Among patients with refractory cardiogenic shock (CS), venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides complete cardiocirculatory support; however, it is associated with high rates of vascular and bleeding complications and peripheral limb ischaemia1. Transcaval (TC) aortic access is a percutaneous technique to deliver large-bore catheter devices from a femoral vein into the abdominal aorta in patients with inadequate peripheral arterial access2. In the setting of CS, TC access may enable VA-ECMO in patients with diseased iliofemoral arteries that are unsuitable for accommodating arterial cannulas. To date, the use of TC access for VA-ECMO is limited to only single case reports and has not been previously investigated3. Here we report the in-hospital outcomes of the largest case series to date of TC-VA-ECMO.
A total of 22 consecutive patients who were in refractory CS but whose peripheral arterial access was unsuitable underwent VA-ECMO cannulation via TC access between 2018 and 2023 at a large tertiary shock centre. Our technique for TC-VA-ECMO without the use of preplanning contrast computed tomography (CT) has been previously described3. Left ventricular (LV) unloading, when indicated, was performed per operator discretion using a transaortic microaxial flow pump or transeptal left atrial venoarterial cannula placement (LAVA-ECMO), as previously described3. A schema of the various configurations of TC-VA-ECMO compared with a regular VA-ECMO is illustrated in Figure 1. Vascular and bleeding complications and their relatedness to TC access were reported according to a modified version of the Valve Academic Research Consortium (VARC)-2 definitions2. The study was approved by the Institutional Review Board of Henry Ford Health.
Baseline clinical, haemodynamic, and procedural characteristics of patients who underwent TC-VA-ECMO are reported in Table 1. The mean age was 60.9±16.0 years, and 50% were female. All patients were in CS stage D or E according to the Society for Cardiovascular Angiography and Interventions’ classification. All cases were performed without dedicated CT planning. In most of the patients, the aetiology of CS was valvular (40.9%), and 4 patients (18.2%) had a cardiac arrest at the time of presentation. LV unloading was performed in most of the cases (81.8%) using a LAVA-ECMO configuration.
In-hospital death occurred in 12 patients (54.6%), of which 4 deaths occurred from non-cardiovascular causes. TC access and VA-ECMO cannulation was successful in all patients. TC-related major vascular complications occurred in 4 patients (18.2%), all due to retroperitoneal haematomas associated with a haemoglobin drop ≥3 g/dL. There were 7 major or life-threatening VARC-2 bleeds (31.8%), of which 5 were related to VA-ECMO. There were no cases of acute lower limb ischaemia. ECMO decannulation was attempted in 15 patients, for all of whom TC closure was successful. For closure, an Amplatzer duct occluder (Abbott) was used in 10 patients, and a covered stent was used as the primary closure in 4 patients to achieve immediate closure of the cavoaortic shunt.
TC access has emerged as an alternative vascular access for large-bore sheaths but has been primarily investigated in the setting of transcatheter aortic valve replacement2. In this series, TC access for VA-ECMO appeared to be feasible and associated with acceptable rates of vascular complications. Rates of all-cause mortality and bleeding complications were in line with recent trials of VA-ECMO1. As TC obviates the need for peripheral arterial access, no cases of acute limb ischaemia were observed. Importantly, TC track access and closure was technically successful in all patients. In this series, LV unloading was mostly applied in a LAVA-ECMO configuration. A strategy of TC-LAVA-ECMO allows complete cardiocirculatory support with additional active LV unloading without the need for peripheral arterial access; conversely, a strategy of conventional VA-ECMO plus an additional LV venting device would require 2 large-bore arterial access sites, potentially further increasing the risk of bleeding and vascular complications. Compared with the recent ECLS-SHOCK trial, which reported similar rates of in-hospital death, our series mostly included patients with non-ischaemic CS of which valvular heart disease-Ârelated CS was the most common aetiology. Our strategy could be particularly attractive for these patients.
