of this study show that in patients with persistent CGS secondary to acute MI 30 mins after attempted or successful revascularisation of the culprit lesion, early implementation of VA-ECMO resulted in numerically lower rates of 30-day and 1-year mortality. The overall recruitment to the trial was significantly impacted by the COVID-19 pandemic, and less than 10% of the initially planned recruitment was completed. As a result, no definitive conclusions can be drawn from these data. However, the results do indicate a trend to a benefit from early use of VA-ECMO in this setting, supporting further clinical trials in this area. The potential benefit would have to be compared with the higher potential complications associated with the use of VA-ECMO in this setting, as shown in the results of this study. Thus, any future studies in this area would have to demonstrate any benefit from VA-ECMO outweighing the potential risks and complications associated with the use of this highly invasive mechanical circulatory support device.
During the recruitment period, only 13% of the screened patients were considered eligible for inclusion to the trial. As outlined in Figure 1, the most common reasons for screening failures were out-of-hospital cardiac arrest without ROSC or bystander CPR within 10 minutes (20%) and recovery from CGS after PCI (18%). With regard to the out-of-hospital cardiac arrest patients, the study aimed to include patients who would be most likely to receive the greatest benefit from use of VA-ECMO. Patients who received continuous CPR with no ROSC (which would effectively constitute ECMO-CPR rather than the use of VA-ECMO in CGS) or patients who had a prolonged period following cardiac arrest without CPR were considered to have a worse prognosis and lower chances of neurological recovery and were thus excluded from the trial, as the use of VA-ECMO is unlikely to be of benefit. Similarly, recovery of CGS following PPCI was also excluded, as, in such patients, VA-ECMO is unlikely to confer additional benefit. It is likely that use of an MCS device in patients with CGS complicating acute MI may be beneficial to a distinct cohort of CGS patients whose outcome from the shock state can be improved by the use of VA-ECMO or another MCS device, and, where any additional treatment is not likely to be futile. The timing of the use of VA-ECMO, either before or after the PCI attempt, remains an area of interest and uncertainty. Although patients can improve with PCI alone, as shown in the recruitment data of this trial, there are retrospective data potentially indicating a benefit from early haemodynamic support and stabilisation prior to PCI with an MCS device. Such a strategy of upfront VA-ECMO would need to confirm a benefit beyond any potential risks of using VA-ECMO, and this remains an active area of research with forthcoming trials, such as in the ECLS-SHOCK trial (Clinical.Trials.gov: NCT03637205)8. Determining which patients are most likely to benefit from VA-ECMO or other MCS devices is another aspect of further research in this area, possibly through post hoc analyses of larger trials. Such information could be of benefit in future trials.
This study allowed physicians to undertake standard therapy as per their usual practice in the management of patients with CGS following PPCI. Although there are some data to indicate how such patients can be managed, these patients can be a heterogenous population in terms of shock state post-PPCI and their response to therapy. Thus, a pragmatic design was used allowing physicians to tailor treatment according to response with standard therapy while assessing the additional benefit of VA-ECMO in the intervention arm of the trial. This strategy also accounted for heterogeneity in clinical practice that can occur across intensive care units in Europe recruiting patients to a trial.
In addition, the trial only permitted LV unloading with an IABP. There are several methods of unloading the LV while the patient is on a VA-ECMO, including potentially using an Impella (Abiomed) or atrial septostomy. However, to date, there are no compelling data to support one modality over the other or to indicate in which patients or when to unload the LV9. The trial was not pragmatically designed to investigate any additional benefit of LV unloading in the context of peripheral VA-ECMO nor to determine the optimal modality of LV unloading. The use of an Impella for LV unloading was discouraged, as this may have confounded any effect of VA-ECMO in the intervention arm of the trial, especially as Impella may also have a role in the management of cardiogenic shock10; this is being evaluated in the setting of CGS complicating acute MI in the DanGer Trial (ClinicalTrials.gov: NCT01633502)11.
