
Abstract
Aims: Our aim was to describe our protocol for emergency percutaneous implantation of femoral veno-arterial extracorporeal membrane oxygenation (VA ECMO) in the catheterisation laboratory and to compare its effectiveness and safety with implantation in the intensive care unit and the operating room.
Methods and results: Our retrospective observational study enrolled 56 consecutive patients undergoing VA ECMO implantation in the catheterisation laboratory (n=23), the intensive care unit (n=8) and the operating room (n=25). Among patients undergoing catheterisation laboratory implantation, 11 patients had profound cardiogenic shock but preserved arterial pulsations, and 12 patients had refractory cardiac arrest undergoing automated mechanical chest compression. Using our fluoroscopy-guided protocol, arterial and venous cannulas were successfully implanted and the desired ECMO flow obtained in each patient. There was no vessel perforation/dissection. Moderate/severe GUSTO or BARC 3 and 5 bleeding occurred in 13%. Ipsilateral limb ischaemia occurred in one of eight patients (13%) with upfront perfusion sheath implantation, and in two of three patients (75%) in whom this strategy was not used (p=0.15). There was no infection at the site of cannula implantation. Complications related to implantation in the catheterisation laboratory were comparable to surgical implantation in the operating room and percutaneous implantation in the intensive care unit using ultrasound guidance.
Conclusions: Fluoroscopy-guided emergency implantation of femoral VA ECMO by an interventional cardiologist in the catheterisation laboratory is effective and safe for both patients in cardiogenic shock and those in refractory cardiac arrest.
Introduction
Because of improved emergency prehospital care, there is a growing number of patients with severe haemodynamic collapse surviving to hospital admission who are candidates for immediate circulatory support. Veno-arterial extracorporeal membrane oxygenation (VA ECMO), which appeared as an attractive option for complete heart and lung replacement, is therefore increasingly utilised in patients with profound cardiogenic shock1,2, massive pulmonary thromboembolism3,4 and refractory cardiac arrest5. Due to significant developments in ECMO technology, classic surgical implantation by cutdown and vessel exposure is gradually being replaced by ultrasound-guided percutaneous techniques6,7 performed either in the prehospital setting, the emergency department8 or the intensive care unit9.
Because mature “STEMI networks” are nowadays treating an increasing number of patients with out-of-hospital cardiac arrest and profound cardiogenic shock of diverse aetiologies, many candidates for emergency VA ECMO implantation are transported directly to the catheterisation laboratory (cathlab). Since fluoroscopy allows superior guidance for cannula placement, the cathlab may represent an appropriate environment also for emergency VA ECMO implantation10. Herein, we describe the effectiveness and safety of our protocol for emergency VA ECMO implantation in the cathlab both in patients with profound cardiogenic shock but preserved arterial pulsations, and in patients with refractory cardiac arrest undergoing automated mechanical chest compression. Subsequently we compare the safety of cathlab implantation with implantation in the cardiac intensive care unit and in the operating room.
Patients and methods
This was a retrospective observational study which enrolled consecutive patients with profound cardiogenic shock and refractory cardiac arrest undergoing emergency VA ECMO implantation in either the cathlab, the intensive care unit or the operating room. The decision for VA ECMO was at the discretion of the cardiac intensive care physician and interventional cardiologist. VA ECMO in cardiogenic shock was used in patients with potentially reversible cardiac dysfunction as well as in patients with a remote likelihood of recovery of cardiac function if they were candidates for heart transplantation or a long-term ventricular assist device. Patients with refractory cardiac arrest were candidates for VA ECMO if collapse was witnessed, if lay bystander basic life support was immediately initiated, if delay to advanced life support was <5 minutes, and if the predicted interval between the start of advanced life support and VA ECMO support was <60 minutes. Contraindications were significant pre-arrest comorbidities and advanced age (>65 years). Implantation in the cathlab was considered if the patient was admitted directly to the cathlab, if the index event occurred in the cathlab, and in other patients if the cathlab was immediately available. Otherwise, percutaneous implantation in the intensive care unit using ultrasound guidance was performed7. The decision for surgical implantation in the operating room was considered in patients with significant peripheral artery disease in whom femoral or axillary chimney graft was used11-13. Surgical implantation was not considered in patients with refractory cardiac arrest. VA ECMO implantation was not performed in the prehospital setting or in the emergency department.
