Abstract
There has been a significant increase in the use of short-term percutaneous ventricular assist devices (pVADs) as acute circulatory support in cardiogenic shock and to provide haemodynamic support during interventional procedures, including high-risk percutaneous coronary interventions. Although frequently considered together, pVADs differ in their haemodynamic effects, management, indications, insertion techniques, and monitoring requirements. This consensus document summarizes the views of an expert panel by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the Association for Acute Cardiovascular Care (ACVC) and appraises the value of short-term pVAD. It reviews the pathophysiological context and possible indications for pVAD in different clinical settings and provides guidance regarding the management of pVAD based on existing evidence and best current practice.
Preamble
This consensus document summarizes the views of an expert panel endorsed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the Association for Acute Cardiovascular Care (ACVC) and appraises the importance of short-term percutaneous ventricular assist device (pVAD). It reviews the pathophysiological context, initiation, and indications for pVAD in different clinical settings and provides guidance regarding the management of pVAD based on existing evidence and best current practice.
Introduction
There has been a significant increase in the implementation of short-term percutaneous ventricular assist device (pVAD) in recent years, aiming to improve outcomes in cardiogenic shock (CS) and high-risk percutaneous coronary intervention (HR-PCI). These devices aim to reduce cardiac stroke work and myocardial oxygen demand whilst maintaining systemic and coronary perfusion.1,2 Although frequently considered interchangeable, the indications, management and evidence supporting the use of various types of pVAD differ significantly.3 This Joint European Association of Percutaneous Cardiovascular Interventions (EAPCI)/Association for Acute Cardiovascular Care (ACVC) expert consensus document reviews the pathophysiological context and indications for pVAD in different clinical settings and provides guidance regarding the clinical management of patients requiring pVAD.
Pathophysiology of shock and haemodynamic response to pVADs
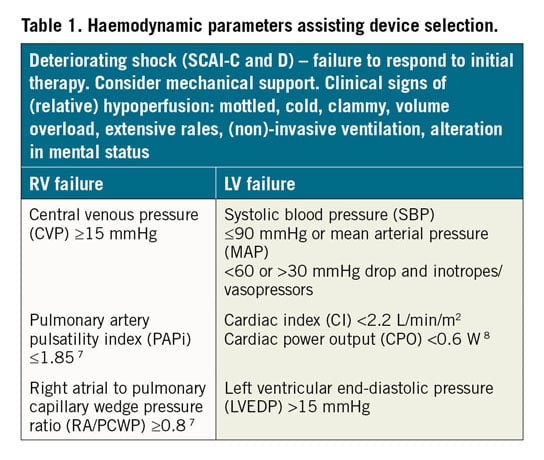
Understanding the pathophysiological background of haemodynamic changes during disease and in response to support is vital for selection and monitoring, troubleshooting, and assessment of pVAD performance. Different options for pVAD are currently available (see Figure 2 for their possible selection based on left and right ventricular (RV) support; Supplementary material online, Table S1 for comparison among different pVAD). The phenotype and severity of CS additionally dictate device selection, including RV and/or left ventricular (LV) support, with/without oxygenation.4 The position and shape of ventricular pressure-volume (PV) loops are preload and afterload-dependent5 with normal PV loops bound by the end-systolic PV relationship (ESPVR) and enddiastolic PV relationship (EDPVR) (Figure 1). The ESPVR is relatively linear, with the slope Ees (end-systolic elastance) and the volume-axis intercept (Vo), shifting with changes in contractility. The EDPVR is non-linear and defines the diastolic properties of the ventricle. Afterload can additionally be depicted on the PV plane by the ‘effective arterial elastance’ (Ea) line. The Ea line starts on the volume axis at the end-diastolic volume intersecting the ESPVR at the ventricular end-systolic PV point of the PV loop (Figure 1A).6 Based on pulmonary artery catheter measurements, numerous haemodynamic parameters can be measured, allowing calculation of cardiac index, systemic vascular resistance, pulmonary vascular resistance, and pulmonary artery pulsatility index, all of which may contribute to device selection. Several different haemodynamic variables are associated with worse outcome in RV dysfunction which may also assist in device selection (Table 1).
