Abstract
Aims: This technical report describes the essentials and practical implantation technique of the completely percutaneous PulseCath iVAC 2L left ventricular assist device.
Methods and results: Percutaneously inserted mechanical left ventricular assist devices are used for circulatory support during cardiogenic shock or high-risk percutaneous coronary interventions. The PulseCath concept is a novel pulsatile design that consists of an extracorporeal membrane pump connected to a large-bore catheter which is inserted across the aortic valve retrogradely into the left ventricle. A genuine intra-aortic balloon pump (IABP) console drives the pulsatile pump.
Conclusions: The percutaneous PulseCath iVAC 2L is driven by a genuine IABP console and provides more left ventricular support.
Introduction
Percutaneously inserted mechanical left ventricular assist devices (LVAD) are used for circulatory support during cardiogenic shock or high-risk percutaneous coronary interventions1-3. Cardiology and cardiac surgery societies on both sides of the Atlantic have issued joint guidelines on myocardial revascularisation and myocardial infarction, including prudent recommendations on the use of LVADs4,5. Data to support LVADs in patients with cardiogenic shock or undergoing high-risk PCI are scarce, yet these devices remain an essential part of the complete armamentarium of a tertiary care facility dealing with challenging patients who have complex coronary pathology. Compared with continuous flow systems, pulsatile LV support results in higher systemic perfusion pressure, theoretically augmented coronary perfusion and more unloading of the right heart and pulmonary circulatory system6.
The net haemodynamic benefits with the intra-aortic balloon pump (IABP) appear modest at best, with a cardiac output increase up to 0.5 L/min7. The iVAC 3L™ (PulseCath BV, Amsterdam, The Netherlands) is a membrane pump connected to a 21 Fr catheter that is inserted across the aortic valve retrogradely into the left ventricle: it is driven by a genuine IABP console and can generate a pulsatile blood flow up to 3 L/min8. The PulseCath iVAC 3L requires surgical access to the right subclavian artery. This technical report illustrates the newest-generation PulseCath iVAC 2L and its conversion to a true percutaneous transfemoral LVAD.
Technical specifications
DESCRIPTION
The PulseCath iVAC 2L has three essential components: 1) the membrane pump, 2) a bi-directional flow catheter, and 3) a patented rotating two-way valve (Figure 1, Figure 2). The extracorporeal membrane pump is transparent and contains a blood chamber and an air chamber divided by a thin flexible membrane. The blood chamber is connected to the bi-directional flow catheter and the air chamber to a mainstream IABP console, which acts as the pneumatic driver of the pump (Moving image 1). The total chamber volume is 40 mL. The pump can expel a maximum volume of blood of 21 ml. The bi-directional flow catheter is composed of nitinol wire-reinforced polyurethane, measures 95 cm and has a 17 Fr (5.9 mm) outer diameter. The inlet tip is made of stainless steel. At 6 cm from the inlet tip, the catheter has an integrated two-way valve that pivots around two axes. A connector piece at the other end of the catheter connects to the membrane pump.
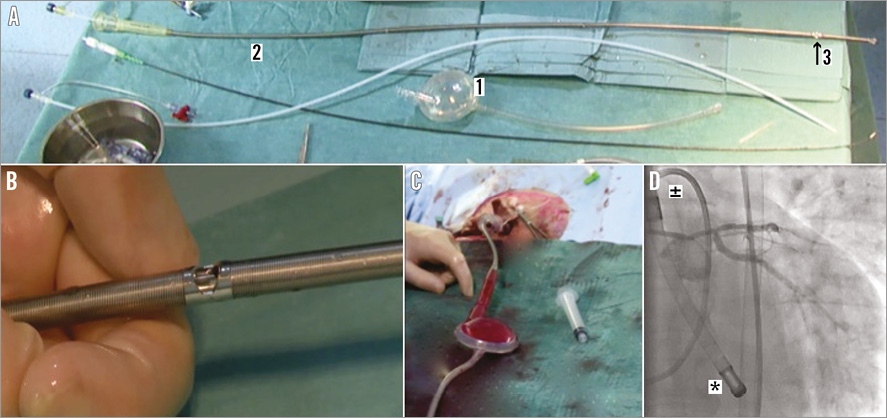
Figure 1. The PulseCath iVAC 2L. A) Extracorporeal transparent membrane pump with a blood chamber and an air chamber divided by a thin flexible membrane (1), bi-directional flow catheter (2), and rotating two-way valve (3). B) Close-up of the rotating two-way valve. C) Extracorporeal transparent membrane pump filled with blood. D) PulseCath iVAC 2L in situ with the distal tip (*) in the left ventricle and the two-way valve above the aortic valve (±).