In patients with cardiogenic shock and preserved arterial pulsations, puncture of the common femoral artery was performed and a 6 Fr sheath inserted. Infrarenal angiography using a 6 Fr pigtail catheter was performed to assess the iliofemoral arteries. If severe obstructive disease or inadequate arterial diameter according to the planned cannula size was documented, surgical implantation was considered. The decision for upfront implantation of a distal perfusion sheath to prevent limb ischaemia6 was initially left to the operator. Subsequently, due to limb ischaemia in the first two out of three patients, upfront implantation became mandatory. In brief, after ipsilateral anterograde puncture of the superficial femoral artery facilitated by retrograde angiography using an already implanted femoral sheath, a J-tipped wire was advanced and an 8 Fr, 11 cm flexible metal sheath (Arrow®; Teleflex Medical Europe Ltd., Athlone, Ireland) was implanted. Adequate sheath position was confirmed by anterograde (Figure 1A) or retrograde (Figure 1B) angiography. A front wall puncture of the ipsilateral or contralateral femoral vein was then performed, a 6 Fr sheath was inserted and a 180 cm Amplatz Extra Stiff Wire (Cook Medical, Bloomington, IN, USA) was advanced to the superior vena cava. After progressive tapered dilatation using a standard percutaneous insertion kit (Maquet Getinge Group, Rastatt, Germany), a 21-25 Fr, 55 cm multiple-stage drainage venous HLS cannula (Maquet Getinge Group) was advanced till the tip reached the inferior part of the right atrium (Figure 2A). The wire and introducer were removed, backflow of venous blood allowed and the cannula clamped. A bolus of 5,000 IU of unfractionated heparin was administered. The same Amplatz wire was then advanced through a retrograde femoral arterial sheath into the descending aorta, progressive tapered dilatation performed and a 17-23 Fr, 23 cm arterial HLS single-stage cannula (Maquet Getinge Group) was implanted. The tip was advanced to the aortic bifurcation (Figure 2B). The introducer and the wire were removed, arterial backflow allowed and the cannula clamped. The side port of the distal perfusion sheath was then connected to the side port of the arterial cannula. After removal of air using a 50 ml syringe with normal saline, arterial and venous cannulas were sequentially attached to the ECMO console (CARDIOHELP; Maquet Getinge Group), primed during cannula implantation (Figure 3). The cannulas were unclamped, and ECMO flow initiated and gradually increased until the desired levels were reached. After fluoroscopic confirmation of adequate position, both cannulas were sutured to assure a stable position. If the patient had an intra-aortic balloon pump (IABP) before ECMO implantation, IABP was maintained if the contralateral iliofemoral artery was adequate for arterial cannula implantation (Figure 3).
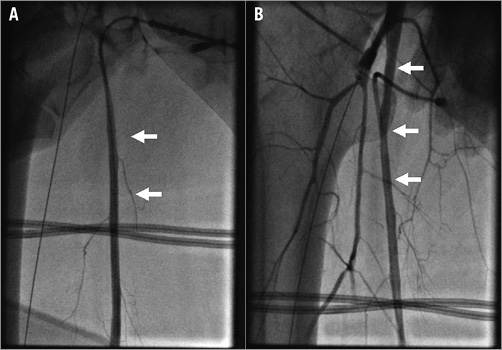
Figure 1. Confirmation of distal perfusion sheath position. A) Anterograde angiography. B) Retrograde angiography. White arrows indicate superficial femoral artery.
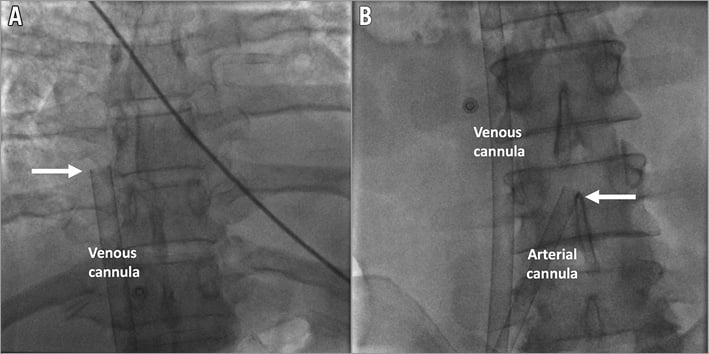
Figure 2. Fluoroscopic confirmation of venous cannula position with the tip reaching the inferior part of the right atrium (A) and the arterial cannula with the tip at the aortic bifurcation (B). White arrows indicate the tips of the cannulas.