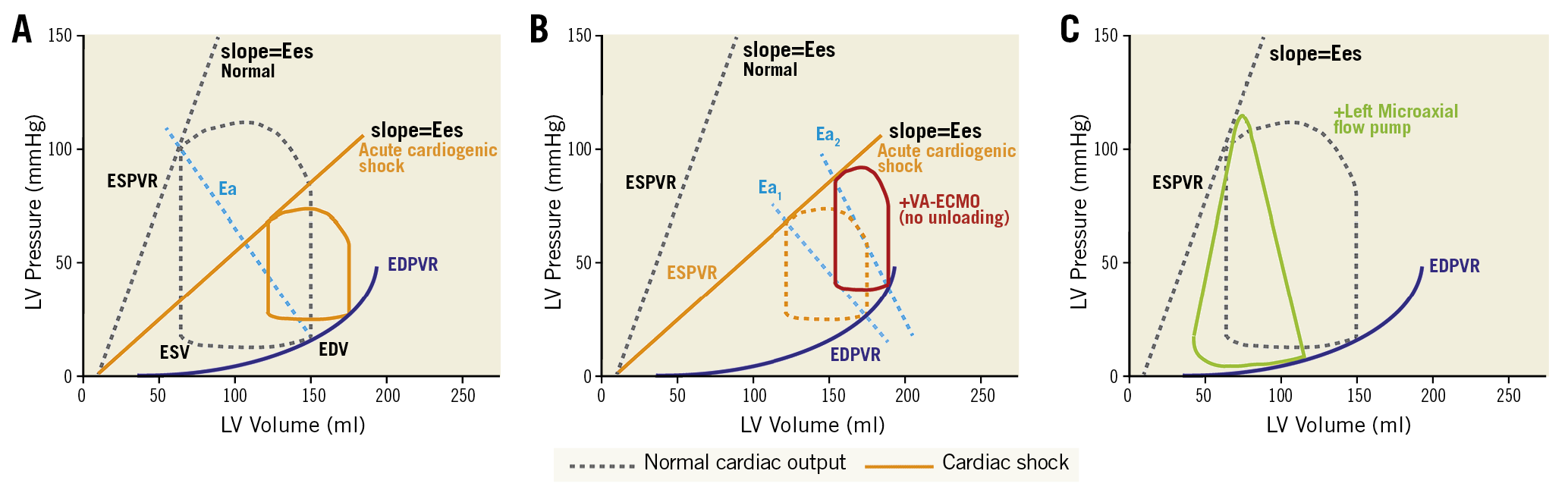
Figure 1. Pressure-volume loops. (A) Normal PV-loop and PV-loop in acute cardiogenic shock, the slope (Ees) shifts with changes in contractility. (B) PV-loop in VA-ECMO supported cardiogenic shock. PV-loop becomes narrower and is associated with an increase in EDPVR. (C) PV-loop in a left ventricular microaxial flow pump supported configuration, resulting in loss of normal isovolumetric periods, reduced EDPVR and conversion of the typical PV-loop to a triangular shape. Ea: arterial elastance; EDPVR: end-diastolic pressure-volume relationship; EDPVR: end-diastolic pressure-volume relationship; EDV: end-diastolic volume; Ees: end-systolic elastance; ESPVR: end-systolic pressure-volume relationship; ESV: end-systolic volume
Current models of left-sided pVAD comprise three different circuit configurations: right atrium to aorta (e.g. veno-arterial extracorporeal membrane oxygenation, VA-ECMO); left atrium to aorta (e.g. the TandemHeart, LivaNova London, UK); or left ventricle to aorta (Impella, Abiomed, Danvers, MA, USA; PulseCath iVAC2L, PulseCath BV, Amsterdam, The Netherlands; HeartMate PHPTM, St. Jude Medical/Abbott Vascular, St. Paul, MN, USA) (Figure 2).5,9 Peak flow rates range between 2.0 and 7.0 L/min, depending on the circuit and cannula(e) diameter(s). Devices may be used alone, in combination, and some allow/mandate concomitant use of an oxygenator within the circuit.
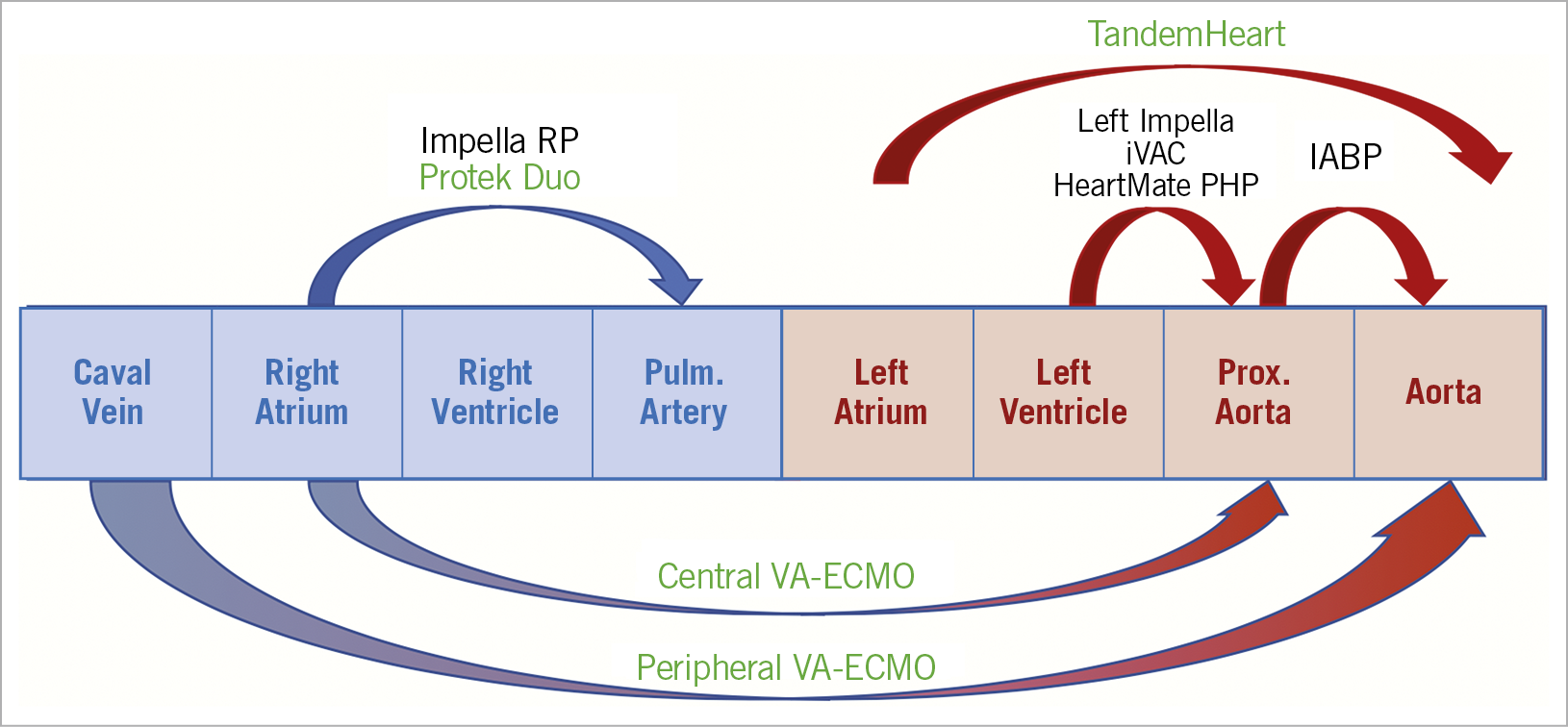