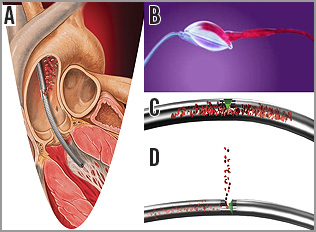
Figure 2. Cartoon illustration of the PulseCath iVAC 2L. A) Catheter across the aortic valve with its distal tip in the left ventricle and expulsion of blood above the aortic valve into the ascending aorta. B) Extracorporeal transparent membrane pump with blood entering the blood chamber. C) Bi-directional flow catheter with the valve (in green) closed towards the ascending aorta; blood will be directed from the left ventricle to the extracorporeal pump. D) Bi-directional flow catheter with the valve (in green) open to the ascending aorta; blood will be directed from the extracorporeal pump to the ascending aorta.
CONCEPT
The catheter is inserted through the common femoral artery. The catheter is advanced retrogradely across the aortic valve into the left ventricle. The catheter is de-aired and connected to the extracorporeal membrane pump, which itself is connected to the IABP console. Using ECG triggering, blood is aspirated from the left ventricle through the inlet tip into the membrane pump during systole and ejected from the pump through the catheter valve into the ascending aorta during diastole. The pivotal valve function at the outflow port is synchronised with aortic valve closure and precludes interference with aortic valve functioning. The resulting pulsatile support generates additional output with (analogous to a typical IABP) systolic unloading of the left ventricle and diastolic counterpulsation (Figure 3).
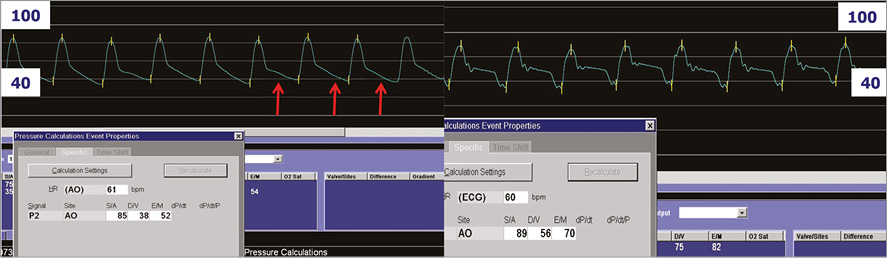
Figure 3. Pulsatile support with the PulseCath iVAC 2L. Left: pre support; right: with support. Note diastolic augmentation (red arrows) and resultant increase in mean arterial pressure.
HAEMODYNAMIC EFFECTS
The PulseCath iVAC 2L can expel up to 21 mL per beat. At higher heart rates (>80 bpm) the net stroke volume will drop because the diastolic phase decreases and the pump no longer has sufficient time to eject its maximum volume. The iVAC 2L can generate a maximum output of 2.2 L/min in vitro. In vivo the pump generates an output of up to 1.5 L/min. Figure 4, Figure 5 and Figure 6 illustrate the typical haemodynamic changes during PulseCath iVAC 2L support. The mean systemic arterial pressure and cardiac output increase; mean pulmonary arterial pressure and mixed venous oxygen saturation remain stable. Of note, no significant haemolysis is perceived.
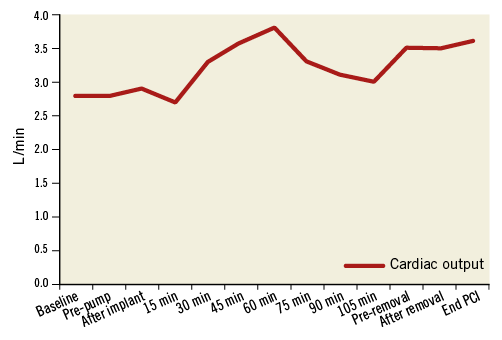
Figure 4. Change in cardiac output with PulseCath iVAC 2L support.
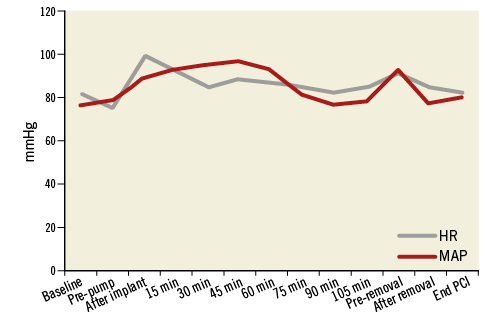
Figure 5. Change in heart rate (HR) and mean arterial pressure (MAP).
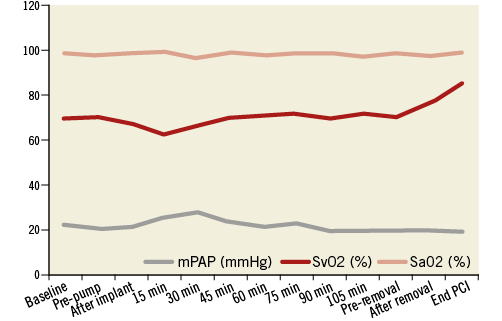
Figure 6. Change in mean pulmonary artery pressure (mPAP), arterial oxygen saturation (SaO2) and mixed venous oxygen saturation (SvO2).
