
Abstract
Aims: The aim of the study was to identify independent correlates of survival in patients undergoing PCI for refractory cardiogenic shock due to myocardial infarction (RCS-MI) with the need for extracorporeal life support (ECLS).
Methods and results: This observational single tertiary centre study enrolled 106 consecutive patients (52.7±10.4 years) with ECLS placed before or after the PCI. Half of the patients had triple vessel disease and PCI was attempted whenever possible (74.5%). The 30-day mortality rate was 63.2%. Left main culprit vessel disease (19% of patients) (adj. HR [95% CI]: 2.31 [1.27-4.18], p=0.006) and sepsis-related organ failure assessment ≥13 (adj. HR 2.17 [1.25-3.75], p=0.005) were independently associated with 30-day mortality. The use of intra-aortic balloon pump (IABP) combined with ECLS was an independent protective factor (adj. HR 0.48 [0.28-0.80], p=0.006). Neither complete (p=0.66) nor successful (p=0.69) myocardial revascularisation was associated with 30-day survival.
Conclusions: RCS in MI patients often reveals a severe multivessel coronary artery disease with no impact of early percutaneous coronary revascularisation on clinical outcome. The survival advantage of IABP when combined with ECLS further suggests that achieving an early effective haemodynamic support should be the major goal in this young patient population.
Abbreviations
CI: confidence interval
CABG: coronary artery bypass graft
CAD: coronary artery disease
ECLS: extracorporeal life support
IABP: intra-aortic balloon pump
IQR: interquartile range
LVAD: left ventricular assist device
LVEF: left ventricular ejection fraction
MI: myocardial infarction
OR: odds ratio
PCI: percutaneous coronary intervention
RCS: refractory cardiogenic shock
SOFA: sepsis-related organ failure assessment
VTI: velocity time integral
Introduction
Cardiogenic shock is the most severe complication of myocardial infarction (MI), arising in 5-10% of patients, and is associated with a mortality rate as high as 40%1 or even higher in refractory cardiogenic shock due to MI (RCS-MI). Acute left ventricular failure is the main cause of CS whereas mechanical complications are less common. The European guidelines recommend emergency revascularisation with either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery and complete revascularisation if there is multivessel disease2. The use of an intra-aortic balloon pump (IABP) may be considered as a haemodynamic support in selected patients with severe mitral insufficiency or ventricular septal defect. Mechanical left ventricular assist devices (LVADs), including percutaneous short-term mechanical circulatory support devices, have been evaluated in RCS-MI with mitigated results3. These devices are recommended as rescue therapy to preserve organ perfusion as a bridge either to recovery of myocardial function or to heart transplantation4, or even as a bridge to LVAD destination therapy. The decision is usually made on an individual basis by a shock team when available.
The benefit of myocardial revascularisation in these seriously ill patients presenting with RCS-MI remains unknown. Early haemodynamic stabilisation is often the first goal5. The aim of the retrospective Pitié-Salpêtrière Cardiogenic Shock Registry was to determine the independent predictors of mortality. In the present analysis, we selected RCS-MI presenters who were also implanted with extracorporeal life support (ECLS).
Methods
PATIENT SELECTION AND STUDY DESIGN
All consecutive patients presenting with an RCS-MI treated with an ECLS and addressed to the catheterisation laboratory of our institution between 2007 and 2013 were considered. Acute MI was defined according to the third universal definition with a rise in cardiac troponin above the 99th percentile upper reference limit with symptoms of ischaemia, new or presumed new significant ST or T-wave changes or new left bundle branch block. Early revascularisation was the primary strategy in all cases with PCI as the preferred option and thrombolysis as an alternative when PCI could not be performed within 120 minutes of symptom onset. Patients without coronary artery disease (CAD) and those who did not undergo coronary angiography were excluded (Figure 1).
