
Abstract
The HeartMate PHP (percutaneous heart pump) is a second-generation transcatheter axial flow circulatory support system. The collapsible catheter pump is inserted through a 14 Fr sheath, deployed across the aortic valve expanding to 24 Fr and able to deliver up to 5 L/min blood flow at minimum haemolytic risk. As such, this device may be a valuable adjunct to percutaneous coronary intervention (PCI) of challenging lesions in high-risk patients or treatment of cardiogenic shock. This technical report discusses: (i) the HeartMate PHP concept, (ii) the implantation technique, (iii) the haemodynamic performance in an in vitro cardiovascular flow testing set-up, and (iv) preliminary clinical experience. An update on the device, produced by St. Jude Medical/Abbott Laboratories, can be found in the Appendix.
Introduction
Percutaneous mechanical circulatory support (MCS) is a valuable adjunct to percutaneous coronary intervention (PCI) of challenging lesions in high-risk patients or treatment of cardiogenic shock. MCS is intended to prevent or reverse haemodynamic collapse and shock. MCS adoption in clinical practice is not only patient dependent but also centre specific. Indeed, a recent analysis of the National Cardiovascular Data Registry underscored variability in the use of MCS across hospitals and revealed a clustering of use in a selected number of (tertiary care) institutions1. Several pulsatile and continuous flow support devices that can be inserted percutaneously are commercially available. The intra-aortic balloon pump (IABP) offers diastolic augmentation to increase myocardial perfusion and reduce afterload during systole to promote modest forward flow of 0.3 to 0.5 L/min2. The PulseCath® iVAC2L (PulseCath bv, Amsterdam, the Netherlands) is a new pulsatile device that is inserted across the aortic valve into the left ventricle (LV) and driven by a genuine IABP console to generate a pulsatile flow of 1.5 L/min on top of the existing cardiac output3. The Impella (Abiomed Inc., Danvers, MA, USA) was the first commercial percutaneously inserted continuous axial flow system to unload the ventricle by pumping aspirated blood from the LV into the ascending aorta4. The Impella 2.5 and CP® can generate antegrade flow of up to 2.5 L/min and 3.5 L/min, respectively. The HeartMate PHP™ (percutaneous heart pump) (St. Jude Medical, St. Paul, MN, USA) is a new continuous flow support design that can produce an output up to 5 L/min. It obtained CE mark approval in July 2015 for the support of patients undergoing high-risk PCI. In this technical report, we describe the novel design features of the PHP, procedural instructions and haemodynamic performance.
Technical specifications
DESCRIPTION
The HeartMate PHP is a catheter-based percutaneous blood pump with collapsible axial flow impeller and cannula at the distal end. The key feature of the HeartMate PHP is the expansion of its distal end from 13 Fr to 24 Fr upon unsheathing by the operator, as shown in Figure 1 and Figure 2. An external motor drives the elastomeric impeller via a flexible drive shaft that resides inside the inner sheath. Radial alignment of the impeller is maintained by saline-lubricated hydrodynamic bearings located at the proximal end of the cannula. The 5.5 cm long cannula consists of a super-elastic nitinol supported cage and haemocompatible thin film Thoralon® coating (Thoratec, Pleasanton, CA, USA). The tip of the cannula is supported by a polymer flexible atraumatic tip that serves to facilitate device insertion over the guidewire. The 102 cm 14 Fr outer sheath is reinforced with a stainless steel braid to provide adequate structural support during device placement and removal. The inner sheath has variable elastic modulus at the distal section to allow radial flexibility in tracking the catheter through the vasculature and positioning across the aortic valve into the left ventricle. Inner and outer sheathing are designed as a system to provide positional stability and prevent cannula migration during operation.
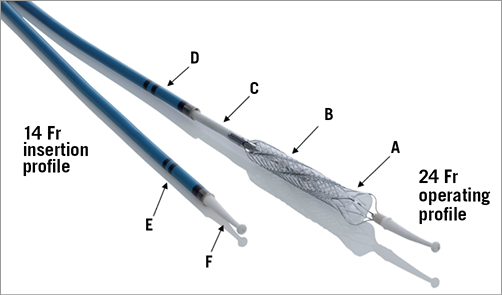
Figure 1. HeartMate PHP catheter and expandable impeller and cannula mechanism. Thoralon coated nitinol cannula (A), elastomeric impeller (B), inner sheath (C), outer sheath (D), dual radiopaque markers (E), flexible atraumatic tip with guidewire lumen (F).
