Abstract
Percutaneous mechanical circulatory support devices, such as intra-aortic balloon pump (IABP), active left ventricular assist devices (LVAD) or extracorporeal life support (ECLS), are treatment options for selected patients in cardiogenic shock, undergoing cardiopulmonary resuscitation, or high-risk percutaneous coronary intervention and coronary artery bypass grafting. Potential benefits include the maintenance of organ function and the reduction of intracardiac pressures, volumes, and oxygen consumption. On the other hand, they are invasive, resource intensive, and can be associated with serious complications. Thus, their potential benefits must be weighed against the inherent risks. Despite the lack of sufficient scientific evidence, the use of mechanical circulatory support devices has risen considerably in recent years. This educational article covers practical issues of IABP, LVAD, and ECLS with respect to patient and device selection, implantation technique, potential complications, and future perspectives.
Background
Percutaneous mechanical circulatory support devices, such as the intra-aortic balloon pump (IABP), active left ventricular assist devices (LVAD) or extracorporeal life support (ECLS), are treatment options for selected patients in cardiogenic shock, undergoing cardiopulmonary resuscitation (CPR), or high-risk percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG).
This educational article focuses on the various percutaneous mechanical circulatory support devices available and covers practical issues with respect to device and patient selection, implantation technique, potential complications, and future perspectives.
Devices
INTRA-AORTIC BALLOON PUMP
IABP is the most widely used device for mechanical circulatory support. After publication of the IABP-SHOCK II trial in the year 2012 implantation rates declined1. IABP, which is made of a polyurethane membrane mounted on a vascular 7.0-8.0 Fr catheter, is positioned in the descending thoracic aorta just distal to the left subclavian artery (Figure 1). The device is timed to inflate and deflate according to the cardiac cycle. The IABP increases the diastolic blood pressure and lowers the end-systolic pressure without affecting the mean blood pressure and without effects on cardiac output, cardiac power index, serum lactate or any effect on the doses of catecholamines2,3. Recent advances in technology, including enhanced automation, flexible treatment algorithms, improved insertion speed, and a smaller catheter shaft diameter allowing sheathless insertion, may theoretically permit improved support at reduced complication rates. Data to support this hypothesis are lacking.
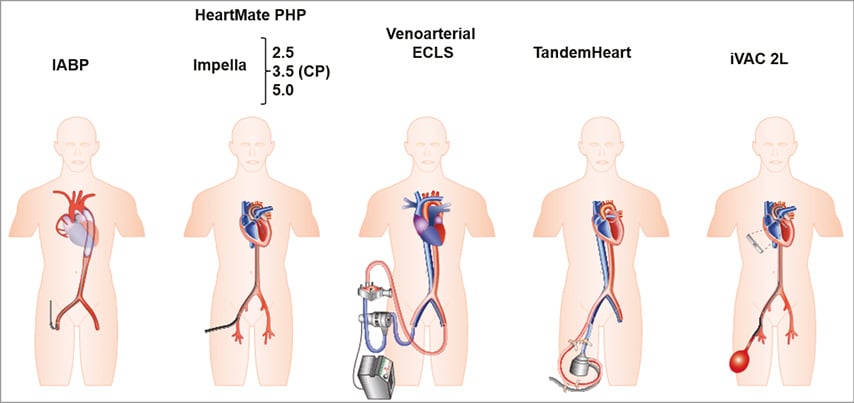
Figure 1. Schematic drawings of current percutaneous mechanical support devices. Adapted from reference 13, with permission to be used from Oxford University Press.
DEVICE-RELATED COMPLICATIONS OF IABP
IABP is associated with rare but relevant complications such as major bleedings, stroke, local and systemic infections, and vascular complications. In comparison to LVAD or ECLS, IABP has the lowest complication rates.