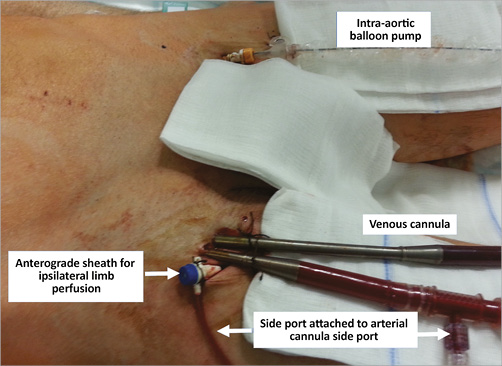
Figure 3. Right groin with anterograde sheath for ipsilateral limb perfusion attached to the arterial cannula via the side port. Venous cannula and concomitant intra-aortic balloon pump in the left groin maintained after ECMO implantation.
In patients with refractory cardiac arrest without spontaneous arterial pulsation, ongoing automated chest compression was not interrupted (Figure 4A). A femoral puncture was made blindly and, when blood flow was obtained, a J-tipped wire was advanced and its position documented by fluoroscopy according to the spine. Vein puncture was recognised if the wire passed left of the spine towards the right atrium and superior vena cava and arterial puncture if the wire passed right of the spine into the descending aorta. After the first wire was advanced to either the venous or arterial site, a 6 Fr sheath was inserted to secure the access. An ipsilateral groin puncture, which was more lateral in case of initial vein puncture or more medial in case of initial arterial puncture, was then performed, a second J-tipped wire advanced and a 6 Fr sheath inserted if the wire had an adequate fluoroscopic course according to the first wire and the spine (Figure 4B). After arterial and venous accesses were secured, cannulas were implanted and connected to the ECMO console as previously described. When ECMO flow was started, mechanical compression was immediately stopped. Because of the higher priority of re-establishing ECMO flow, no upfront implantation of distal limb perfusion was performed. This was carried out subsequently if limb ischaemia evolved using either percutaneous or surgical techniques.
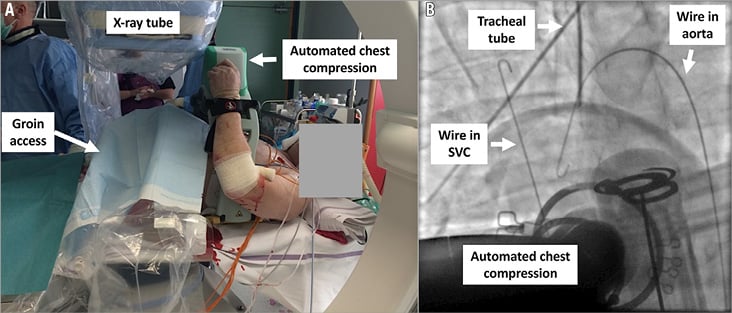
Figure 4. Implantation of VA ECMO in a patient with refractory cardiac arrest and ongoing automated mechanical chest compression using fluoroscopy (A). Fluoroscopic confirmation of adequate venous and arterial wire positions before implantation of cannulas (B).
After VA ECMO implantation in the cathlab, coronary angiography/PCI was performed if a coronary cause was suspected. Pulmonary angiography or contrast computed tomography (CT) followed by thrombolysis or thrombectomy was performed in patients with suspected massive pulmonary thromboembolism. In patients with refractory cardiac arrest, systematic CT of the head, thorax and abdomen was performed to document possible brain oedema/intracranial bleeding or injuries related to prolonged mechanical chest compression. Patients were then admitted to the cardiac intensive care unit and treated according to the current principles of intensive care. VA ECMO was removed in the operating room by a cardiovascular surgeon.