Figure 2. Different options for pVAD. Arrows indicate which part of the circulation is supported by the pVAD-modality. Devices in green can add blood oxygenation next to mechanical support. IABP: intra-aortic balloon pump; VA-ECMO: veno-arterial extracorporeal membrane oxygenation
The haemodynamic response to different pVADs is discussed in the Supplementary material online.
pVAD in high-risk PCI
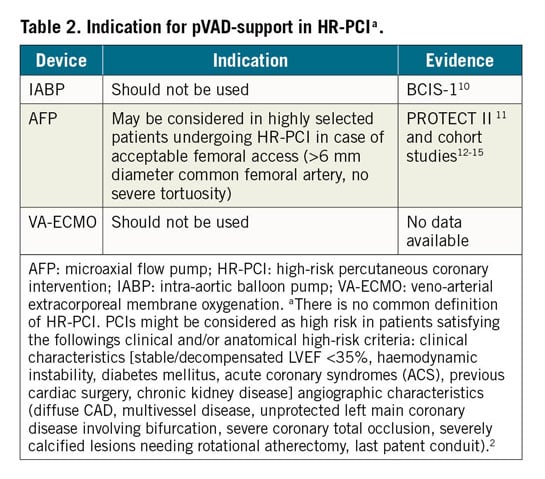
The rationale and indications (Table 2) for pVAD in high-risk percutaneous coronary intervention (PCI) are described in the Supplementary material online. The aims of pVAD in the setting of HR-PCI are to initiate haemodynamic support in very high-risk patients before the intervention, to prevent profound hypotension/low cardiac output (CO) episodes, and allow sufficient time to achieve optimal and complete revascularization (Table 2).1,16
pVAD in high-risk myocardial infarction without cardiogenic shock
The rationale and indications (Table 3) for pVAD in high-risk acute myocardial infarction (AMI) without CS are described in the Supplementary material online. In high-risk AMI, unloading of the left ventricle can be initiated prior to reperfusion in order to rapidly reduce wall tension and potentially reduce myocardial damage.19,20 No data from randomized trials or long-term outcomes of a pre-emptive unloading strategy are available, however, the DTU (Door to Unload) trial is currently enrolling patients (Table 3).