Limb ischaemia is the most common vascular complication but the incidence has been reduced by the availability of new catheters with smaller diameters and sheathless insertion. Aortic dissection, retroperitoneal bleeding, femoral haematomas, arteriovenous fistulas, and femoral pseudoaneurysms can develop after any femoral access procedure, including IABP therapy. Embolisation of aortic atherosclerotic components to peripheral vascular beds may occur and can induce ischaemia in the affected territories. Finally, visceral arteries can be compromised due to improper balloon sizing and positioning. Non-vascular complications such as infections are rare. Gas embolisation is usually without clinical consequence due to the use of helium, which is quickly eliminated by the respiratory system if leaked. In contrast to these clinical observations, the randomised IABP-SHOCK II trial did not observe a higher rate of potentially IABP-related complications in the IABP-treated patients3.
EXTRACORPOREAL CARDIAC LIFE SUPPORT/EXTRACORPOREAL MEMBRANE OXYGENATION
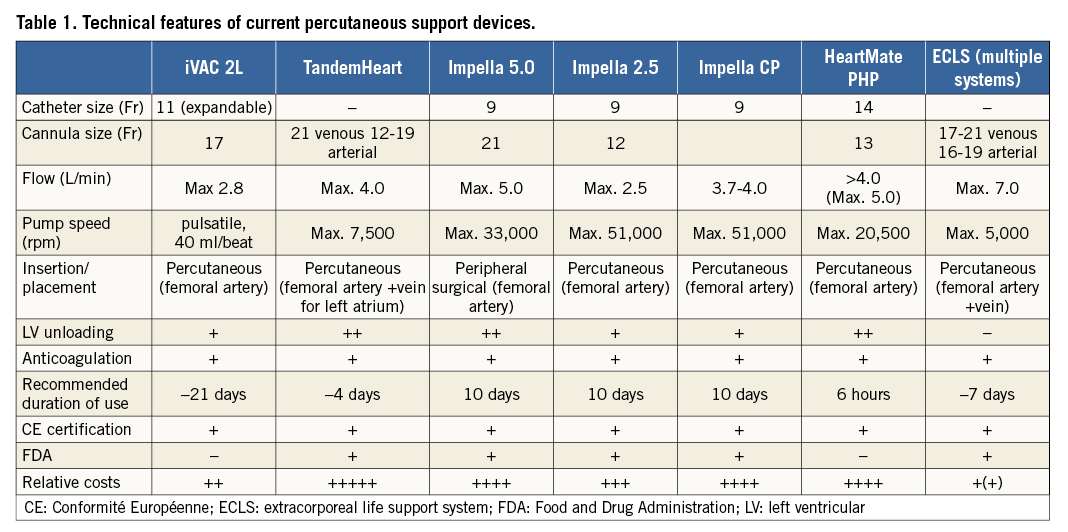
Since the introduction of the first cardiopulmonary bypass system with oxygenation in 1953, further advances have led to the development of percutaneous devices. Such systems consist mainly of an external blood pump and a membrane oxygenator (Figure 1). A 16-19 Fr arterial cannula in the descending aorta and an 18-21 Fr venous cannula advanced into the right atrium are usually used. Blood is withdrawn from the right atrium, pumped subsequently through a heat exchanger and the membrane oxygenator, and ultimately returned into the femoral artery. The pump usually provides a continuous flow with maintenance of a pulsatile arterial pressure unless the circulation is completely supported by a cardiopulmonary bypass device. ECLS can be used in patients with right ventricular, left ventricular (LV), or biventricular failure at very high blood flow rates leading to a support >5 L/min (Table 1).
DEVICE-RELATED COMPLICATIONS OF ECLS
Due to its high invasiveness, the incidence of complications of ECLS is very high. Frequent complications include lower extremity ischaemia (16.9%), stroke (5.9%), major bleeding (40.8%), and significant infection (30.4%) as shown in a recent meta-analysis4. These rates may be lowered by increasing experience in percutaneous implantation and by obligatory insertion of an antegrade perfusion cannula. Further, dislodgement of the arterial cannula is a rare but fatal complication. Finally, the increase of afterload due to ECLS may lead to LV distention, especially when LV ejection is not preserved or aortic valve insufficiency is present. LV distention leads to impairment of LV recovery but may also be associated with thrombosis of the aortic valve and even intraventricular clotting in rare cases. The most promising non-surgical venting mechanism appears to be insertion of an additional LVAD with LV unloading properties (e.g., Impella®; Abiomed Europe, Aachen, Germany). Again, the benefits and risks of venting mechanisms have not been studied extensively and are only theoretical.