The primary endpoint was successful cannula implantation in the cathlab with establishment of the desired ECMO flow. Safety endpoints were related to ECMO implantation and included vessel rupture/dissection, development of limb ischaemia, significant bleeding at the cannula site (defined as GUSTO moderate/severe14 and BARC 3 and 515) and infection at the cannula site. These safety endpoints related to ECMO implantation in the cathlab were subsequently compared to percutaneous implantation in the intensive care unit and surgical implantation in the operating room.
STATISTICAL ANALYSIS
Numerical variables are expressed as mean±standard deviation and continuous variables as proportions. For comparison of numerical variables related to VA ECMO implantation in the cathlab, cardiac intensive care unit and the operating room, ANOVA was used. For comparison of categorical variables, Fisher’s exact test was used. A p-value of <0.05 was considered as significant.
Results
From May 2010 to July 2015, a total of 56 consecutive patients underwent VA ECMO implantation. Implantation was performed in either the cathlab, the cardiac intensive care unit or the operating room (Figure 5). Among 23 patients undergoing implantation in the cathlab, there were 11 patients with cardiogenic shock and 12 patients with refractory cardiac arrest and ongoing chest compression. Arterial and venous cannulas were successfully implanted and the desired ECMO flow obtained in each patient. None of the patients was referred for surgical implantation.
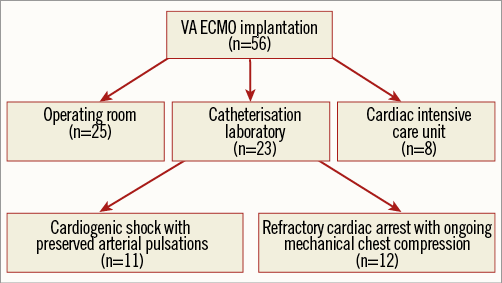
Figure 5. Flow chart showing consecutive patients undergoing emergency VA ECMO implantation at the University Medical Center Ljubljana (Slovenia) between May 2010 and July 2015.
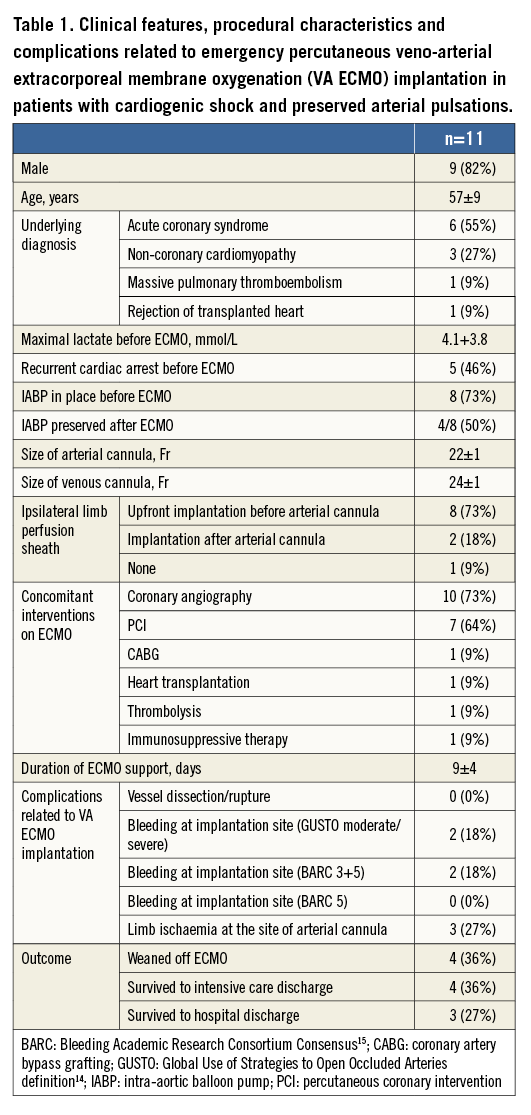
The majority of patients with cardiogenic shock were already on IABP (73%), which was maintained after ECMO implantation in half of the patients (Table 1). A distal limb perfusion sheath was implanted before arterial cannula implantation in 73% of patients and after arterial cannula implantation in 18% of patients. Concomitant interventions on ECMO included coronary angiography, PCI, CABG, thrombolysis and heart transplantation. The average duration of ECMO support was nine days. Complications related to ECMO implantation were GUSTO moderate or severe/BARC 3 and 5 bleeding at the site of the arterial cannula in 18% of patients and ipsilateral limb ischaemia in 27% of patients. Of note, limb ischaemia evolved in only one of eight patients (13%), with upfront perfusion sheath implantation, and in two of three patients (75%) in whom this strategy was not used (p=0.15). There was no infection at the site of the cannula implantation. Three patients (27%) survived to hospital discharge. One patient, who underwent cardiac transplantation while still on VA ECMO, completely recovered. The remaining two patients are currently on optimal medical treatment and enrolled in the advanced heart failure programme.