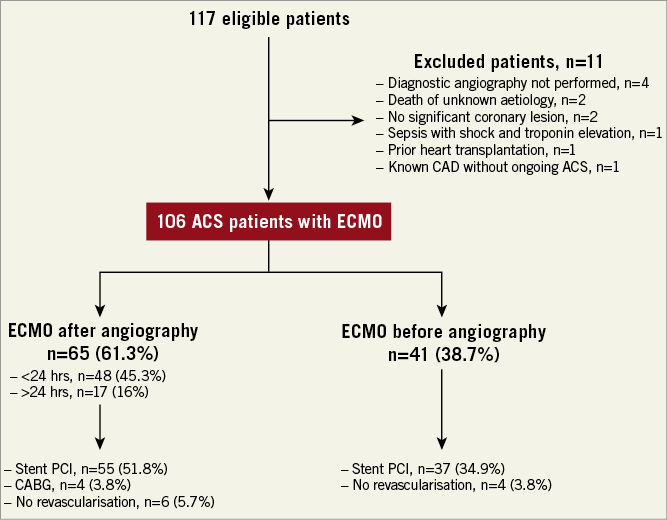
Figure 1. Flow chart.
Coronary angiographies were performed by experienced operators. Complete angiographic revascularisation was defined as successful dilation of all stenoses of ≥50% in the proximal halves of the right coronary artery, left circumflex artery, left main coronary artery, left anterior descending coronary artery, and any previous grafts. Angiographically, PCI success was defined for the culprit vessel as a residual stenosis of less than 50% of the luminal diameter and post-angioplasty TIMI flow ≥2/3. Clinical success of revascularisation was defined as the angiographic success combined with the resolution of the ST-segment deviation or life-threatening arrhythmia and no referral for coronary artery bypass grafting during hospital stay.
ECLS was indicated as short-term mechanical circulatory support for RCS, defined as the combination of left ventricular ejection fraction <25%, cardiac index <2.2 L/min/m2, blood pressure <90 mmHg for >30 min or need for catecholamines to maintain systolic pressure over 90 mmHg, and impaired organ perfusion despite a high dose of catecholamines (dobutamine ≥20 μg/kg/min and norepinephrine >1 μg/kg/min). Precluding criteria for ECLS were life expectancy <1 year and prolonged cardiac arrest (>60 min). Cardiac arrest >30 minutes refers to the timing of CPR.
Our shock team provides a 24-hr/7-day service together with an ECMO mobile unit coordinated by the SAMU for out-of-hospital patients. In refractory cardiac arrest, ECLS was implanted as soon as possible. All ECLS devices were surgically inserted wherever the patient was with a median time of 15 minutes allowing circulatory support within 20 minutes after onset of implantation. An additional 7 Fr catheter was systematically inserted into the femoral artery to prevent leg ischaemia. Pump speed was adjusted to obtain blood flow of 3.5-4.5 L/min. Intravenous (IV) unfractionated heparin was given to maintain the activated partial thromboplastin time at 1.5-2 times normal. The decision to wean off ECLS, bridging to long-term mechanically assisted circulatory support or heart transplantation was made by the Heart Team. Weaning off ECLS was performed according to established echocardiographic criteria including an aortic velocity time integral (VTI) ≥10 cm, LVEF ≥20-25%, and lateral mitral annulus peak systolic velocity ≥6 cm/s when ECLS flow was reduced to <1.5 L/min6. All ECLS were retrieved surgically.
The use of IABP (CS100®; Datascope/Maquet, Getinge Group, Rastatt, Germany) was left to the discretion of the treating physicians and was inserted into the femoral artery on the opposite side to ECLS. Pumping was set at a 1:1 ratio with electrocardiographic triggering and maintained until weaning from ECLS.
Patient characteristics, treatment and outcome data were extracted from our ECLS database and patients’ medical records. Patients were followed up to 30 days.
OBJECTIVES AND OUTCOMES
The primary objective was to evaluate mortality and identify the independent predictors of mortality. The primary study endpoint was 30-day mortality. The secondary outcome was in-hospital mortality. Major bleedings according to the BARC definitions were also recorded (type 3 to 5)7.
STATISTICAL ANALYSIS
Continuous variables are presented as mean (standard deviation) or median (IQR) and categorical variables as number (percentage). Baseline characteristics were compared between survivors and non-survivors by means of the chi-square (χ²) or Fisher’s exact test. The Student’s t-test or the Mann-Whitney-Wilcoxon test (normality tested with the Shapiro-Wilk test) was used for quantitative variables. Variables were considered for multivariable analysis if associated with one-month mortality on univariate analysis with a p-value <0.2. Collinearity between the explanatory variables was examined and associated variables were not included in the model. Because a change of clinical practices could result in time-related confounding, we considered two periods (before 2011, and 2011 and after) for adjustment in the multivariable analysis.