PERCUTANEOUS LEFT VENTRICULAR ASSIST DEVICES
Currently established and available LVAD include the TandemHeart™ (Cardiac Assist, Inc., Pittsburgh, PA, USA), the microaxial Impella 2.5, 5.0, and CP systems (Abiomed Europe), the paracorporeal pulsatile device iVAC 2L® (PulseCath BV, Arnhem, The Netherlands), and the most recently introduced HeartMate percutaneous heart pump, HeartMate PHP™ (St. Jude Medical, Pleasanton, CA, USA) (Figure 1).
TANDEMHEART
This system is a left atrial-to-femoral arterial LVAD device (Figure 1). A detailed description of the mode of action and implantation procedure of the TandemHeart has been published previously5. In brief, after septal puncture a venous inflow cannula is inserted into the left atrium and an arterial cannula (17 Fr) is afterwards inserted into the femoral artery. Oxygenated blood is withdrawn from the left atrium and returned to the lower abdominal aorta via the arterial cannula in the femoral artery. The redirection of blood reduces LV preload, LV wall stress, and LV pressures. The system is capable of delivering flow up to 4.0 L/min at 7,500 rpm (Table 1).
IMPELLA
The Impella pump is a non-pulsatile axial flow pump that consists of a suction cannula with a turbine positioned in the LV to propel blood into the ascending aorta (Figure 1). As mentioned above, three versions are available: Impella 2.5, 5.0, and CP (Table 1). The Impella 2.5 and CP can be inserted via a standard catheterisation procedure through the femoral artery, into the ascending aorta, across the aortic valve, and into the LV. The inlet area, located at the distal tip of the cannula, has four openings that allow blood to be drawn into the inlet and channelled through the cannula. Implantation of the larger Impella 5.0 is very similar except that a surgical cut-down is required. The axial flow pump systems produce an unloading of the LV and a reduction in LV wall stress.
HEARTMATE PHP
This axial flow device system consists of a covered nitinol cannula with integrated impeller which is introduced percutaneously over a 13 Fr introducer into the femoral artery (Figure 1). The major design feature is a collapsible elastomeric impeller and nitinol cannula making this device the lowest profile insertion cannula with the highest flow. Once placed across the aortic valve, the cannula can be expanded to 24 Fr and allows a continuous mean flow of >4 L/min at modest operating speeds (Table 1). Thus, LV end-diastolic pressures and LV volumes are reduced. For removal, the system can be collapsed to the initial 13 Fr. Currently, data are limited for this device with only a small registry trial with 46 patients undergoing high-risk PCI (SHIELD I). The results have not yet been published.
iVAC 2L
The iVAC 2L system is introduced percutaneously through the femoral artery and can provide a pulsatile support of approximately 2 L/min using an extracorporeal membrane pump via a 17 Fr cannula (Figure 1, Table 1). In the systolic phase of the heart, blood is aspirated from the LV through the catheter lumen into the membrane pump. During the diastolic phase, the pump ejects the blood back through the catheter, subsequently opening the catheter valve and delivering the blood to the ascending aorta through the side outflow port, thereby creating an “extra heart beat”. Data are limited to small case series, and the clinical impact of this device needs to be investigated further6.
DEVICE-RELATED COMPLICATIONS OF PERCUTANEOUS LEFT VENTRICULAR ASSIST DEVICES
Most of the complications mentioned above for ECLS can also occur with the different types of percutaneous LVAD. Current evidence suggests that there are fewer complications with the Impella device than with the TandemHeart or ECLS. This might be due to the smaller cannulas used by the Impella 2.5, Impella CP and the avoidance of extracorporeal circulation.