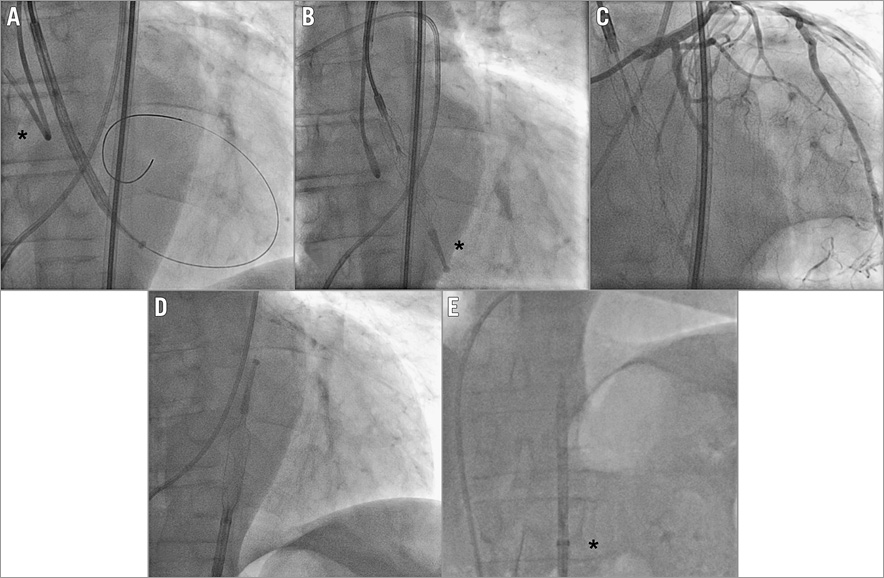
Figure 2. Angiographic appearance of the HeartMate PHP. Device insertion across the aortic valve in its sheathed 14 Fr-compatible composition: the * mark identifies the guiding catheter seating on the aortic valve (A). Expansion of the device to 24 Fr: * identifies the atraumatic tip (B). Coronary angiography while operating HeartMate PHP in situ (C). Resheathing of the HeartMate PHP in the descending aorta (D). Retrieval of the re-collapsed device in the 14 Fr sheath (*) (E).
CONCEPT
Moving image 1 and Figure 3 illustrate the HeartMate PHP system components and device insertion. Prior to the procedure, the device is connected to the console, which provides saline lubrication and controls the impeller speed. The collapsible 24 Fr nitinol cannula is pulled into the outer sheath, resulting in a 14 Fr compatible insertion profile. The assembly is inserted through the common femoral artery and advanced in retrograde fashion over-the-wire across the aortic valve into the LV. Radiopaque markers are aligned with the aortic valve to ensure optimal device positioning. The outer sheath is then retracted to expand the cannula and flexible impeller to the 24 Fr operating profile, with the cannula inlet within the left ventricle and the cannula outlet in the ascending aorta. The touchscreen on the console is used to activate the impeller motor. The impeller speed setting is adjusted between 16,000 and 20,500 rpm according to the patient’s haemodynamic requirements.
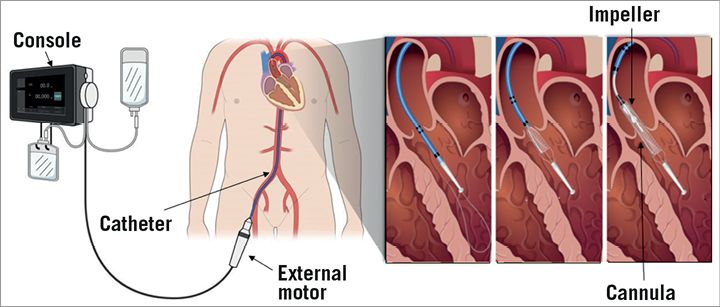
Figure 3. HeartMate PHP system components. Percutaneous device insertion through the femoral artery and expansion of the collapsible impeller section across the aortic valve illustrated with inserts.