Independent predictors of 30-day mortality were identified with multivariable stepwise Cox proportional hazards analysis with an exit p-value threshold set at 0.1. Proportional hazards assumption was verified for all variables. Results are reported as adjusted hazard ratio (adj. HR) with 95% confidence interval (95% CI). The Kaplan-Meier method was used to build survival curves for variables of interest (complete revascularisation, left main coronary disease, IABP). A p-value <0.05 was considered significant unless otherwise specified. SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA) was used.
Results
BASELINE CHARACTERISTICS
The patient population (n=106) consisted predominantly of young active smoking males (Table 1). Abnormal ST segment was observed in the vast majority and cardiac arrest lasting more than 30 minutes was reported in almost half of them, highlighting the very high risk of this population. Severe CAD at presentation was common with significant left main disease in one out of five patients. PCI was attempted in 75% of patients and reperfusion was successful in 72% of them. Reperfusion delay was four hours after symptom onset. Troponin peak was high, suggesting an extended myocardial damage, and lactate levels were elevated at presentation (≥2.0 mmol/L) in 85% of patients.
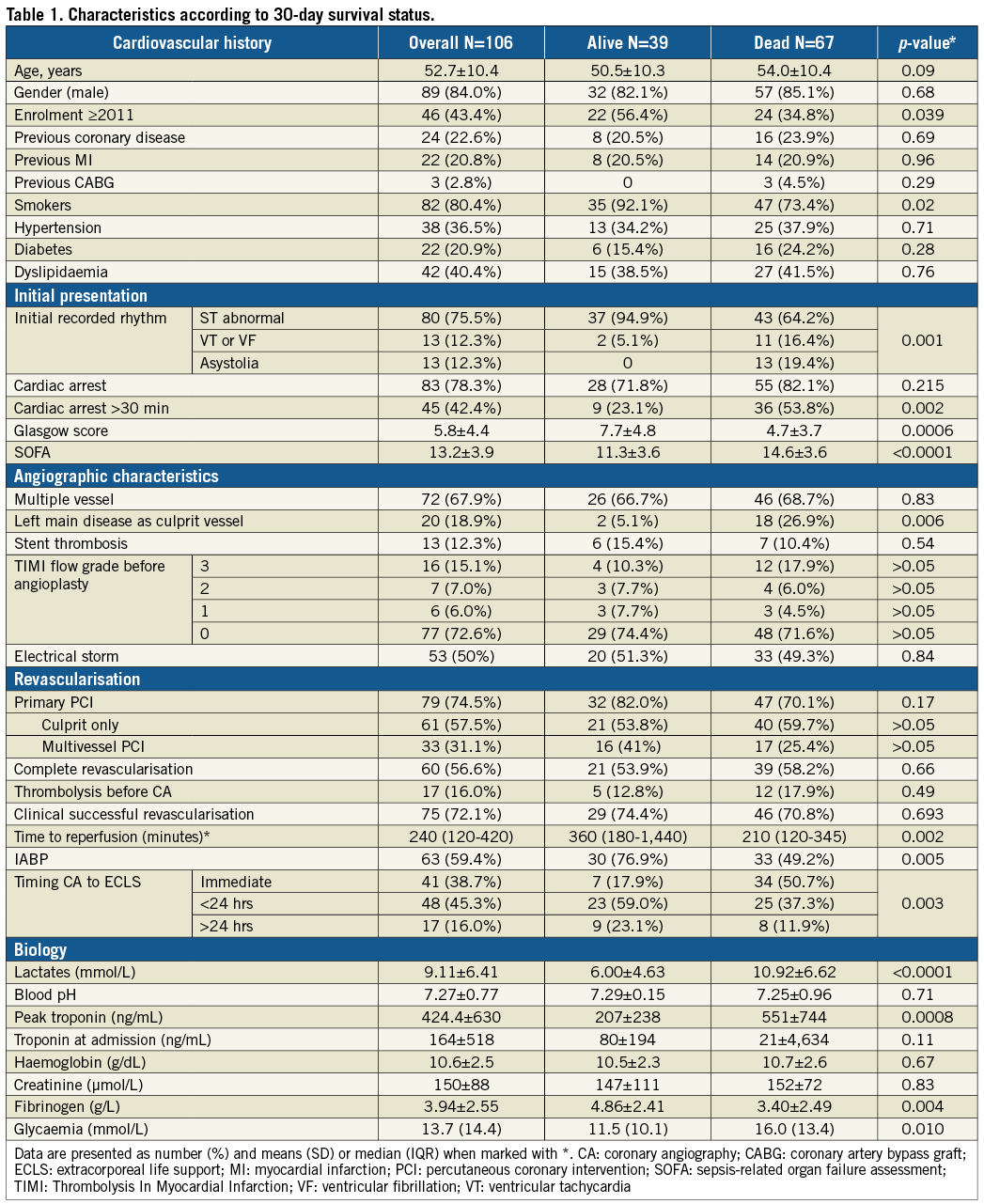
Coronary angiography was performed as soon as possible and at the time of ECLS implantation in 38.7% of patients in whom RCS occurred shortly before or during angiography/PCI (Supplementary Table 1, Figure 2). IABP was used in 59.4% and was implanted at the time of angiography or PCI in all patients. Early ECLS implantation (45.3%) was performed in patients at much higher risk with more profound multiple organ failure and higher 30-day mortality versus ECLS implantation during or after angiography (Figure 2).