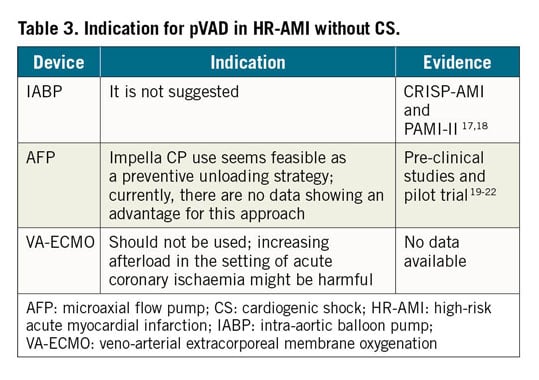
Left-sided pVAD in cardiogenic shock
The rationale and indications (Table 4) for pVAD in CS are described in the Supplementary material online. Left-sided pVADs primarily aim to restore CO in patients with CS or in case of refractory cardiac arrest. There are, however, no randomized clinical trials addressing optimal timing or selection of pVAD in CS. Outside the cardiac arrest setting, usual practice is to initiate pVAD in CS as soon as possible, and before the onset of multi-organ failure. In patients with CS complicating AMI, registry data (uncontrolled and with inherent selection bias) suggest higher survival rates with device placement before revascularization than after in patients with AMI and CS.27 These findings have been supported by preclinical data.20 Recent data in a larger cohort of CS patients have challenged the concept of pre-emptive device placement.34 Until high-quality data are available, decisions regarding the timing of pVAD initiation (as with every pMCS), are therefore based on the risk/benefit assessment, including severity of shock and the burden of comorbidity, as evaluated by the multidisciplinary shock team.
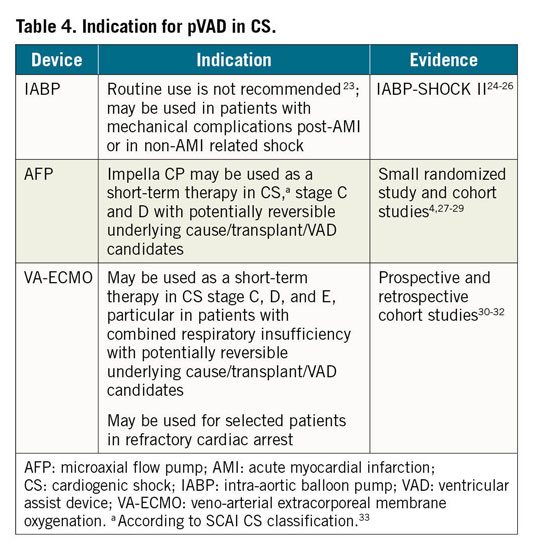
Right-sided pVAD in cardiogenic shock
The rationale and indications (Table 5) for RV-pVAD in CS are described in the Supplementary material online. No clinical trials exist that address the optimal timing of RV-pVAD placement in patients with acute RV failure. Furthermore, there are no parameters that have been demonstrated to predict RV failure or requirement for RV-pVAD after initiation of LV-pVAD. The decision to initiate RV-pVAD should be based on decisions made by the multidisciplinary shock team.
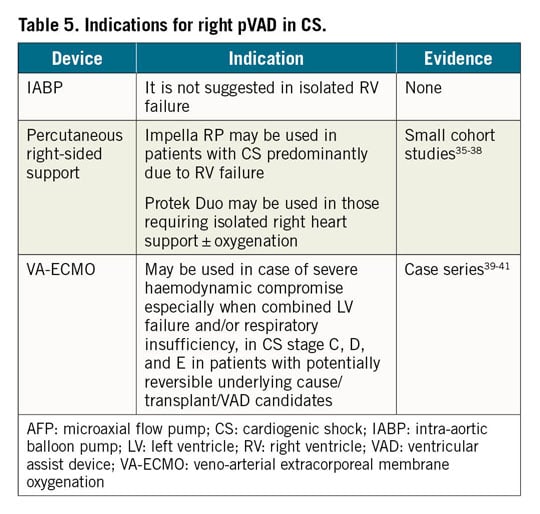
Biventricular pVAD in cardiogenic shock
The rationale and indications (Table 6) for biventricular pVAD in CS are described in the Supplementary material online. Acute, primary biventricular support (vs. delayed, secondary) should be implemented before the onset of multiorgan failure in selected patients as a strategy to buy time for recovery or bridge to other therapies.
Clinical monitoring and ongoing management of patients requiring pVAD
The monitoring of cardiac function and tissue perfusion is pivotal to optimize treatment and recognize potential complications of pVAD. This is described in Table 7 and the Supplementary material online.