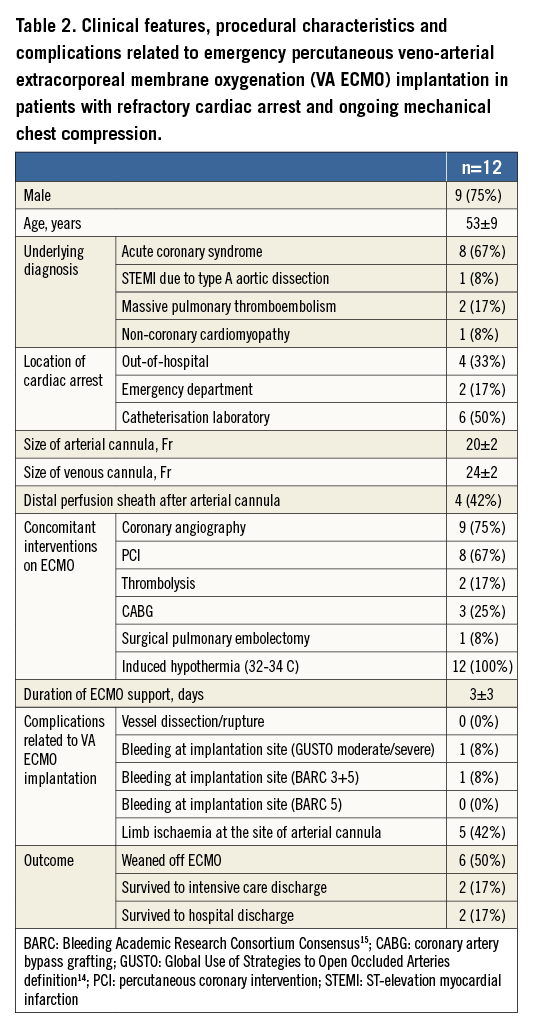
In 12 patients with refractory cardiac arrest, the location of the event was either out-of-hospital, in the emergency department or in the cathlab (Table 2). Four patients received distal perfusion after implantation of an arterial cannula because of the development of limb ischaemia. Coronary angiography and revascularisation were performed in the majority of patients, and two patients received thrombolysis due to massive pulmonary embolism. The average duration of ECMO support was three days. The most frequent complication related to ECMO implantation was ipsilateral limb ischaemia (42%). There was no infection at the site of cannula implantation. Two patients (18%) undergoing CABG and surgical pulmonary embolectomy while on ECMO survived to hospital discharge with a good neurologic condition and recovered completely. Because of brain death, one patient became an organ donor.
