
Abstract
Aims: Our aim was to test the feasibility and safety of the transfemoral PulseCath iVAC 2L (PulseCath, Amsterdam, The Netherlands).
Methods and results: Circulatory support devices are helpful adjunctive tools to perform high-risk percutaneous coronary interventions (PCI). The PulseCath iVAC 2L is a novel pulsatile circulatory support system capable of generating output of up to 2 L/min. We performed a prospective clinical pilot study enrolling 14 patients who underwent high-risk PCI under protection with the iVAC 2L. Median age was 74 (56-84) years. Implantation of the iVAC 2L was successful in 13 (93%) patients. Median device flow was 1.4 (1.1-2.0) L/min. Total support time was 67 (23-149) minutes. The use of iVAC 2L support was associated with a better mean arterial pressure and cardiac output during the procedure. Angiographic success was 100%. There was one major procedural complication related to the 19 Fr access sheath. There were no major adverse events at three-month follow-up.
Conclusions: Circulatory support with the iVAC 2L device is feasible and safe in patients undergoing high-risk PCI.
Abbreviations
AKIN: Acute Kidney Injury Network
ANOVA: analysis of variance
BARC: Bleeding Academic Research Consortium
CI: cardiac index
CK-MB: creatinine kinase MB fraction
CO: cardiac output
HR: heart rate
IABP: intra-aortic balloon pump
LBBB: left bundle branch block
LM: left main coronary artery
LVEF: left ventricular ejection fraction
MACE: major adverse cardiac events
MAP: mean arterial blood pressure
MPAP: mean pulmonary artery pressure
MSCT: multi-slice computed tomography
NS: not significant
PCI: percutaneous coronary intervention
STS: Society of Thoracic Surgeons
SvO2: (mixed) venous oxygen saturation
3VD: three-vessel coronary disease
TTE: transthoracic echocardiography
ULN: upper limit of normal
Introduction
Catheter-based circulatory support devices can be used to prevent or stabilise haemodynamic compromise in patients undergoing high-risk percutaneous coronary interventions (PCI)1,2. Pulsatile and continuous flow devices exist. Most experience has been gathered with the pulsatile intra-aortic balloon pump (IABP) and the axial continuous flow Impella® device (Abiomed, Danvers, MA, USA). The benefit of circulatory support in high-risk PCI is unproven, yet use of the IABP enhances haemodynamic stability and the Impella 2.5 provides even superior haemodynamic support3,4. However, especially in cardiogenic shock from acute myocardial infarction, the benefit of the IABP on global haemodynamics and tissue perfusion has recently been questioned5-7. Guidelines on myocardial revascularisation provide prudent recommendations on the use of circulatory support with PCI8,9.
The PulseCath iVAC 2L® (PulseCath B.V., Amsterdam, The Netherlands) is a next-generation pulsatile support system driven by any standard IABP console and generating pulsatile blood flow up to 2 L/min (Figure 1), depending on preload and afterload conditions and heart rate10.
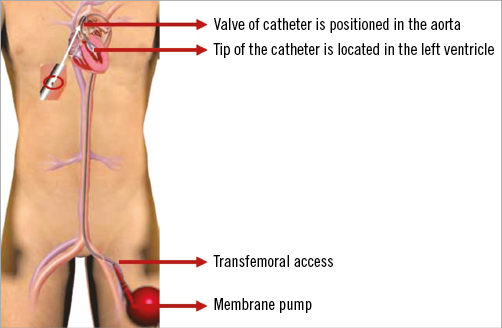
Figure 1. The iVAC 2L in position. The catheter is inserted via the femoral artery, with the iVAC tip in the left ventricle and the iVAC valve in the ascending aorta.
The aim of this pilot study was to assess the feasibility and safety of iVAC 2L circulatory support in patients undergoing elective high-risk PCI.
Methods
STUDY DESIGN
The study was a prospective single-centre, single-arm feasibility study. The Institutional Research Board/Ethics Committee approved the protocol, and written informed consent was obtained from each patient. The study complied with the Declaration of Helsinki. Data from this study were also used in the clinical evaluation required for CE-mark approval for left ventricular assistance for up to 24-hour use. PulseCath B.V. provided the iVAC 2L “free of charge” along with technical support throughout the study. The authors are responsible for the conduct and results of the study and the preparation of the manuscript.