Complications and their management
Complications associated with pVAD are potentially serious, life-threatening and may be related to the device itself, its insertion or from device-induced alteration of homeostasis or organ function, or anticoagulation (Figure 3). The most frequent complication is bleeding (related to vascular cannulation, full anticoagulation, or device-induced alteration in the coagulation pathways).50,51,52,53,54 Other complications include infection,55,56 haemolysis,57,58 limb ischaemia,59 device failure, and central nervous system haemorrhage or infarction.60,61 The incidence of heparin-induced thrombocytopenia is relatively low (0.36%, n=21/5797) in VA-ECMO and does not impact survival.62
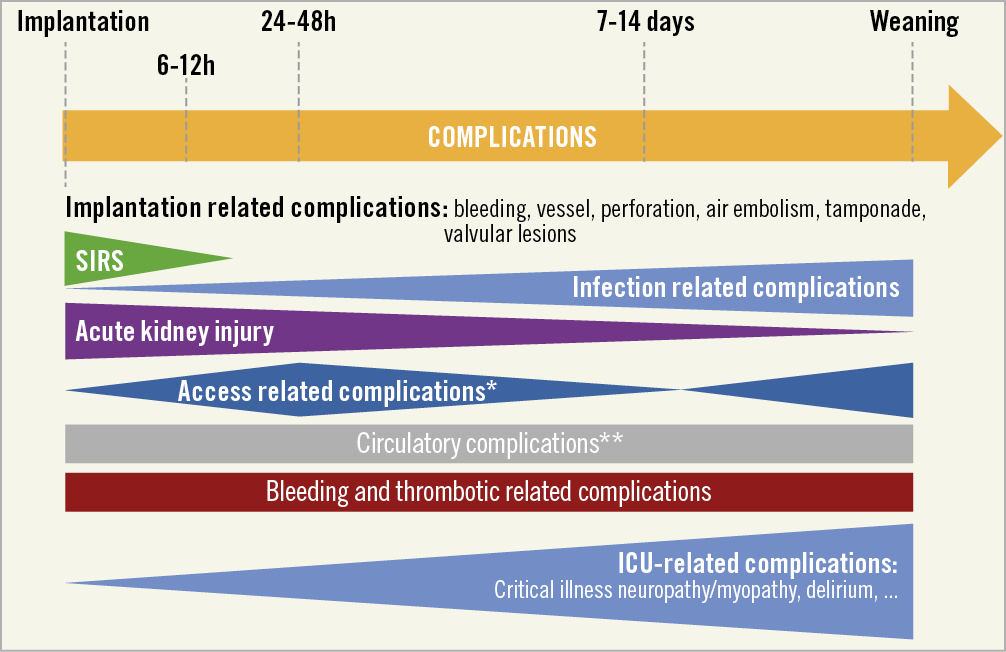
Figure 3. Complications associated with pVAD. Most frequent complications associated with pVADs depending on timepoint of implantation and weaning. *Indicates problems like bleeding, leg ischaemia, dissection or pseudoaneurysm; **Indicates problems as Harlequin-syndrome, cannula dislocation, afterload and/or preload mismatch. ICU: intensive care unit; SIRS: systemic inflammatory response syndrome
SPECIFIC, DEVICE-RELATED COMPLICATIONS AND THEIR MANAGEMENT
Impella devices are associated with the highest incidence of haemolysis among pVAD (5-10% in registry data).57,58 Accurate placement, and reduction of pump speed may decrease haemolysis and associated acute kidney injury. In a retrospective analysis of patients with AMI-related CS, the use of Impella was associated with more frequent bleeding (10.4% vs 1.7%, P<0.01), sepsis (38.2% vs. 17.4%, P<0.01) and peripheral vascular complications (9.6% vs. 3.5%, P=0.05) compared with matched patients from the IABP-SHOCK II trial supported with intra-aortic balloon pump (IABP).28 In a propensity-matched registry-based retrospective cohort study of patients with AMI complicated by CS, Impella was associated with more major bleeding (31.3% vs. 16.0%, P<0.001) compared with matched patients supported with IABP.34 In another retrospective analysis of 48306 patients, undergoing PCI with pVAD, when analysed by time-periods or at hospital/patient-level, Impella use was associated with: bleeding [odds ratio (OR)=1.10] and stroke (OR=1.34), although a similar, non-significant result was observed for acute kidney injury (OR=1.08).63
Specific complications of the TandemHeart include air embolism and cardiac perforation, tamponade, and atrial septal defect from transseptal cannulation.64 Drainage cannula displacement in the right atrium may cause massive shunting of deoxygenated blood to the arterial circulation.