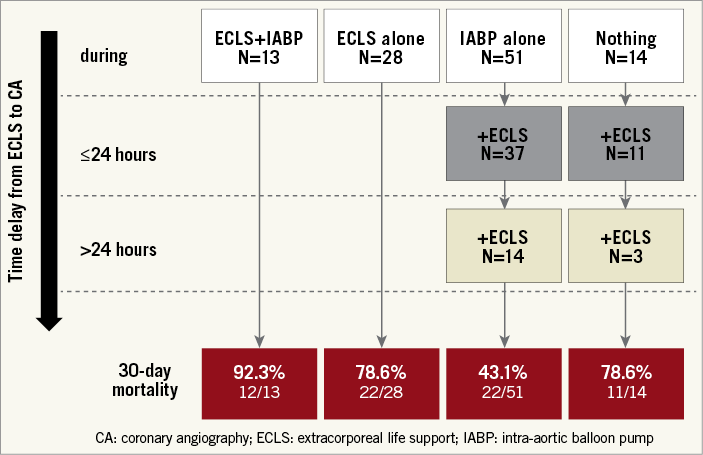
Figure 2. Time delay from ECLS/IABP implantation to coronary angiography and one-month clinical outcome.
Patients presenting with cardiac arrest (n=83) less frequently had multiple vessel disease, IABP use, more severe multiple organ failure, more frequently received ECLS before or at the time of coronary angiography and had higher 30-day mortality than those without cardiac arrest.
OUTCOMES
In-hospital and 30-day mortality was 61.3% and 63.2%, respectively. Patient characteristics according to 30-day vital status are described in Table 1. A total of 69.2% (27/39) of the one-month survivors recovered and were weaned off ECLS, while 25.6% (10/39) were bridged to long-term haemodynamic support or heart transplantation (n=2). Significantly more patients recovered and were weaned off ECLS with than without IABP (eight [19%] vs. 24 [37.5%], p=0.043). Recovery in one-month survivors did not differ according to whether revascularisation was successful or not, despite a trend towards better recovery when revascularisation was successful (8/10 [80%] vs. 19/29 [65.5%], p>0.05, respectively).
The rate of major bleedings according to BARC definitions was 34.9% and was not associated with survival, probably due to the high background mortality in this cohort and the small sample size. There was no difference in major bleeding in patients who received IABP versus those who did not.
INDEPENDENT CORRELATES OF SURVIVAL
Age, smoking status, initial recorded rhythm, cardiac arrest >30 min, sepsis-related organ failure assessment (SOFA), left main coronary disease, primary PCI, IABP, timing of ECLS regarding coronary angiography, lactate level, troponin peak, fibrinogen level and year of enrolment ≥2011 were associated with one-month mortality (p-value <0.2) (Table 2).