Implantation step by step
PREPARATION
The HeartMate PHP requires a 14 Fr arterial sheath (e.g., Fast-Cath™ Introducer; St. Jude Medical). The status of the iliofemoral arteries in general and the puncture location at the level of the common femoral artery in particular needs to be addressed in terms of size (minimum 5 mm), calcification and tortuosity. In elective cases the access site can be screened with a multislice computed tomography (MSCT) scan. Ultrasound and invasive contrast angiography are additional tools to determine safe arterial access. Given the larger bore access, we recommend fluoroscopy or ultrasound-guided access to avoid posterior wall, too high or too low punctures. In elective cases, a suture-based preclosure technique can be applied with either one ProStar or two ProGlide® devices (Abbott Vascular Devices, Redwood City, CA, USA). Once the 14 Fr arterial sheath is inserted, the aortic valve is crossed with a pigtail catheter. A 260 cm 0.018” stiff guidewire is positioned through the pigtail catheter into the left ventricular apex (Moving image 1).
INSERTION
The pigtail catheter is exchanged over the 0.018” extra support wire for the prepped assembly. The radiopaque markers help position the device accurately, ensuring that the cannula inlet is positioned in the mid-cavity of the LV below the aortic valve and the cannula outlet distal to the impeller is in the ascending aorta above the aortic valve. The extra support wire is then removed and the motor is attached. The nitinol cannula is then unsheathed and the system is connected to the driving console. The impeller is activated and the operator adjusts the speed and flow. The console controls the pump speed and monitors system performance. An integrated saline system ensures that saline is flowing through the catheter to maintain lubricity of the rotating components. The targeted activated clotting time is 250 seconds or greater.
RETRIEVAL
At the end of the procedure the console is switched off and the entire assembly is pulled back into the descending aorta. After the nitinol cannula is resheathed, the entire system is removed from the body. Finally, the 14 Fr sheath is removed and the arteriotomy is closed with the initially constructed preclosure.
PERFORMANCE AND HAEMODYNAMIC EFFECTS
In vitro experiments demonstrate that the PHP unloads the LV with reductions in end-diastolic volume (EDV) and end-diastolic pressure, increases the mean systemic pressure and increases the overall output. Pressure-volume loops indicate a shift to the left due to reduction of EDV and stroke volume as the pump increases.
Figure 4 illustrates the characteristic pressure-flow relationship for the HeartMate PHP using a blood analogue solution. The flow rate of the HeartMate PHP is inversely proportional to the difference in pressure between the aorta and the left ventricle at a given set speed. When the differential pressure is 60 mmHg, the HeartMate PHP can generate an output of up to 5 L/min at a speed of 20,500 rpm. Reduction of the pump speed will cause a leftward shift across the pressure-flow curves, resulting in less flow. At lower differential pressures, the HeartMate PHP provides full haemodynamic support at low impeller speeds. During the cardiac cycle, as the pressure differential across the left ventricle and aorta changes periodically, the pump flow responds by cycling between the peak flow rate at the systole and lower flow rate during diastole.
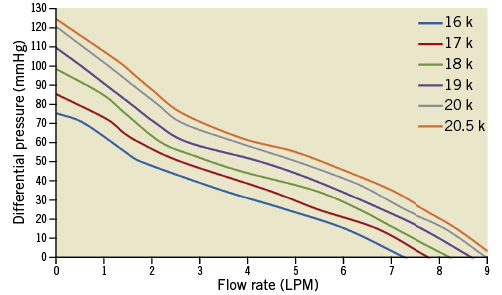
Figure 4. Pressure-flow relationships of HeartMate PHP tested in blood analogue solution (viscosity: 3.6 cP) over various operating speeds.