VA-ECMO provides retrograde blood flow in the aorta and increases LV afterload and left ventricular end-diastolic pressure, that may induce pulmonary oedema and potentially myocardial ischaemia.65,66 Combining VA-ECMO and IABP (to unload the left ventricle) was associated with lower mortality in a meta-analysis based on observational data.45 The addition of an Impella to VA-ECMO decompresses the left ventricle may also improve outcome.45,67,68,69 Direct venting of left-cardiac chambers or percutaneous balloon atrial septostomy are other strategies used to offload the left ventricle in VA-ECMO. Since retrograde ECMO flow competes with the native heart ejection, in case of lung failure, deoxygenated blood may be directed to the upper part of the body resulting in heart and brain hypoxia. This situation is termed differential hypoxia, the North-South or Harlequin Syndrome.70,71,72 Veno-arterial-venous ECMO (which splits the reinfused blood by a Y-connector into an arterial and a venous cannula, additionally returning oxygenated blood to the right atrium) can provide circulatory and adequate pulmonary support in this setting.72 Veno-veno-arterial ECMO (VV-ECMO) configuration (completely offloading the right heart and reducing LV ejection) is another option. The incidence of major vascular complications with VA-ECMO can exceed 15% with significant impact on patient prognosis.73 Insertion of a distal perfusion cannula into the superficial femoral artery, positioned using contrast-enhanced Doppler ultrasound or invasive angiography, may prevent limb ischaemia.74 Ultrasound-guided percutaneous cannulation is the preferred option in VA-ECMO, and associated with less local infection (16.5% vs. 27.8%, P=0.001), similar rates of limb ischaemia (8.6% vs. 12.4%, P=0.3), sensory-motor complications (2.6% vs. 2.3%, P=0.8) and improved 30-day survival (63.8% vs. 56.3%, P=0.03) compared to surgical cannulation in a propensity-matched study including 532 patients receiving VA-ECMO.75
Antithrombotic pharmacology: anticoagulation and antiplatelet therapy
Up to 80% of patients on VA-ECMO suffer from major bleeding requiring transfusion and up to 16% develop intracranial haemorrhage.76 The precarious balance between bleeding and thrombotic complications, is a significant challenge and strongly influences pVAD-induced morbidity and mortality.51,77,78 A well-balanced antithrombotic strategy is mandatory.
Anticoagulation with unfractionated heparin (UFH) is the standard of care due to its short half-life, rapid on- and offset, low cost and ready availability.79 Other anticoagulation strategies (bivalirudin, argatroban) have been reported, especially in the context of heparin-induced thrombocytopenia.80,81 Due to their long half-life and renal excretion, the use of non-vitamin-K-oral-anticoagulants and low-molecular-weight heparins should be avoided.82 Monitoring UFH in patients on pVAD is challenging. Although activated clotting time (ACT)-guided monitoring is common it should be avoided due to its high variability, the non-specificity for heparin and the lack of widespread availability of ACT monitoring outside the catheterization lab.79,83 The activated-partial-thromboplastin-time (aPTT, most frequently used) and/or anti-Xa assays (the gold standard although not widely available) are preferred.84 In patients with sepsis, disseminated intravascular coagulation, liver failure or unexplained aPTT-prolongation, anti-Xa-testing should be used. The use of thromboelastography-guided UFH-monitoring has been evaluated, aiming to take platelet interactions and fibrinolysis into account, but further validation is pending.85 Antithrombin-monitoring should be considered when heparin-resistance is suspected.86
Randomized studies for specific heparin dose-regimens for anticoagulation are lacking. The Impella anticoagulation-guidelines favour therapeutic anticoagulation levels in all non-bleeding patients on pVAD.87 Nevertheless, a more individualized approach, well-balanced with the risk of bleeding, is suggested.88 Supplementary material online, Table S3 describes various devices with recommended antithrombotic strategies. pVAD-patients with underlying atrial fibrillation, mechanical valves or fresh (venous or arterial) thrombi should additionally receive therapeutic anticoagulation in the absence of major bleeding. No anticoagulation in (left-sided) pVAD-supported patients can be considered in major, life-threatening bleeding but the high risk of acute circuit failure and/or systemic embolization/thrombosis must be taken into consideration. An important number of pVAD-supported patients will have an additional indication for dual antiplatelet therapy because of PCI with stent implantation. Here, UFH should be combined with low dose aspirin plus clopidogrel (triple antithrombotic therapy) or with clopidogrel alone (dual antithrombotic therapy) depending on the individual bleeding risk of the patient. Prasugrel and ticagrelor are not recommended in a triple therapy strategy due to their increased bleeding hazards when compared with clopidogrel.89
In addition to determining the optimal UFH-dose, optimizing the platelet count and fibrinogen levels, and any bleeding source control is mandatory (surgical control, topic tranexamic, and/or adrenaline application in the cannula or mucosal bleeds or circuit change in case of consumption coagulopathy).
Although bleeding and thrombotic complications are the most frequent cause of morbidity and mortality in pVAD-supported patients, evidence from randomized clinical trials is scarce. Large, prospective multicentre trials are urgently needed to investigate the optimal anticoagulation management strategies during pVAD support.
Pharmacological support
Catecholamines are a standard part of the armamentarium of pVAD-supported patients although few data on safety and outcome are available to recommend inotrope/vasopressor selection and use.90 In CS, norepinephrine is the first-line vasopressor. Although vasopressin significantly increases mean arterial pressure (MAP), it has a lesser effect on cardiac index compared to norepinephrine.91,92 Its theoretical advantage at low dose (pulmonary vasoconstriction) deserves further investigation. MAP should be titrated according to the clinical scenario—maintaining organ perfusion pressure, whilst avoiding excessive increases in after-load. Following pVAD initiation, pressor support should be reduced to the lowest dose possible, and relative hypotension may/may not be tolerated, depending on other organ involvement (e.g. cerebral perfusion pressure in the context of post-cardiac arrest management).