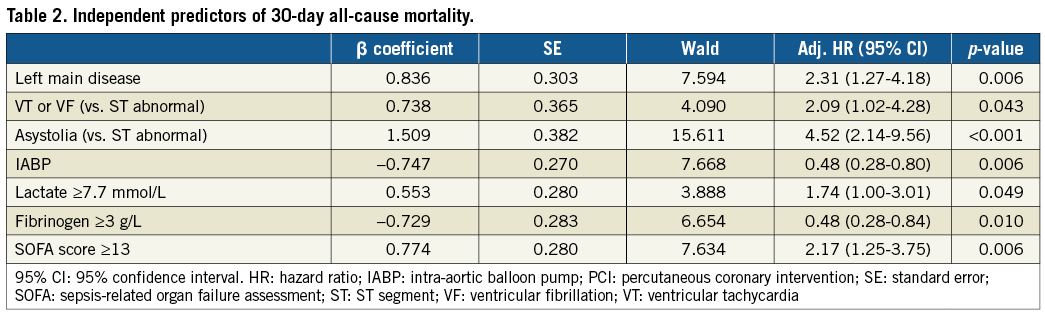
Survivors were younger, had less severe coronary anatomy, had a lower SOFA score and higher Glasgow score, more frequently had a shockable rhythm and more frequently underwent PCI and IABP implantation as compared to non-survivors. Survival did not differ according to the completeness of coronary revascularisation (Figure 3A). The frequency of use of IABP did not differ between the before and from and including 2011 periods (p=0.757) in our cohort.
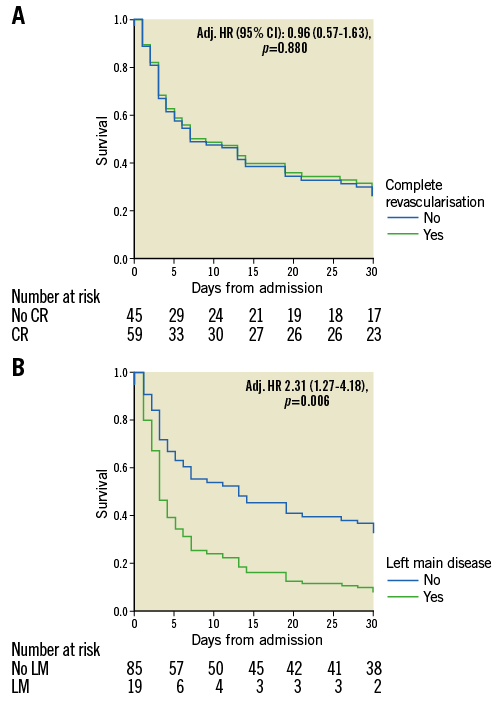
Figure 3. Kaplan-Meier curves of cumulative survival with and without complete revascularisation (A) and with and without left main culprit vessel disease (B).
Blood lactate, fibrinogen and SOFA score were transformed into binary variables to satisfy the proportional hazards assumption. Left main culprit vessel disease (Figure 3B) and admission SOFA score ≥13 were independently associated with 30-day mortality (Table 2), whereas the use of IABP (Figure 4) combined with ECLS was identified as an independent protector to 30-day mortality. Time delay from ECLS to coronary angiography was not independently associated with 30-day mortality even if forced into the model (adj. HR 0.81 [0.39-1.67], p=0.561).
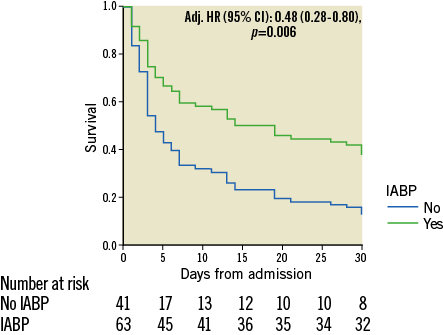
Figure 4. Kaplan-Meier curve of cumulative survival with and without IABP implantation.