PATIENTS
Inclusion and exclusion criteria are listed in Table 1. The Heart Team, including at least one interventional cardiologist and one cardiothoracic surgeon, reached consensus for PCI and identified all patients at risk for procedural haemodynamic compromise (criteria: PCI of single remaining vessel, unprotected left main [LM], or three-vessel coronary disease [3VD] with left ventricular ejection fraction [LVEF] ≤35%). At baseline, all patients underwent transthoracic echocardiography (TTE) and echo Doppler of the femoral arteries to determine the quality of the access vessel. A common femoral artery minimum diameter of 6 mm is required to accommodate the 19 Fr expandable or re-collapsible SoloPath® (Terumo Corp., Tokyo, Japan) sheath. Pre-procedural CT angiography of the iliofemoral arterial tree was recommended. PCI was routinely performed by radial artery access. Postoperatively, patients underwent echocardiography at the ICCU to investigate damage to the aortic valve or increase in aortic regurgitation. Patients were followed up for 90 days post procedure.
iVAC 2L
The principles of the PulseCath iVAC 2L have been described previously10. In brief, the iVAC 2L insertion kit consists of a catheter, a membrane pump and a catheter protector. The thin-walled catheter has a total length of 100 cm and a diameter of 17 Fr (5.9 mm diameter) (Figure 2). The catheter is composed of an inlet tip, an integrated two-way valve and a connector for connection to the membrane pump. The transparent membrane pump consists of a blood chamber and an air chamber, which are divided by a flexible membrane (total volume=40 mL). The blood chamber side is connected to the catheter, while the air chamber side is connected to a pneumatic helium-filled IABP driver. The iVAC actively aspirates blood from the left ventricle during systole and ejects this blood in the ascending aorta in diastole creating a counterpulsation effect and an additional circulatory support up to 2 L/min.
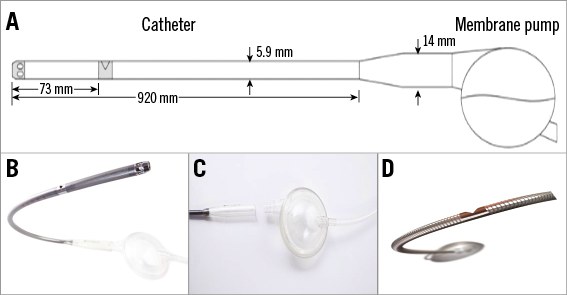
Figure 2. Technical details. Technical details of the iVAC 2L catheter and the membrane pump (A), and detailed pictures of the inlet tip (B), membrane pump (C), and integrated two-way valve (D).
IMPLANTATION TECHNIQUE
Ultrasound-guided access to the common femoral artery was obtained in all patients. A vascular preclosure technique was used (two Perclose ProGlide® Suture-Mediated Closure systems; Abbott Vascular, Santa Clara, CA, USA). A 19 Fr SoloPath sheath was inserted. Heparin was then administered aiming for an activated clotting time >250 seconds. The aortic valve was crossed with a pigtail catheter; an Amplatz Super-Stiff™ guidewire (Boston Scientific, Marlborough, MA, USA) was shaped and inserted through the pigtail into the left ventricular apex. The pigtail was then exchanged for the 17 Fr PulseCath iVAC 2L catheter. Its distal tip should be seated halfway into the left ventricular cavity and the valve housing with its side opening in the ascending aorta. After introduction, the membrane pump was filled with heparinised saline and air-free connected to the catheter and the IABP console. In this study, we used the CARDIOSAVE® IABP console (Maquet Cardiovascular, Fairfield, NJ, USA). The two-way valve would guide the blood during pump aspiration from the tip of the catheter to the membrane pump, and during pump ejection from the membrane pump to the side opening in the valve housing, thus generating pulsatile flow. At the end of the procedure, the patient was weaned off the device, and the device was removed. The femoral arteriotomy was closed using the closure technique mentioned above.