Implantation step by step
PREPARATION
The PulseCath iVAC 2L requires an 18 Fr femoral sheath. A thorough knowledge of the iliofemoral arterial tree is thus crucial to confirm eligibility and avoid access-site complications. Conventional contrast angiography or even a multi-slice computed tomography (MSCT) scan à blanc without contrast may prove to be a valuable screening tool. The minimum lumen arterial diameter should exceed 6 mm to accommodate the 18 Fr sheath. Furthermore, excessive tortuosity and vessel wall calcification should be avoided. Multiple access techniques are possible including fluoroscopy-guided puncture and use of a landmark pigtail coming from the contralateral side. We prefer echo-guided access to guarantee access above the femoral bifurcation, avoid calcified regions and posterior wall puncture, and minimise radiation exposure to patient and operator. We first introduce a 6 Fr arterial sheath and make a contrast angiogram to confirm appropriate access and vessel characteristics. We then use a pre-closure technique with either one Prostar® or two ProGlides® (Abbott Vascular Devices, Redwood City, CA, USA). A stiff 0.035” guidewire is inserted and an 18 Fr arterial sheath, typically the SoloPath™ Balloon Expandable TransFemoral Access System (Terumo Corp., Tokyo, Japan), is navigated into the iliofemoral arterial trajectory. A 6 Fr pigtail catheter is used to cross the aortic valve and a pre-shaped 0.035” stiff guidewire (e.g., Amplatz Super Stiff™ Guidewire; Boston Scientific, Marlborough, MA, USA) is positioned in the apex of the left ventricle (Moving image 1).
INTRODUCING THE iVAC 2L CATHETER
The bi-directional flow catheter is pre-loaded with a dedicated dilator and advanced over the 0.035” stiff guidewire until the stainless steel inlet tip has entered the left ventricle. The stiff guidewire and dilator are carefully removed, leaving the tip of the catheter in the LV apex. As the dilator is removed from the catheter, blood bleeds back and de-airs the system. The catheter is then temporarily squeezed with a surgical clamp. The chamber of the membrane pump is filled with heparinised saline. With a 60 mL syringe a wet-to-wet connection is made between the filled pump and the catheter. After confirmation of an air-free connection, the clamp is relieved and the IABP console is activated. The chamber will fill with arterialised blood. The pulsatile membrane mechanics can be visually monitored and assessed for thrombus formation. The target activated clotting time is between 250 and 300 seconds.
RETRIEVAL
At the end of the procedure the IABP console is switched off and the arterial tubing is clamped. With gradual continuous force the catheter can be retrieved from the body. Finally the 18 Fr SoloPath sheath can be removed and the arteriotomy closed with the initially constructed pre-closure.
COMPLICATIONS
As mentioned above, the requirement of an 18 Fr arterial sheath with the PulseCath iVAC 2L mandates proper pre-procedural planning with imaging and meticulous access-site preparation to avoid major access-site complications. Adequate per-procedural anticoagulation is essential to avoid thromboembolic phenomena but should be balanced with the risk of excessive bleeding. As with all LV support systems haemolysis should be monitored. Results from ongoing clinical studies will shed light on the incidence of device-specific procedural complications.
Conclusion
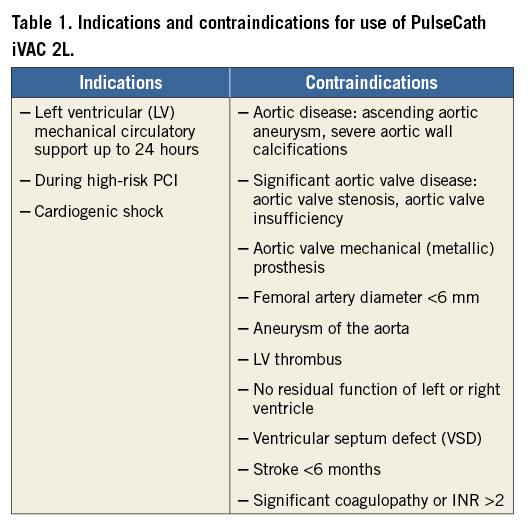
The PulseCath iVAC 2L is a next-generation LVAD that can be safely introduced in a completely percutaneous fashion. It offers a pulsatile alternative to the continuous flow LV support systems and may have some theoretical benefits. It obtained CE mark approval for left ventricular circulatory support up to 24 hours on February 21st 2014. Table 1 displays indications and contraindications. A genuine IABP console drives the device. The assembly can generate an additional output of 1.5 L/min on top of the existing cardiac output and, as such, significantly outperforms the IABP. Clinical studies to assess safety and efficacy are ongoing.
| Impact on daily practice Percutaneous left ventricular assist devices are used to perform high-risk percutaneous coronary interventions and as an adjunct in the treatment of cardiogenic shock. The PulseCath iVAC 2L is a next-generation pulsatile LVAD that is driven by a genuine intra-aortic balloon pump console and generates an additional output of 1.5 L/min on top of the existing cardiac output. As such it outperforms the standard IABP and may be a valuable addition to the overall treatment armamentarium in patients requiring temporary circulatory support. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. PulseCath principle concept.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. PulseCath principle concept.