Discussion
In this observational study, we found that the left main involvement was frequent (19% of patients) and independently associated with mortality in the acute setting of a refractory cardiogenic shock related to myocardial infarction. The second major finding was that percutaneous coronary revascularisation was not associated with survival benefit. This was observed irrespective of whether it was complete and/or successful. Finally, there was an improved survival when intra-aortic balloon pump was combined with ECLS, which was placed in all patients either before or after coronary intervention.
This is the first study reporting on the lack of association between coronary revascularisation and survival, although left main culprit artery disease was found to be a major prognostic factor. The presence of multiple vessel disease did not alter the prognosis in our cohort, highlighting further left main disease as the main coronary anatomy feature threatening survival. Post-PCI TIMI ≥2/3 flow was obtained in more than 90% of patients with left main disease and did not differ according to multiple versus simple vessel disease. One possible explanation accounting for such a discrepancy between the severity of the underlying CAD and the lack of benefit of revascularisation could be the very short period available for opening an occluded left main and the very large amount of suffering myocardium.
Early revascularisation is recommended as the primary treatment of acute MI with haemodynamic instability8. It seems that, in patients with RCS-MI, revascularisation does not have the ability to reverse myocardial injury fully when jeopardised myocardium is extended, as in our study population. In addition, complex PCI procedures may result in additional reperfusion injury, and do not immediately offload the injured ventricle and reverse systemic inflammatory response syndrome and multi-organ dysfunction syndrome, which are common in RCS-MI. All these factors may have accounted for such an unusually low success rate of 72% as compared with that of primary PCI, although this is in line with previous reports9. Furthermore, the risk in these gravely ill patients may not be that the non-infarct myocardium is subtended by potentially vulnerable distant lesions but that potentially recoverable myocardium in the peri-infarct area needs support while trying to recover. Although our patient population differs drastically from that of the CULPRIT-SHOCK trial where the clinical benefit of an early and complete revascularisation approach was evaluated in cardiogenic shock patients responding to inotropes, our results are consistent with the lack of benefit of complete revascularisation and its potential harm10. CULPRIT-SHOCK substudies on patients with left main disease and/or ECLS, very common features in our study population, are awaited.
Alternative strategies are necessary to improve outcomes in patients with RCS-MI. No short-term mechanical circulatory support device demonstrated a reduction of mortality in patients with cardiogenic shock due to myocardial infarction11,12 including refractory cardiogenic shock. Percutaneous left ventricular assist devices such as TandemHeart™ (CardiacAssist, Inc., Pittsburgh, PA, USA) and Impella® (Abiomed, Danvers, MA, USA) have been evaluated in small randomised trials that failed to convert the observed haemodynamic improvement into decrease of mortality11,13. This may be explained by the selection of too gravely ill patients. Percutaneous ECLS is expanding as a salvage therapy given full circulatory support (up to 4.0 L/min), rapid improvement in tissue oxygenation in situations of cardiogenic shock combined with severe pulmonary oedema, and rapid and easy implantation at the patient’s bedside at a low cost11,13.
International guidelines do not recommend the routine use of IABP for cardiogenic shock due to MI after the IABP-SHOCK II randomised trial1, despite previous reports of improved haemodynamic status14,15. Comparable to our study, almost half of the patients in the IABP-SHOCK II trial experienced cardiac arrest. A recent meta-analysis16 reported better survival when IABP was associated with ECLS5,16. The preventive effect of IABP on left ventricle overload caused by the arterial cannula of ECLS17 has been further characterised by a decreased hydrostatic pulmonary oedema and more days off mechanical ventilation18. As opposed to previous reports, we observed a reduction in mortality and a higher rate of recovery when IABP was added to ECLS19, a finding supportive of the systematic use of IABP in these young patients presenting with RCS-MI and in whom ECLS is placed.
Time delay from cardiac arrest to ECLS has been reported to be a major factor for worse outcomes20,21 because late haemodynamic restoration could favour systemic inflammatory response syndrome and multi-organ dysfunction syndrome. We did not observe any significant association between the time delay from coronary angiography to ECLS and mortality. Mortality was high in our study (63%) but comparable to previously published data22,23, probably reflecting the severity of the condition of the patients included in this real-life cohort.