Although we attempted to ensure no crossover therapy between the standard and VA-ECMO groups, there were 5 patients who were randomised to VA-ECMO but did not receive the study intervention. This was mainly due to difficulties in peripheral cannulation (3/5), with 2 of the 5 cases due to patient refusal or withdrawal of consent. Anticipating this potential for patients not being able to receive the allocated treatment, an “as-treated” analysis was also undertaken which, again mindful of the low numbers of participants, continues to demonstrate a potential, if non-significant, benefit for VA-ECMO in those patients that received this treatment. Again, this would need to be confirmed in larger-scale randomised controlled trials; however, the principle of a prospective “as-treated” analysis would be important to mitigate the impact of potential crossovers in such trials.
In contrast to other studies that have suggested no benefit of VA-ECMO use in refractory CGS1213, the patients randomised to VA-ECMO within this trial received ECMO at a median of 4.8 hrs from the time of onset of CGS. This is commensurate with timings of other studies that suggested a benefit from VA-ECMO in this setting1415. Thus, the potential benefit derived from VA-ECMO or other mechanical support devices is likely driven by early haemodynamic support before maladaptive physiological responses have occurred because of the low cardiac output state, which inevitably leads to multiple organ failure and consequent mortality.
The EURO SHOCK trial only included patients who had persistent CGS 30 mins after revascularisation. While some have suggested that upfront use of a mechanical support device before revascularisation may lead to a greater derived benefit from these devices, as alluded to from a subset of data from the USpella Registry10, there is a potential risk of including patients who would otherwise recover from PCI without further intervention, potentially exposing such patients to high risk complications associated with invasive devices. Indeed, from the screening logs of this trial, 18% of patients who were not recruited had early resolution of CGS following revascularisation.
The recently reported ECMO-CS trial showed no difference in the primary composite endpoint of death from any cause, resuscitated circulatory arrest and implementation of another circulatory support device at 30 days between those patients who received immediate VA-ECMO and those who did not (63.8% in the immediate VA-ECMO group, 71.2% in the non-early VA-ECMO group; HR 0.72, 95% CI: 0.46-1.12; p=0.21). Similarly, no difference was seen in the individual components of the primary endpoint nor adverse secondary outcomes16.
Although the ECMO-CS study seems at odds with the indication of early improvement seen in outcomes with VA-ECMO from this study, it should be noted that 39% of patients enrolled to the non-early VA-ECMO arm of ECMO-CS did receive VA-ECMO or another mechanical support device due to a deterioration in their clinical condition. The mean time to VA-ECMO insertion in these crossover patients was 1.9 days. This crossover effect has been cited in other trials involving the use of ECMO, such as the EOLIA trial17, as being a possible reason for diluting any potential benefits of ECMO use and a reason why this was discouraged in the standard therapy group in EURO SHOCK. In addition, ECMO-CS did not exclusively include patients with CGS secondary to acute myocardial infarction – only 74 patients of the 117 analysed patients that were recruited had CGS secondary to acute MI. Therefore, recovery and subsequent weaning would be dependent on recovery from the underlying cause of shock, whereas in acute MI patients, VA-ECMO use is envisaged to support organ perfusion in the time following PCI where revascularised myocardium can recover.
The finding of a numerically lower rate of 30-day mortality in the VA-ECMO group in EURO SHOCK is commensurate with similar reported benefits in other studies. Sheu et al showed a significantly lower 30-day mortality in patients who received VA-ECMO in the catheterisation lab following confirmation of refractory shock, despite IABP use, compared with IABP alone14.
A small pilot study randomising patients with CGS to immediate VA-ECMO or standard therapy showed a numerically lower rate of 12-month mortality in the VA-ECMO group18. However, as with the EURO SHOCK trial, this study recruited 42 patients, therefore, the number of patients was too low to draw meaningful conclusions. In addition, 12-month mortality was a secondary outcome measure in this study; the primary outcome was improvement in LV function at 30 days, as measured by echocardiography, and the study showed no benefit of VA-ECMO in this respect19. Also, it is noteworthy that at 30 days, only 1 patient had died in the control group, implying that this study had recruited lower-risk CGS patients.