A complication specific to the TandemHeart device is the dislocation of the venous cannula from the left to the right atrium. Furthermore, bleeding complications, need for subsequent blood component transfusions, and most probably also the rate of infections are significantly more frequent with this active device7. Other rare complications include the persistence of an atrial septal defect after removal of the venous cannula and pericardial tamponade.
The Impella as well as the HeartMate PHP device may lead to aortic insufficiency, aortic valve injury, arrhythmias due to the location in the LV, cardiac tamponade or cerebral vascular accidents/stroke. Haemolysis may be relevant in particular for the Impella due to the high rpm, which can occur in up to 10% of patients treated.
Due to a lack of clinical trials, no specific complications are known regarding the iVAC 2L and the HeartMate PHP device. However, complications similar to those seen with the Impella device can be expected.
Indications
Haemodynamic support, invasiveness, and complications appear to display a linear relation with the currently available devices (Figure 2). Due to the high complication rate, aggressive devices with higher flow rates should be reserved for severe clinical conditions such as cardiogenic shock, whereas less invasive devices with a subsequent better safety profile but limited support may be chosen more liberally (Figure 3). In daily clinical routine, IABP, LVAD, and ECLS are mainly used in cardiogenic shock. Moreover, ECLS is increasingly used in refractory CPR. Furthermore, prophylactic IABP or LVAD therapy is used to support high-risk PCI. Finally, preoperative or postoperative IABP insertion is a widely accepted therapeutic option in high-risk patients undergoing CABG.
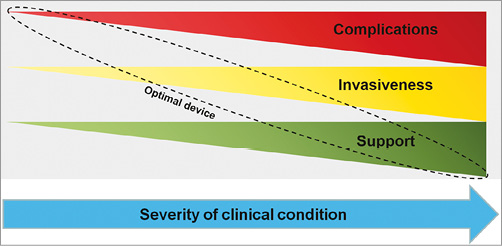
Figure 2. Sketch displaying the relationship between complications, invasiveness, and support of current percutaneous mechanical support devices.
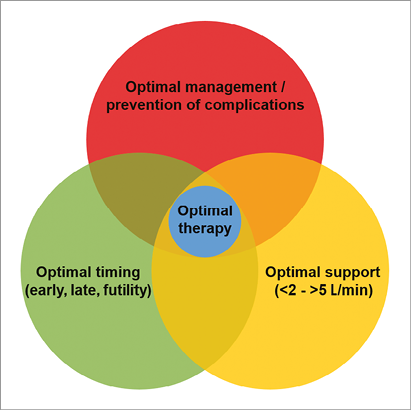
Figure 3. Considerations on use of mechanical support.
HIGH-RISK PERCUTANEOUS CORONARY INTERVENTION
The use of IABP or LVAD is controversial in patients with high-risk PCI features. This can be mainly explained by the fact that data on the use of prophylactic IABP or LVAD use in high-risk PCI are scarce. To date, there are only two randomised studies including a total of 749 patients (PROTECT II: n=448, BCIS-1: n=301)8,9. Second, there is no clear definition of high-risk PCI, leading to many different definitions. Furthermore, neither the PROTECT II trial nor the BCIS-1 study observed a significant benefit for IABP or Impella over standard high-risk PCI without assisted circulation with respect to the primary study endpoints. However, the BCIS-1 trial comparing elective IABP versus standard PCI showed a mortality benefit at five-year follow-up10. This is most likely a chance finding due to the lack of a pathophysiologically plausible explanation. The PROTECT II trial comparing elective Impella to IABP in the setting of high-risk PCI was stopped for futility. The primary endpoint, defined as the 30-day incidence of major adverse events, was not different for patients with IABP or Impella support, but trends for improved outcomes mainly related to soft endpoints without any effect on hard endpoints were observed for Impella-supported patients at 90 days.
Due to the broad range of high-risk PCI definitions and weak data, there are no meaningful guideline recommendations which address the current potential indications for the use of IABP or LVAD in high-risk PCI settings.