Limitations
This work has major limitations. First, this is a retrospective observational study with all the known methodological flaws; however, literature on these patients treated with ECLS and revascularisation is scarce. Confounders were handled by adjusting the results in the multivariate logistic regression analysis but residual unknown confounders cannot be ruled out. Importantly, when considering the relationship between ECLS and mortality, one should keep in mind the great patient heterogeneity in terms of RCS severity and of time delay from ECLS to coronary angiography. While all patients underwent ECLS and/or IABP, it was sometimes needed at initial presentation while delayed in others due to worsening of haemodynamic conditions. Finally, some patients developed RCS after coronary angiography while in the intensive care unit and then received ECLS. Unfortunately, the limited sample size does not allow any robust conclusion with respect to clinical outcome according to patient flow. Time delay from ECLS to coronary angiography was not significantly associated with mortality in the multivariable Cox regression analysis. Whether statistical models taking into account all possible alternatives of mechanical circulatory support devices, time delay of implantation and severity of patients’ conditions are relevant remains to be established. The reported mortality reduction of combined IABP with ECLS, although supported by physiological arguments, should be further evaluated in a prospective randomised trial to address this question. A diagnostic coronary angiogram is recommended in these patients when haemodynamic stabilisation has been obtained, and PCI should be performed if indicated to improve myocardial salvage or recovery. Patients presenting with RCS should receive ECLS as soon as possible, aiming at restoring haemodynamic stability, together with IABP to prevent LV overload and pulmonary oedema.
Conclusions
Although there is a strong association between the presence of left main coronary artery disease and mortality in patients with refractory cardiogenic shock due to myocardial infarction, the use of percutaneous coronary revascularisation is not associated with survival improvement in this young population requiring ECLS. Means aiming at restoring haemodynamic stability should be prioritised.
| Impact on daily practice Urgent percutaneous coronary intervention is not associated with better survival in patients with RCS-MI in need of haemodynamic mechanical support. The use of intra-aortic balloon pump is associated with better survival as a consequence of a more effective haemodynamic support with avoidance of left ventricle overload. The degree of organ failure is a strong determinant of survival. Means aiming at restoring haemodynamic stability should be prioritised. It is likely that early mechanical circulatory support has a better chance of improving outcome than PCI but this warrants further investigation. Whether coronary revascularisation improves myocardial recovery when successful in survivors warrants further investigation. |
Conflict of interest statement
G. Montalescot reports receiving research grants to the institution or consulting/lecture fees from ADIR, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, BMS, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Cardiovascular Research Foundation, Celladon, CME Resources, Daiichi Sankyo, Eli Lilly, Europa, Elsevier, Fondazione Anna Maria Sechi per il Cuore, Gilead, ICAN, Janssen, Lead-Up, Menarini, Medtronic, MSD, Pfizer, Sanofi-Aventis, The Medicines Company, TIMI Study Group, and WebMD. J-P. Collet reports receiving research grants from BMS, and consulting/lecture fees from AstraZeneca, BMS, and Sanofi-Aventis. J. Silvain reports receiving research grants to the institution from the Fondation de France, consulting/lecture fees from Actelion, Amed, AstraZeneca, Bayer, Daiichi Sankyo, Eli Lilly, Gilead Science, Sanofi-Aventis, Amgen, Algorythm, and Iroko Cardio, and travel support from Amgen, AstraZeneca and St. Jude Medical. M. Kerneis reports receiving research grants from Sanofi, Servier, and Fédération Française de Cardiologie, and lecture fees from AstraZeneca and Bayer. A. Combes is the primary investigator of the EOLIA trial (NCT01470703), a randomised trial of VV-ECMO supported by Maquet, and has received honoraria for lectures from Maquet. J-S. Hulot reports receiving research grants to the institution from ANR, Fédération Française de Cardiologie, France Génomique, Fondation Simon et Cino Del Duca, Leducq Foundation, and Sanofi, and consulting/lecture fees from Novartis. G. Lebreton and P. Leprince report receiving lecture fees from HeartWare, Thoratec, Maquet, LivaNova (Sorin), Xenios (Medos), and Medtronic, and proctoring fees from HeartWare. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Characteristics according to the timing of ECLS placement.
To read the full content of this article, please download the PDF.