PRELIMINARY CLINICAL EXPERIENCE
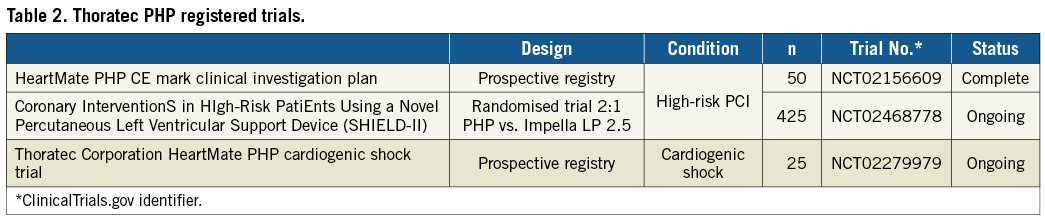
The HeartMate PHP has been successfully inserted in eight patients who underwent elective high-risk PCI at the Erasmus Medical Center in Rotterdam, the Netherlands, as a part of the broader SHIELD I CE Mark study. Patient profiles are shown in Table 1. The average duration of the procedure was 76 minutes, ranging between 15 and 131 minutes. The average impeller speed was 17,400±900 rpm. Cardiac output and mean arterial pressure during HeartMate PHP support increased, whereas pulmonary capillary wedge pressure remained stable (Figure 5). Of note, no clinically significant haemolysis, gross haematuria, new or worsening aortic sufficiency or end-organ dysfunction was observed. Table 2 displays currently ongoing multicentre clinical trials that will shed further light on safety and efficacy of the HeartMate PHP in patients undergoing high-risk PCI and in cardiogenic shock.
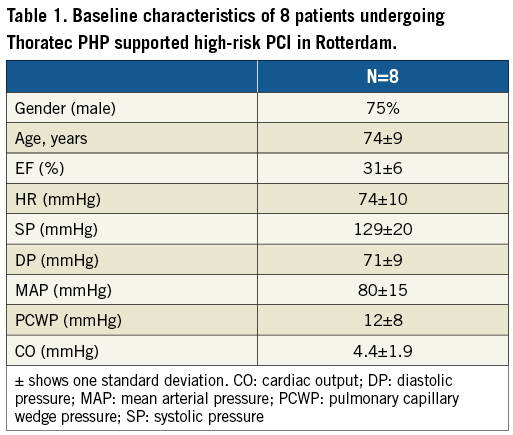
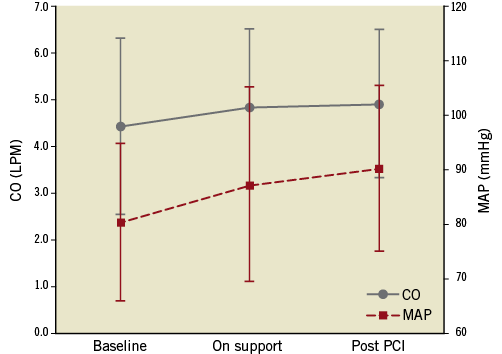
Figure 5. Change in cardiac output (CO, solid line) in L/min (left, y-axis) and mean arterial pressure (MAP, dashed line) in mmHg (right, y-axis) before, during and after HeartMate PHP support in eight consecutive patients in Rotterdam. Error bars indicate one standard deviation.
Conclusions
The HeartMate PHP is a next-generation axial flow pump capable of generating up to 5 L/min blood flow without creating significant haemolysis. As such, it may be the most powerful percutaneous MCS device of its class. It is 14 Fr sheath compatible and expands to 24 Fr at the level of the aortic valve to deploy the nitinol cannula and the flexible impeller. Preliminary experience is favourable, and multicentre clinical trials are currently ongoing to address the safety and efficacy of the HeartMate PHP further.
Appendix
UPDATE
In an update following the “temporary pause” by Abbott Laboratories of the use of the HeartMate PHP, Dr Van Mieghem added the following comment:
In the Rotterdam practice with PHP-supported high-risk PCI cases, I did not encounter any issues with the PHP device. The ease of use and the dynamic PHP support at the moment the patient needed it most stand out as compelling features. The device consistently and automatically increased its output when patients entered a critical phase in the procedure, e.g., during prolonged balloon inflations in the left main stem of patients with poor LV function. That said, one needs to be cautious and respect meticulous device preparation and handling, and patient selection. Still, to enter daily clinical practice, devices need to be reliable and physician-proof.
Guest Editor
This paper was guest edited by Holger Thiele, MD; University Heart Center Luebeck, Medical Clinic II, Luebeck, Germany.
Conflict of interest statement
K. Fitzgerald, C. Parker, P. Muller, and O. Dur are employees of St. Jude Medical. N. Van Mieghem is an advisor for PulseCath. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
Moving image 1. HeartMate PHP preparation, insertion, deployment and retrieval.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. HeartMate PHP preparation, insertion, deployment and retrieval.