Thirty-day all-cause mortality in the EURO SHOCK control group was 61.1%, suggesting that the population recruited to the trial were not “lower-risk” CGS patients. This is consistent with data on 30-day mortality reported from other retrospective analyses2. It is likely that we were able to exclude patients who would recover with revascularisation alone by allowing an appropriate window of time following PPCI and before randomisation20.
The findings of this study do indicate potential benefit from early use of VA-ECMO in CGS patients. There are currently 2 other large scale randomised controlled trials, ECLS-SHOCK and the ANCHOR trial (ClinicalTrials.gov: NCT04184635) comparing mostly upfront use of VA-ECMO prior to revascularisation and VA-ECMO compared with standard therapy, respectively. These trials should provide further insight into the use of VA-ECMO in CGS patients.
In evaluating the impact of the use of VA-ECMO in survival from CGS, it is important to ascertain whether survival is associated with a good quality of life. Although we attempted to assess quality-of-life outcomes at 30 days with both the EQ-5D-3L and Minnesota Living with Heart Failure Questionnaire (MLHFQ), there were low response rates for both questionnaires at follow-up. The low number of responses is a result of the overall number of patients recruited to the trial and the consequent number of patients surviving in each group at 30 days, as well as the expected response rate from such questionnaires. Given the high 30-day mortality associated with CGS, it is imperative to ensure a high a rate of completion of questionnaires in both the control and intervention arms at follow-up to ensure data that can inform whether any potential mortality benefit also translates into sustained or improved quality of life. Strategies that could be employed to obtain optimal response rates could include ensuring completion of questionnaires at the time of follow-up in the clinics and limiting the number of questionnaires that patients are asked to complete. Due to the small numbers of completed questionnaires, it is difficult to draw any meaningful conclusions on the impact on quality of life for patients surviving CGS and having had VA-ECMO.
Limitations
The key limitation of this trial is the low recruitment, due mainly to the COVID-19 pandemic. Trial recruitment was stopped early, predominantly because of the impact of the COVID-19 pandemic. Trial recruitment was suspended at different time periods during the trial in participating centres due to the need to utilise ECMO resources for COVID-19 patients. Consequentially, the reported results are significantly underpowered to draw meaningful conclusions on the utility of VA-ECMO in this setting.
Although there were no prespecified weaning criteria for patients placed on VA-ECMO, we decided to allow intensive care physicians to undertake weaning as they felt appropriate according to the clinical condition of the patient; this allowed for a more pragmatic reflection on how patients presenting in such circumstances are treated if placed on VA-ECMO and how they should be evaluated in the trial. All centres involved in the trial were experienced ECMO centres.
Conclusions
Due to the limited number of patients recruited to the trial, no definite conclusions could be drawn from the available data on the use of VA-ECMO in CGS complicating acute myocardial infarction. There could be a potential role for VA-ECMO in CGS; however, the efficacy and safety of VA-ECMO in this setting would need to be assessed in larger RCTs. These data may contribute to future meta-analyses of forthcoming RCTs comparing the use of VA-ECMO with standard care in CGS patients following acute myocardial infarction.
Impact on daily practice
Although underpowered because of poor recruitment, the findings from the trial support further randomised clinical trials into the early use of VA-ECMO in cardiogenic shock complicating acute myocardial infarction that does not improve following primary PCI of the culprit lesion.
Acknowledgements
We thank the patients and staff who supported this study.