PREDEFINED ENDPOINTS
Feasibility was defined as the ability to introduce the system and to reach adequate LV support (defined as flow >1.0 L/min). The iVAC 2L flow was measured with a clamp-on flow probe attached to the 17 Fr catheter just distal to its connection to the membrane pump and a SonoTT™ Ultrasonic FlowMeter (em-tec GmbH, Finning, Germany). Clinical success was achieved when the patient received proper circulatory support and was successfully weaned from the device within 24 hours after insertion. The application of the iVAC 2L catheter was considered safe when its use was free of serious adverse events, defined as death, damage to cardiac structures, major vascular complications and life-threatening bleedings. Bleeding was reported according to the BARC criteria11. We defined significant bleeding as a type 2-5 bleeding (a type 1 bleeding is not actionable and does not necessitate or prolong hospitalisation)11. Access-site complications were defined as a vascular complication at any access site resulting in haematoma or leg ischaemia requiring surgical or percutaneous intervention, false aneurysm (on ultrasound imaging), or femoral artery occlusion. Major procedural complications included prolonged hypotension, ventricular tachycardia/fibrillation requiring defibrillation, permanent damage to cardiac structures as noted by TTE or respiratory arrest requiring assisted ventilation. Prolonged hypotension was defined as a mean arterial pressure less than 60 mmHg for at least 10 minutes despite fluid resuscitation or requirement of inotropic/vasopressor support to maintain the mean arterial pressure above 60 mmHg. Acute kidney injury was defined according to the Acute Kidney Injury Network (AKIN) criteria12. A (clinically relevant) periprocedural myocardial infarction was defined as a rise of peak CK-MB measured within 48 hours of the procedure to ≥10x the local laboratory upper limit of normal (ULN), or to ≥5x ULN with new pathologic Q-waves in ≥2 contiguous leads or new persistent left bundle branch block (LBBB)13. MACE was defined as the composite of all-cause death, myocardial infarction, and revascularisation occurring at 90 days.
HAEMODYNAMIC PARAMETERS
We measured mean arterial pressure (MAP), heart rate (HR), mean pulmonary artery pressure (MPAP), wedge pressure, cardiac output (CO), cardiac index (CI), and mixed venous oxygen saturation (SvO2). The latter haemodynamic parameters were measured with a pulmonary artery catheter by continuous CO and SvO2 monitoring (Swan-Ganz™ CCOmbo; Edwards Lifesciences, Irvine, CA, USA). Cardiac power index (CPI) was computed as MAP×CI/45114. All parameters were measured at baseline under stable conditions (two equal baseline measurements), during support (directly after implantation of iVAC 2L as well as every 15 minutes after implantation), and finally after device removal.
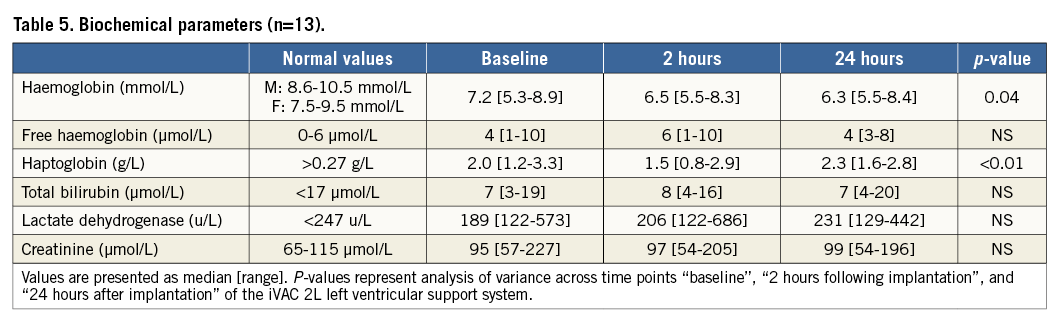
BIOCHEMICAL PARAMETERS
Haemoglobin, free haemoglobin, haptoglobin, bilirubin, lactate dehydrogenase, and creatinine were measured at baseline, two hours, and 24 hours following implantation of the iVAC 2L. Cardiac markers (hs-troponin T and CK-MB) were measured every six hours following the procedure until the peak was reached.