Inotropic support may be required to enhance ventricular contractility but may alter ventricular loading and precipitate arrhythmia. In the case of univentricular pVAD, inotropy may be required to maintain adequate function of the non-supported ventricle. In CS, dobutamine is the inotrope of choice in patients93 as epinephrine has shown to be associated with a worse metabolic profile and patient outcomes.94,95,96
A randomized trial of norepinephrine versus epinephrine in patients with AMI-related CS demonstrated a higher incidence of lactateacidosis, tachycardia and refractory CS in the epinephrine-group, although many received concomitant dobutamine.96 In case of RV failure, phosphodiesterase-type-3 inhibitors (i.e. milrinone) may be preferred for their inodilators effects, despite lacking randomized trials.97 The long-acting calcium-sensitizer levosimendan (0.05-0.1 μg/kg/min) may be used given its inotropic and vasodilatory effect. However, hypotension and supraventricular arrhythmias may occur.98 Although levosimendan has shown beneficial haemodynamic effects during pVAD-weaning, further validation is needed.
Weaning from pVAD
The potential for weaning from pVAD should be evaluated daily from 24 to 48 h after the initiation of support. Several clinical features may predict the likely duration of pVAD support and likelihood of cardiac recovery including age, underlying pathology and presence/absence of pulmonary hypertension. Although the patient’s condition/pVAD complications may demand accelerated weaning/device explantation, the cornerstones guiding elective weaning include clinical, biochemical, echocardiographic parameters and right heart catheterization, depending on the clinical context, and all indicating resolution of cardiac/non-cardiac pathophysiological derangement.
Measures of ‘off-pump’ LVEF, end-diastolic diameter, pulmonary capillary wedge pressure (PCWP), together with tissue Doppler and strain imaging on echocardiography are widely used to predict successful long-term pVAD explantation.99 Similar parameters have been proposed to predict weaning from VA-ECMO. Here, in stable patients (low dose vasopressor ± inotropic support without pulmonary congestion/hypoxemia or other significant uncontrolled medical conditions) echocardiographic signs of improved LV function (LVEF >20-25%, velocity-time integral >10 cm and lateral mitral annulus peak systolic velocity >6 cm/s) during reduced flow (1-2 L/min) with no significant fall in MAP predict weaning success.100,101 There are many proposed VA-ECMO weaning algorithms, but none has been shown to be superior in randomized studies. In case of cardiorespiratory failure, where cardiac function has recovered, but the lungs remain severely impaired, downgrading to VV-ECMO may be an option. If the right ventricle is also significantly compromised, but the left ventricle has recovered, an oxy-right ventricular assist device may be an option.
The literature on weaning from other pVAD is limited, with recommendations/weaning algorithms based on expert consensus (Figure 4). Principles are, however, similar to VA-ECMO; the patient must be stable with a pulsatile arterial waveform (MAP >60-65 mmHg) on low-dose vasopressor ± inotropic support without pulmonary congestion/other conditions that may preclude successful weaning including arrhythmia, acid-base/metabolic disturbance, and mechanical complications. In left-sided support, PCWP should be near normal (preferably <15 mmHg) in a patient without former heart failure, before weaning is considered. There are no validated echocardiographic cut-off values that predict successful weaning in either left- or right-sided pVAD.