The limitations of the current report are its observational, retrospective design and the lack of an independent clinical event adjudication committee. In addition, our findings may lack external validity for centres that are less experienced in TC access. Finally, the rates of major or life-threatening bleeding, despite the use of TC access, remain high. Larger studies evaluating the use of an alternative access for mechanical circulatory support are necessary.
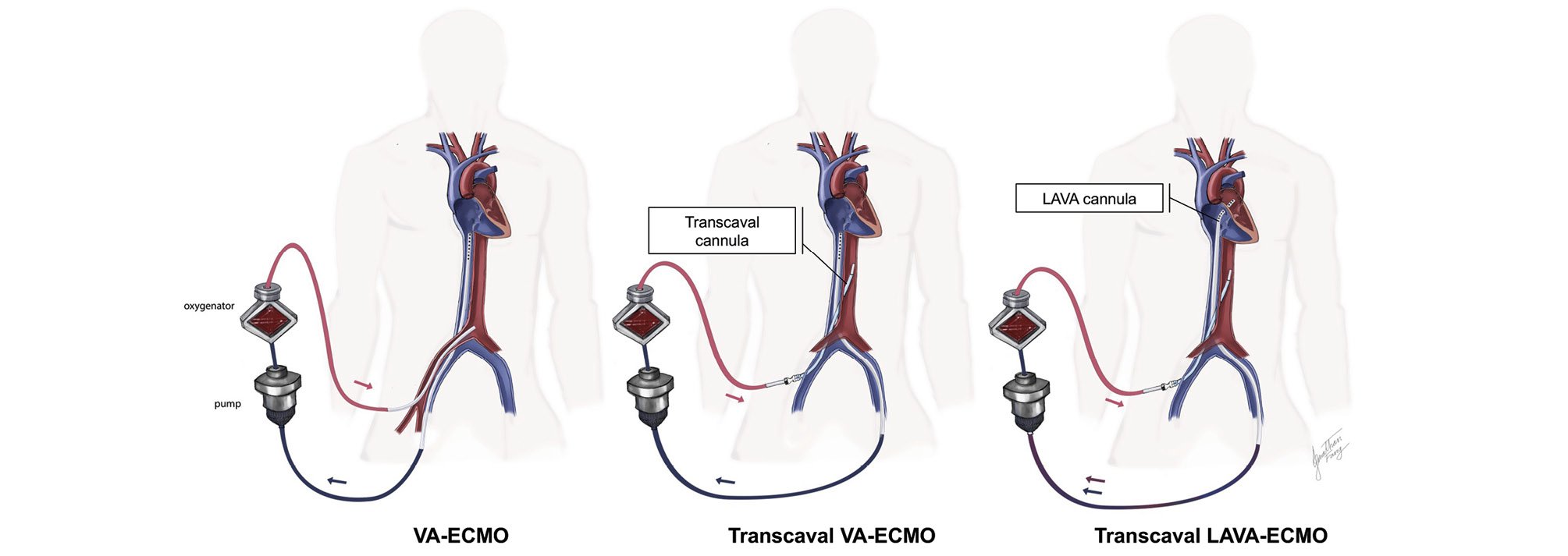
Figure 1. Configuration of TC-VA-ECMO and TC-LAVA-ECMO compared with conventional VA-ECMO. ECMO: extracorporeal membrane oxygenation; LAVA: left atrial venoarterial; TC: transcaval; VA: venoarterial
Table 1. Clinical characteristics, haemodynamics and outcomes of patients who underwent transcaval extracorporeal membrane oxygenation.