STATISTICS
Categorical variables are presented as absolute numbers with percentages. Continuous variables are presented as median and total range. Differences between independent samples were tested with the Mann-Whitney test. Correlations were investigated by inspecting the bivariate scatter plot and with Spearman’s rho. Changes over time were tested with one-way repeated measures analysis of variance (ANOVA), the Wilcoxon signed-rank test or the Friedman test, when appropriate. Polynomial regression was performed to assess correlation between heart rate and iVAC output. A p-value <0.05 was regarded as statistically significant. Analyses were performed using MS Excel 2013, SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Results
PATIENT PROFILE
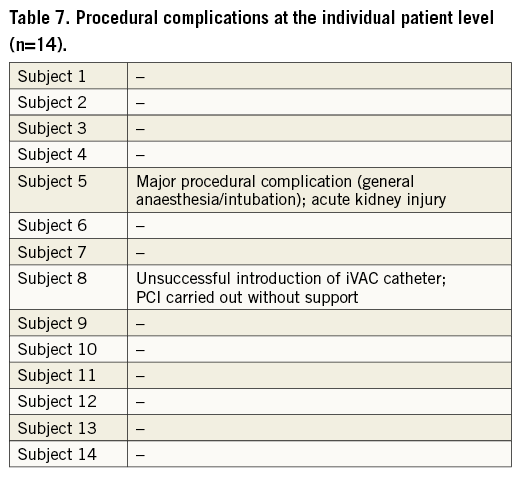
Fourteen patients were included in the study between November 2013 and November 2015. Baseline characteristics are listed in Table 2. Median age was 74 years and median EF was 30%. Median STS PROM score was 5%, median SYNTAX score was 28.5, and 57% of patients were considered inoperable. Seven patients (50%) underwent preoperative multi-slice computed tomography (MSCT), six with contrast angiography and one without contrast, to evaluate the patency and diameters of peripheral arteries. In one patient who did not undergo pre-procedural MSCT assessment, the descending aorta appeared too tortuous and the iVAC catheter could not be advanced into the left ventricle. Both SoloPath and catheter were removed as a unit. The procedure was then successfully completed without left ventricular support. Hence, data from this patient were excluded from (post-) procedural analyses.
PROCEDURE CHARACTERISTICS AND iVAC 2L PERFORMANCE
Procedure characteristics are displayed in Table 3. Total duration of the procedure was 172 (142-278) minutes, including 67 (23-149) minutes iVAC 2L support time. Total time needed for implantation and removal of the system was 27 (16-48) minutes. All patients were supported for ≥30 minutes, seven patients for ≥60 minutes, three patients for ≥90 minutes, and one patient for ≥120 minutes. Left main stem PCI was performed in 62% of patients. A median of three lesions was treated per case. Angiographic PCI success was obtained in all patients. Output of the iVAC 2L was 1.4 (1.1-2.0) L/min (stroke volume 21 [12-26 mL]) and modestly dependent on the heart rate (polynomial regression, R2=0.35) (Figure 3). A heart rate of 70-80 bpm generated optimal performance. Neither baseline wedge pressure nor baseline diastolic blood pressure correlated with iVAC 2L output (after implantation or mean output during support; all p=NS). Haemodynamic stability was present throughout the procedure. Mean aortic pressure increased significantly during iVAC 2L support (Table 4). Figure 4 demonstrates changes throughout the study in MAP (Δ+16 [+6; +36] mmHg during support, vs. +11 [–12; +29] mmHg after removal; p<0.001). Apart from contrast dye, five patients received a bolus (500 [500-1,000] mL) of gelofusine during support. Overall cardiac output increased with a mean of 35% from 3.7 L/min to 5.0 L/min (Δ+1.1 [+0.3; +2.8] L/min during support, vs. +0.6 [–0.3; +2.6] L/min after removal, compared to baseline; p<0.001). Increases in cardiac output in patients who received fluid resuscitation were similar compared to patients who did not receive fluids (during support: +1.2 [+0.3; +1.3] vs. +1.0 [+0.6; +2.8] L/min, respectively, p=NS; after support: +0.8 [0.0; +1.0] vs. 1.1 [+0.6; +2.6] L/min, respectively, p=NS). iVAC support was associated with lower MPAP and higher SvO2.
