
Beginning with the advent of the “heart-lung machine” in the 1950s followed by the development of membrane oxygenators in the 1960s, the field of extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS) has evolved exponentially1. In the 1970s, pioneering work by Hill and Bartlett introduced the use of ECMO as a method to support respiratory failure in both children and adults2,3. In the late 1980s, the Extracorporeal Life Support Organization (ELSO) was founded to advance the engineering, logistics, and clinical protocols associated with the use of ECMO and to develop a global registry to evaluate clinical outcomes. Based on over 50 years of experience, ECMO is now an integral part of any advanced critical care programme.
In contrast to respiratory failure, the use of ECMO to support cardiogenic shock due to predominant left ventricular (LV) failure is a more recent development with limited published experience and data. Over the past decade, the use of percutaneously delivered haemodynamic support devices in cardiogenic shock has grown to include intra-aortic balloon pumps (IABP), axial flow catheters (Impella®; Abiomed Inc., Danvers, MA, USA), left atrial-to-femoral artery bypass (TandemHeart®; Cardiac Assist Inc., Pittsburgh, PA, USA), and veno-arterial ECMO (VA-ECMO)4,5. The two primary goals of acute haemodynamic support systems in cardiogenic shock are: 1) to increase mean arterial pressure (MAP) and vital organ perfusion (circulatory support) and 2) to reduce LV pressure and volume, thereby reducing wall stress, stroke work, and myocardial oxygen consumption (ventricular support).
With the exclusion of IABP therapy, all of these support devices generate continuous blood flow by transferring kinetic energy from a circulating impeller to the blood stream. In contrast to axial flow catheters, both the TandemHeart and VA-ECMO employ inflow and outflow cannulas connected to an extracorporeal centrifugal pump that drains blood from the heart into the arterial system. A major difference between the TandemHeart and VA-ECMO systems for LV support is the location of the inflow cannula, which is placed in the left atrium via transseptal puncture for the TandemHeart system and across the right atrium for VA-ECMO. Since VA-ECMO displaces blood from a large venous reservoir into the arterial system without directly reducing LV preload, the LV is subjected to increased afterload and wall stress (Figure 1)6-8. As a result, VA-ECMO can effectively provide circulatory support (increased MAP), but does not unload the LV. Therefore, in the setting of cardiogenic shock due to LV failure, VA-ECMO solves one half of the haemodynamic support equation but often fails to provide both circulatory and ventricular support.
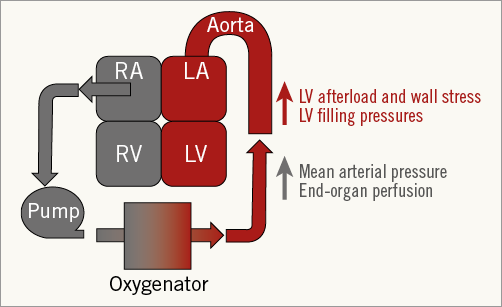
Figure 1. The haemodynamic effects of VA-ECMO.
In this issue of EuroIntervention, de Waha and colleagues conducted a large single-centre retrospective analysis of 83 patients with refractory cardiogenic shock treated with peripherally cannulated VA-ECMO9. Despite a multidisciplinary effort to identify good candidates for VA-ECMO, the authors report a dismal prognosis, with only 18.1% surviving to 18 months and 15.7% surviving with a good functional outcome. In-hospital mortality was also high at 68.7% due primarily to multi-organ failure. It was not possible to wean 43.4% of patients from VA-ECMO due to continued haemodynamic instability. Major complication rates were high, including life-threatening bleeding, thromboembolic events, infection, limb ischaemia, and renal failure. The authors identified age, prior ICD or CRT therapy, and symptomatic heart failure as independent predictors of long-term mortality. Among these predictors, patients aged >75 years had a significantly worse long-term outcome compared to younger patients. Notably, the severity of acute haemodynamic compromise based on circulating lactate levels or a measured cardiac index did not correlate with long-term survival.
The authors further identified that patients with ST-segment elevation myocardial infarction (STEMI) requiring VA-ECMO did not differ in terms of in-hospital or long-term mortality, bleeding rates, or rates of weaning from VA-ECMO when compared to patients receiving VA-ECMO for other reasons. This is an important finding since the clinical outcomes were poor despite successful revascularisation in the vast majority of STEMI patients.
Several aspects of this report are uniquely geared towards practising interventional cardiologists. First, this report focuses specifically on percutaneously delivered VA-ECMO using bifemoral cannulation and mandated use of an antegrade perfusion sheath to limit limb ischaemia. This approach represents the best technical practice currently available for vascular access. Whether central cannulation VA-ECMO using an open thoracotomy would have led to better outcomes than peripheral cannulation remains unknown. Second, none of the patients included in this analysis received any form of LV decompression. As described above, in the setting of impaired LV function, VA-ECMO can increase LV afterload and potentially worsen pulmonary congestion, myocardial ischaemia, and ventricular arrhythmias. Furthermore, no haemodynamic data to assess the effects of VA-ECMO are reported. Thus, we are limited in our ability to understand whether concomitant LV venting with an IABP, axial flow catheter, or left atrial catheter to reduce left heart filling pressures would have improved clinical outcomes10,11. Third, ECMO management requires close attention to cerebral perfusion and the possible need for veno-arterial-venous cannulation (VAV-ECMO) to improve supra-diaphragmatic oxygenation. VAV-ECMO was not reported in this series and may have impacted on both short- and long-term outcomes. Finally, data examining the utility of VA-ECMO during STEMI are limited. Several preclinical and clinical reports indicate that mechanical circulatory support in STEMI may reduce infarct size and clinical outcomes12-14. Based on this analysis, VA-ECMO does not appear to be a viable approach to support patients with STEMI and cardiogenic shock.
Despite system-wide advances in healthcare delivery and device technology, the optimal approach to cardiogenic shock remains an unsolved equation and persists as a major cause of morbidity and early mortality in contemporary clinical practice. The high complication rate combined with poor clinical outcomes in this report do not support widespread use of VA-ECMO in patients with cardiogenic shock. Furthermore, these data highlight the critical need for a scoring system to identify candidates for VA-ECMO that incorporates age and pre-existing comorbidities such as chronic renal insufficiency, valvular heart disease, ejection fraction, symptomatic heart failure, SAPS II score, and prior ICD/CRT therapy.
Conflict of interest statement
N. Kapur receives funding for research support from Maquet, Abiomed, St. Jude and Cardiac Assist and serves on the speakers bureau for Maquet and Abiomed. D. Zisa has no conflicts of interest to declare.