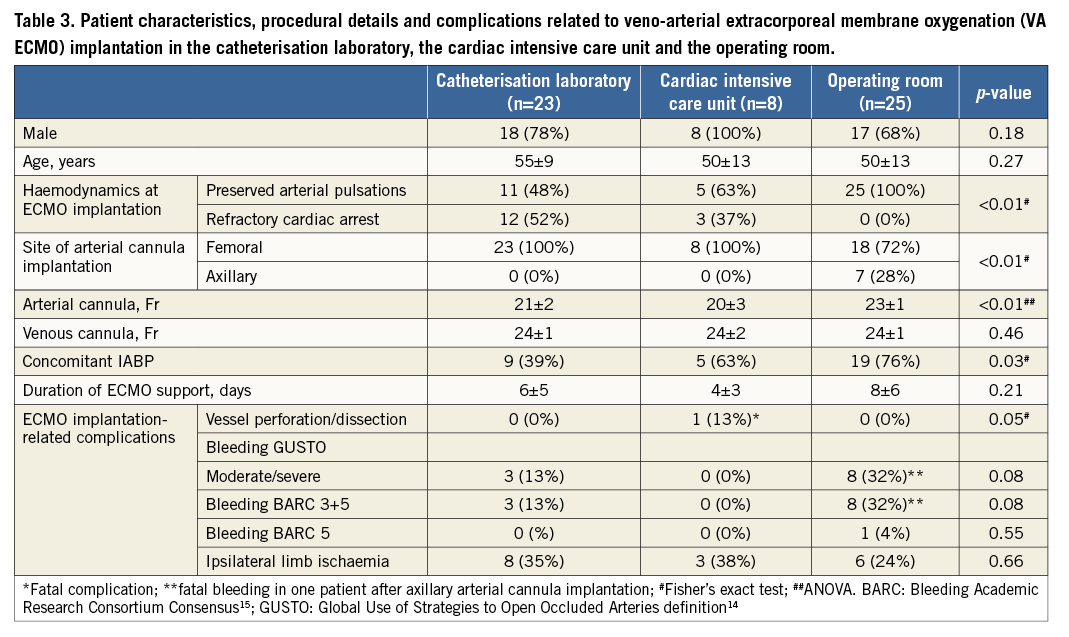
Patients with VA ECMO implantation in the cathlab, in the cardiac intensive care unit and in the operating room were comparable in terms of sex and age (Table 3). Refractory cardiac arrest as an indication for VA ECMO occurred more often in the cathlab group (p<0.01). While femoral artery access was used in all patients in the cathlab and cardiac intensive care unit, the axillary artery was used in 28% of patients in the operating room (p<0.01). Patients in the operating room received larger arterial cannulas, while the size of the venous cannula was the same. Concomitant IABP was used more often in the operating room. The duration of ECMO support ranged from four to eight days and was comparable among the groups. Fatal dissection of the iliofemoral artery and the descending aorta occurred in one patient in the intensive care unit. No vascular complications were documented in the other groups. There was a trend towards more bleeding in patients with implantation in the operating room. BARC 3 bleeding was managed by temporary compression, additional haemostatic suture and temporary discontinuation of heparin. In a patient with BARC 5 bleeding after axillary chimney graft implantation in the operating room, bleeding was fatal before emergency surgical revision. There was no significant difference in the occurrence of ipsilateral limb ischaemia. There was no implantation-related infection with any of the methods.
Discussion
Our retrospective observational study demonstrated that fluoroscopy-guided emergency percutaneous implantation of VA ECMO in patients with profound cardiogenic shock and refractory cardiac arrest in the cathlab is feasible, effective and at least non-inferior in terms of implantation-related complications compared to implantation in the intensive care unit and the operating room. Moreover, since our patients in the cathlab were more often in refractory cardiac arrest, making vessel puncture more difficult, and, contrary to the intensive care and surgical groups, received periprocedural thrombolysis (13%) and antiplatelet/anticoagulation therapy due to PCI (65%), the rate of significant bleeding (13%) is quite acceptable and compares well with a recently published meta-analysis of 1,866 patients with bleeding ranging from 27% to 57%16. Of note, we lost one patient in the surgical group due to massive bleeding from the axillary artery chimney graft, while none of the patients was lost due to bleeding related to implantation in the cathlab. We also had an unfortunate case of fatal retrograde aortic dissection by arterial cannulation in the intensive care unit despite transoesophageal echocardiography guidance. This complication, which is reported in 1.2% to 2.2%16-18, never occurred in the cathlab. Accordingly, interventional cardiology skills in femoral vessel puncture, fluoroscopy-guided wire and cannula placement and systematic use of an extra-stiff Amplatz wire providing adequate support for large cannula implantation are the key safety measures. We believe that fluoroscopic guidance and confirmation of wire position are especially crucial in patients with refractory cardiac arrest and ongoing chest compression because there is usually no difference in blood brightness and pulsativity between the femoral artery and vein. We demonstrated that fluoroscopy can be performed simultaneously with ongoing automated chest compression. This should not be interrupted during ECMO implantation because any interruption decreases forward blood flow and worsens the potential for neurological recovery19. Another frequent complication related to percutaneous femoral VA ECMO implantation is limb ischaemia at the site of the arterial cannula due to compromised distal flow20. Ipsilateral limb ischaemia occurred in one third of our patients regardless of the implantation technique, which is more than the 13% to 22% reported in a previously described large meta-analysis16. The excess of limb ischaemia in our study is probably at least in part related to the large proportion of our patients undergoing implantation during refractory cardiac arrest with no time for upfront implantation of a distal perfusion sheath. Indeed, in our patients with