HIGH-RISK CORONARY ARTERY BYPASS GRAFTING
Prophylactic preoperative use of IABP has been suggested to improve clinical outcome in high-risk patients undergoing CABG: this has been confirmed in meta-analyses11. However, previous studies were underpowered for clinical outcome. Furthermore, they led to inconsistent results and were often limited due to single-centre designs, suboptimal methodology, and non-uniform definitions of high-risk patients. As a consequence, current guidelines do not unequivocally support the preoperative prophylactic use of IABP in high-risk patients undergoing CABG12. The lack of evidence with contradictory data and weak guideline recommendations underline the need for an adequately powered prospective randomised multicentre trial to determine if prophylactic preoperative IABP results in a perioperative mortality reduction in high-risk CABG patients.
CARDIOGENIC SHOCK
Despite modern treatment strategies such as early revascularisation and optimal intensive care management, the mortality of cardiogenic shock still approaches rates up to 50%13. Standard therapy consists primarily of volume management as well as inotropic agents and vasopressors enhancing cardiac output and vascular tone. The haemodynamic benefits of inotropes and vasopressors are counterbalanced by adverse effects such as increased myocardial oxygen demand, arrhythmogenicity, and compromise of tissue microcirculation, which may translate into an increased mortality risk. Mechanical circulatory support systems are an alternative to increase systemic blood flow, avoiding the possible cardiotoxicity and long-term morbidity of inotropes and vasopressors, and are often the only option to achieve haemodynamic stability in cardiogenic shock refractory to standard therapy.
Before 2012, American and European guidelines supported IABP use in cardiogenic shock with a class I recommendation. The IABP-SHOCK II trial, however, has called this into question. In this, the largest randomised multicentre trial in patients with cardiogenic shock, no significant difference between the two treatment groups was observed with respect to the primary endpoint of 30-day mortality (39.7% versus 41.3%; p=0.69). There were also no differences in any of the secondary endpoints, and no subgroups showed a potential advantage of IABP support3. The 12-month follow-up analysis confirmed these negative findings with a mortality of 52% in the IABP group versus 51% in the control group (p=0.91)14. Although IABP support has been in place for nearly five decades, the negative results of IABP-SHOCK II have influenced recent European revascularisation and also the non-ST-elevation acute coronary syndrome guidelines: IABP has been downgraded to a class III A recommendation for routine use in cardiogenic shock12,15.
Data on LVAD in cardiogenic shock are scarce. Since one meta-analysis published in 2009 reported the results of the only three randomised trials comparing percutaneous LVAD (two trials with the TandemHeart, one with the Impella 2.5) to IABP, no additional randomised trials have been conducted7. Patients treated with active LVAD demonstrated higher cardiac index, higher mean arterial pressure, and lower pulmonary capillary wedge pressure. On the other hand, bleeding complications and inflammation were more frequent with LVAD therapy, and there was no difference with respect to 30-day mortality7. Recent observational studies with the Impella device have suggested some benefit with this device in cardiogenic shock16. In the USpella Registry, patients with cardiogenic shock directly treated with Impella prior to PCI had an overall better survival at hospital discharge compared with those treated after PCI, even when adjusting for potential confounding variables16. For the iVAC and HeartMate PHP systems no trials are currently available.
ECLS provides more potent support in comparison to IABP or LVAD. However, data on the safety and efficacy of ECLS in refractory cardiogenic shock are scarce, led to inconsistent results, and are limited due to small sample sizes as well as relatively short follow-up duration17-20. One single-centre non-randomised retrospective analysis showed improved survival rates with ECLS in comparison to historical control20. In a more recent prospective report, in-hospital mortality of ECLS patients was as high as 63.2%. The elderly patient group of >62 years and those with cardiopulmonary resuscitation were even characterised by a mortality of 100%, questioning the unselective use of ECLS18. Despite major advances in technology since its first development, ECLS remains very invasive, resource intensive, and can be associated with serious complications. Nevertheless, ECLS holds some advantages over other devices due to its ability to support right ventricular, LV, or biventricular failure at very high blood flow rates as well as the potential to support patients with concomitant lung injury by its oxygenation properties. These facts emphasise the role of ECLS as a potential bail-out strategy after failure of less invasive devices.