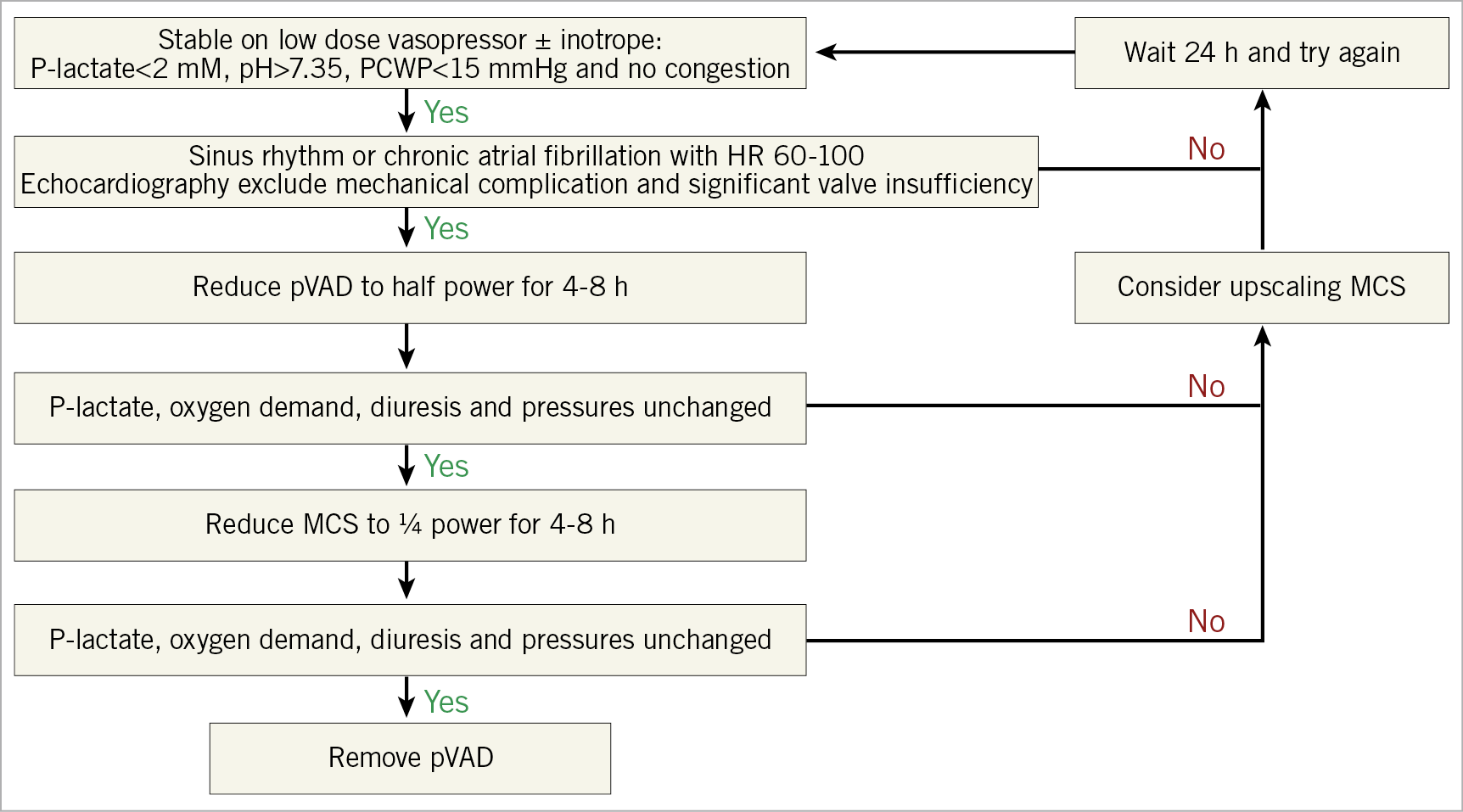
Figure 4. Algorithm for pVAD weaning in cardiogenic shock. MCS: mechanical circulatory support; PCWP: pulmonary capillary wedge pressure
Where a weaning trial is unsuccessful, it is vital to identify and address the cause of weaning failure. When the patient continues to fail to wean, consideration should be made regarding the potential options, including a longer run on the existing device/modification of support to the least injurious to the patient (if cardiac recovery is anticipated), upgrade to more durable circulatory support, or withdrawal of support.59,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117
Futility
Ceilings of care, and determining futility are important, but challenging to set in the context of patients referred for pVAD, especially with the absence strong predictors of outcome at the time of onset of CS, and the need to proceed quickly to pVAD initiation. These challenges are discussed in the Supplementary material online.
Future directions and conclusions
The rapid expansion of pVAD use in the settings of CS and HR-PCI without sufficient evidence from large-scale randomized trials is problematic. Currently, this widespread adoption is based on small series and registries, including industry-sponsored studies alone. Importantly, in particular in CS, the rates of device-related complications remain high. Consequently, there is an urgent need for adequately powered randomized clinical trials and large national/multinational registries to better define those patients who may benefit from pVAD, and how best to evaluate, monitor and manage every aspect of their care, especially in the setting of CS.
GAPS IN KNOWLEDGE AND FUTURE STUDIES
(1) Pathophysiological studies evaluating ventricular unloading in high-risk myocardial infarction and CS.
(2) Randomized clinical trials demonstrating the benefit of pVAD over standard of care in high-risk PCI and CS.
(3) Randomized clinical trials demonstrating the benefit paradigm shift from door to balloon to door to unload.
(4) Large prospective national and international registries evaluating the outcomes of pVAD in a real-world population.
(5) Algorithms and protocols to better define patients population and timing for pVAD.
(6) Protocols and proper education of physicians and healthcare providers to reduce device-related complications.
Conflict of interest statement
A. Chieffo received consulting fees/honoraria from Abiomed, Abbott Vascular, Cardinal Health, Biosensor, Magenta Medical. D. Dudek has served on the Scientific Advisory Board of Impella CP. A. Combes received grants and personal fees from Getinge. J. E. Møller received grants and personal fees from Abiomed and personal fees from Orion Pharma and Novartis. F. Pappalardo received personal fees from Abiomed. G. Tarantini received personal fees from Abiomed and GADA. G. Tavazzi received personal fees from GE Healthcare. N. van Mieghem received grants and personal fees from Abbott Vascular, Medtronic, Boston Scientific, PulseCath BV. N. Werner received personal fees and non-financial support from Abiomed. None of the other authors has relevant conflicts of interest to disclose.
Supplementary data
To read the full content of this article, please download the PDF.