cardiogenic shock, limb ischaemia evolved in only one of eight patients with upfront perfusion sheath implantation and in two of three patients in whom implantation was performed later or not at all. Accordingly, if possible, an ipsilateral distal perfusion sheath should be implanted before arterial cannula introduction to reduce the likelihood of limb ischaemia, also in the emergency setting. Upfront superficial femoral artery puncture is also easier because of preserved flow and pulsativity in the superficial femoral artery, which usually diminishes significantly or even disappears after arterial cannula implantation. For patients with refractory cardiac arrest, ECMO implantation should have priority, but limb ischaemia, if it evolves, should be promptly recognised and treated by surgical or percutaneous implantation of a distal perfusion sheath.
Despite successful puncture and cannula implantation in all our patients, ultrasound-guided intervention may also be very useful in the cathlab. This technique has the potential to reduce complications by evaluating vessel location and morphology prior to attempted puncture, and may serve to estimate appropriate cannula size according to vessel diameter. The main reason that ultrasound was used only in the intensive care unit and not in the cathlab is the lack of ultrasound knowledge and confidence in unassisted femoral puncture by interventional cardiologists. Because of the availability of fluoroscopy, we also did not use transoesophageal echocardiography, which is essential to guide venous wire and cannula positioning in the right atrium in the intensive care unit and operating room.
Despite the absence of major complications related to ECMO implantation in the cathlab, our survival to hospital discharge with good neurological outcome was only 22%. This is at the lower end of reported survival which ranges from 20.8% to 65.4%16. Part of the explanation for this may be that more than half of our patients were in refractory cardiac arrest and obviously at the highest risk with no survival without ECMO support. On the other hand, despite revascularisation, cardiac function remained poor in many patients who, in the absence of donor hearts, would need a ventricular assist device. For financial reasons, such devices are not readily available in our hospital, leading to extended ECMO treatment associated with increasing morbidity and mortality.
Conclusion
The catheterisation laboratory with a skilled interventional cardiologist is an appropriate environment for emergency VA ECMO implantation and represents a valid alternative to implantation in the intensive care unit or the operating room. Moreover, since many patients with severe haemodynamic collapse present with acute coronary syndromes and massive pulmonary embolism requiring immediate cause-related interventions, coronary angiography/PCI and catheter thrombectomy/aspiration may be immediately performed without the need for additional demanding transportation.
| Impact on daily practice Mature “STEMI networks” are nowadays treating increasing numbers of patients with acute coronary syndromes presenting with out-of-hospital cardiac arrest and profound cardiogenic shock who are candidates for immediate haemodynamic support with VA ECMO. The catheterisation laboratory with skilled interventional cardiologists is an appropriate environment for effective and safe emergency VA ECMO implantation. Moreover, since these patients require immediate cause-related interventions, coronary angiography/PCI and catheter thrombectomy/aspiration in case of massive pulmonary embolism may be performed immediately after haemodynamic stabilisation without the need for additional transportation. |
Acknowledgements
We would like to thank the Department of Cardiovascular Surgery, interventional cardiologists and nurses in the catheterisation laboratory for assistance and ongoing support of the project.
Funding
This research was funded by the University Medical Center Ljubljana (Tertiary Research Project Grant No. 20140127).
Conflict of interest statement
M. Noc has received speaker’s honorarium from the Maquet Getinge Group (Rastatt, Germany). The other authors have no conflicts of interest to declare.