The study authors would like to acknowledge the contribution of the following research team members from each institution:
Consorci Institut d’Investigacions Biomediques August Pi i Sunyer, Cardiovascular Institute, Hospital Clinic, Barcelona, Spain: Rut Andrea, Salvatore Brugaletta, María Angeles Castel, Manuel Castellá, Oriol de Diego, Ana García-Álvarez, Marta Farrero, Xavier Freixa, Omar Abdul Jawad-Alisent, Ander Regueiro, Elena Sandoval
Hospital Universitario Vall d’Hebron, Barcelona, Spain: Neus Bellera, Bruno Garcia del Blanco, Angeles Carmona, Ignacio Ferreira
Hospital Universitari Germans Trias i Pujol, Badalona, Spain: Josepa Mauri, Cosme Garcia, Santiago Moreno, Christian Muñoz, Xavier Carrillo
Hospital de la Santa Creu i Sant Pau, Barcelona, Spain: Dabit Arzamendi Aizpurua, Albert Duran Cambra, Antonino Ginel Iglesias, Tobias Koeller, Antonio Serra, Manel Tauron Ferrer, Montserrat Vila Perales
Emergency Medical System (EMS), Catalonia, Spain: F. Xavier Jiménez-Fábrega, Jorge A. Morales, Daniel Vilar
Deutches Herzzentrum Muenchen, Munich, Germany: Monika Neumyer
Ludwig Maximillian Universitaet, Munich, Germany: Monika Baylacher, Leonhard Binzenhöefer
Pauls Stradins Clinical University Hospital and the University of Latvia, Riga, Latvia: Aija Maca-Kaleja
King’s College Hospital, London, UK: Georg Auzinger, Jonathan Byrne, Jonathan Breeze, Iain Carroll, Milena Chee, Richard Fisher, Elton Gelandt, Robert Loveridge, Lisa Morgan, Nilesh Pareek, Sheetal Patale, Tasneem Pirani, Andre Vercueil, Ian Webb, Michael Whitehorne, Chris Willars
Glenfield Hospital, Leicester, UK: Mr Chris Harvey, Dr Caroline Sampson, Dr Susan Dashey, Dr Graziella Isgro, Dr Matthew Charlton, Dr Vasileios Zochios, Ms Elizabeth Wadey, Ms Gail Faulkner, Mel Ferguson
CERC, trial monitoring, Massy, France: Prof. Marie-Claude Morice, Laure Morsiani
Glasgow CTU Team, Robertson Centre for Biostatistics, University of Glasgow, Glasgow, UK: Sharon Keane, Claire Kerr, Mairi Warren, Sarah Boyle.
Accelopment, Zürich, Switzerland: Jacqueline Strehler, Dr Janette Mueller
Funding
EURO SHOCK has received funding from the European Union’s Horizons 2020 research and innovation programme under the grant agreement number 754946-2. Colin Berry is supported by the British Heart Foundation (RE/18/6134217).
Conflict of interest statement
M. Orban reports receiving payments or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Abbott Medical, AstraZeneca, Abiomed, Bayer Vital, Biotronik, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences, and Sedana Medical. T. López-Sobrino reports receiving payments or honoraria for presentations from the University of Barcelona and the European Acute Cardiac Care Association. T. Adriaenssens reports receiving honoraria from Abiomed for speakers’ bureaus. C. Berry is employed by the University of Glasgow, which holds research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, and HeartFlow; and holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Auxilius Pharma, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genetech, GSK, HeartFlow, Menarini, Neovasc, Siemens Healthcare, and Valo Health. D. Adlam has received funding to support a clinical research fellow from Abbott Vascular; funding from AstraZeneca for unrelated research; has conducted unrelated consultancy for GE HealthCare; holds patents for medical devices, including a cardiac assist device (EP3277337A1, PCT/GB2017/050877, UK PATENT APPLICATION NUMBER 2211616.4); and has received royalties from Elsevier Inc. for ECG made Practical and ECG Problems books. M. Flather reports receiving consulting fees from Boehringer Ingelheim for consulting work unrelated to this project; speakers’ fees from Menarini International, AstraZeneca, and Bayer; support for attending meeting from AstraZeneca, and modest honorarium as Deputy Editor of the European Heart Journal ‒ Quality of Care & Clinical Outcomes. The other authors report no conflicts of interest related to the reported study.
Supplementary data
To read the full content of this article, please download the PDF.