REFRACTORY CARDIOPULMONARY RESUSCITATION
Since the introduction of the percutaneous approach, ECLS has been used for patients with refractory out-of-hospital or in-hospital cardiac arrest. Current guidelines on CPR and emergency cardiovascular care recommend that ECLS should be considered in CPR in case of a short time without blood flow and deemed reversibility of the condition leading to cardiac arrest or amenability to heart transplantation (Class IIb, level of evidence C)21. This recommendation is, however, based on observational registry data reporting inconsistent rates of survival ranging from 4 to 64%22-24. Results of a large single-centre trial including more than 300 patients with in-hospital cardiac arrest showed significant differences in short- and long-term survival, favouring ECLS over conventional CPR24. Despite initial stabilisation, long-term survival of patients with refractory cardiac arrest remains low, especially for older patients. This might be partially explained by the high rate of local and systemic complications associated with ECLS and the futile clinical condition itself. Appropriate patient selection thus remains a challenge, and will require further investigation in large randomised multicentre trials, especially regarding the clinical impact of new, small, portable devices such as the Lifebridge® (Lifebridge Medizintechnik AG, Ampfing, Germany) or the CardioHelp® (Maquet Cardiopulmonary AG, Hirrlingen, Germany).
Current and future aspects
Multiple open issues remain in mechanical circulatory support therapy, including device and patient selection as well as optimal timing of device insertion. However, due to the limited evidence, these important questions have to be addressed mainly based on theoretical assumptions.
To date, there are no comparative studies analysing a potential advantage of one LVAD over another. Consequently, potential differing indications arise due to hypothetical considerations based on differing modes of action of LVAD and ECLS. Devices leading to LV unloading such as the TandemHeart, Impella series or the HeartMate PHP might beneficially influence organ recovery in the setting of myocardial infarction and thus appear appealing. LVAD support is theoretically beneficial at early stages of cardiogenic shock to interrupt the vicious circle of ischaemia, hypotension, and myocardial dysfunction to allow recovery of stunned and hibernating myocardium. In this setting, devices with low risk for severe complications should be used despite their limited support. ECLS with its near-systemic flow rates but high rate of severe complications should be restricted to patients with severe haemodynamic instability refractory to optimal medical therapy. In addition, ECLS plays an important role in patients with right ventricular and biventricular failure. Due to its concomitant oxygenation ECLS rapidly improves tissue oxygenation.
Another open question is the optimal timing of device insertion. A potential benefit of early use at the onset of cardiogenic shock could be prevention of multiorgan dysfunction. Early use, however, might lead to complications associated with invasive mechanical circulatory support devices, resulting in an adverse clinical outcome in patients who would still have non-invasive therapeutic options. Timing and appropriate patient selection are thus also influenced by the balance between the efficacy of any device and its device-related complications.
Notably, approximately 60% of cardiogenic shock patients will survive without any active device3. There may also be futile situations where even the best device available will not be able to change clinical outcome. The relationship between these considerations is depicted in Figure 3.
An optimal mechanical circulatory support device should i) be technically easy to implant, ii) allow rapid insertion, iii) be minimally invasive, iv) achieve high haemodynamic support, v) unload the LV, and vi) offer the potential to provide oxygenation. Such a device allowing early use even in patients with mild clinical conditions could lead to a paradigm shift in cardiology, avoiding administration of high doses of inotropes and vasopressors. However, at present percutaneous LVAD and ECLS should be restricted to use in dedicated centres based on an individualised approach with respect to patient selection, device selection, and timing of insertion.
Conclusion
In the last few years, several new devices have entered the market and have shown promising technical aspects. The development of mechanical support devices offers new treatment options for selected patients undergoing high-risk PCI or CABG and those in cardiogenic shock or refractory CPR. Important aspects about device and patient selection as well as timing of insertion need to be evaluated in large randomised multicentre trials.
Conflict of interest statement
The authors have no conflicts of interest to declare.