| TC-ECMO (N=22) | |
|---|---|
| Clinical characteristics | |
| Age, years | 60.9±16.0 |
| Female | 11 (50) |
| Body mass index, kg/m² | 30.7±8.7 |
| Cardiogenic shock aetiology | |
| Ischaemic | 7 (31.8) |
| Valvular | 9 (40.9) |
| Myocarditis | 1 (4.6) |
| Ventricular tachycardia storm | 1 (4.6) |
| Mixed | 4 (18.2) |
| Cardiac arrest at presentation | 4 (18.2) |
| Lactate at presentation | 5.6 [2.7-8.8] |
| Procedural characteristics | |
| Inflow venous cannula site | |
| Right femoral vein | 2 (9.1) |
| Left femoral vein | 20 (90.9) |
| Inflow venous cannula size, Fr | 22.9±1.1 |
| Outflow arterial cannula site | |
| Right femoral vein | 21 (95.5) |
| Left femoral vein | 1 (4.6) |
| Outflow arterial cannula size, Fr | 18.3±1.6 |
| Left ventricular unloading | |
| None | 4 (18.2) |
| Transseptal left atrial cannula | 18 (81.8) |
| Impella | 1 (4.6) |
| Anaesthesia technique | |
| General anaesthesia with mechanical ventilation | 13 (59.1) |
| Conscious sedation | 9 (40.9) |
| Haemodynamic characteristics | |
| Preimplant haemodynamics | |
| Right atrial mean pressure, mmHg | 12 [10-20] |
| Right ventricular systolic pressure, mmHg | 49 [35-62] |
| Pulmonary artery mean pressure, mmHg | 34 [23-40] |
| Pulmonary capillary wedge pressure, mmHg | 28 [18-32] |
| Cardiac index | 1.9 [1.4-2.2] |
| Mixed venous saturation, % | 57 [53-62] |
| Clinical outcomes | |
| In-hospital all-cause death | 12 (54.6) |
| Cardiovascular death | 8 (66.7) |
| Non-cardiovascular death | 4 (33.3) |
| 30-day all-cause death | 13 (59) |
| TC-related VARC-2 major vascular complication* | 4 (18.2) |
| Major or life-threatening VARC-2 bleeding | 7 (31.8) |
| ECMO-related | 5 (22.7) |
| Non-ECMO-related | 2 (9.1) |
| Retroperitoneal haemorrhage | 4 (18.2) |
| Acute limb ischaemia | 0 (0) |
| Acute kidney injury requiring renal replacement therapy | 8 (36.4) |
| Attempted ECMO decannulation | 15 (68.2) |
| Successful decannulation | 15 |
| Successful transcaval closure | 15 |
| Closed with Amplatzer duct occluder device | 10 |
| Closed with aortic covered stent | 4 |
| Exchanged with an Impella 5.0 | 1 |
| Procedures during the same hospitalisation | |
| Heart transplant | 3 |
| Transcatheter aortic valve replacement | 6 |
| Transcatheter mitral valve replacement or repair | 1 |
| Percutaneous coronary intervention | 5 |
| Coronary artery bypass grafting | 1 |
| Ventricular septal defect closure | 1 |
| Categorical variables are reported as n (%). Continuous variables are reported as mean±standard deviation or median [interquartile range]. *Defined according to a modified VARC-2 classification for transcaval vascular complications which includes the presence of a large retroperitoneal haematoma, or a small or moderate retroperitoneal haematoma associated with a haemoglobin drop ≥3 g/dL or requiring blood transfusion, any aortic dissection requiring intervention, any TC-related vascular injury requiring intervention (except aortocaval fistula), distal embolisation requiring intervention, early or delayed endograft therapy for urgent or persistent bleeding, or any new ipsilateral lower extremity ischaemia {2}. ECMO: extracorporeal membrane oxygenation; TC: transcaval; VARC: Valve Academic Research Consortium | |
Conflict of interest statement
P.A. Villablanca is a consultant for Edwards Lifesciences and Teleflex. D.D. Wang is a consultant for Abbott, Boston Scientific, and Edwards Lifesciences; and receives research grant support from Boston Scientific, assigned to her employer Henry Ford Health. B.P. O’Neill is a consultant to Abbott, Edwards Lifesciences, and Medtronic; and receives research support from Edwards Lifesciences, assigned to his employer Henry Ford Health. T. Frisoli is a clinical proctor for Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic. W.W. O’Neill is a consultant to Abiomed, Medtronic, and Boston Scientific. The other authors have no conflicts of interest to declare.