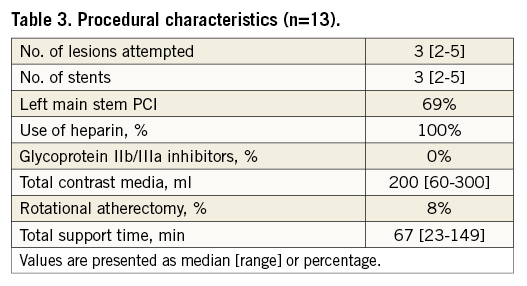
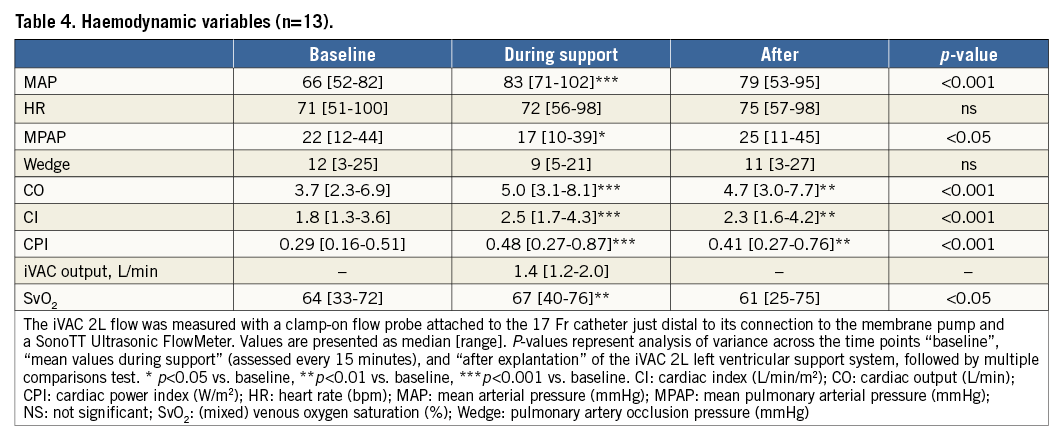
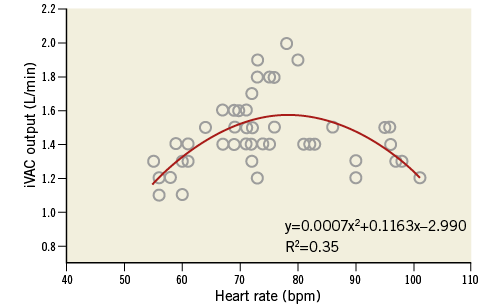
Figure 3. Correlation between heart rate and iVAC 2L output. Pooled data of correlation between heart rate (bpm) and iVAC 2L output (L/min), and polynomial trend line. The iVAC 2L flow was measured with a clamp-on flow probe attached to the 17 Fr catheter just distal to its connection to the membrane pump and a SonoTT Ultrasonic FlowMeter (em-tec GmbH). Data were collected in patients with sinus rhythm each 15 minutes during support. In the model, optimal assist (1.58 L/min) is acquired when the heart rate is 79 bpm.
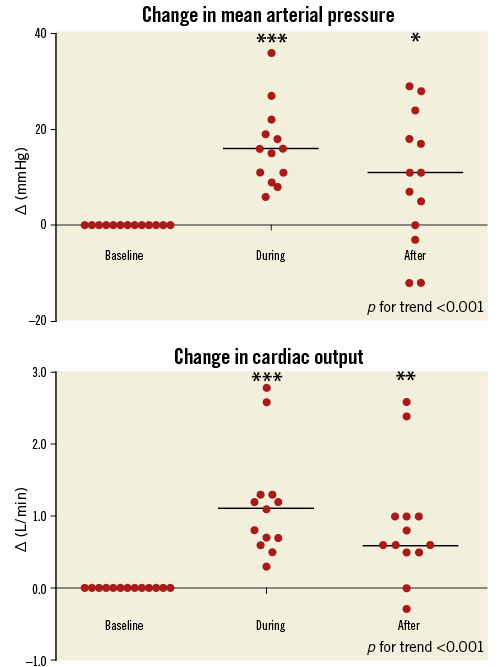
Figure 4. Changes in haemodynamic parameters throughout the study. Mean arterial pressure (A), and cardiac output (B) relative to baseline values (delta) at baseline, during and after iVAC 2L support. Horizontal lines represent medians. * p<0.05, ** p<0.01, *** p<0.001.
In one patient (subject no. 5, a 77-year-old female), introduction of the SoloPath catheter was difficult because of peripheral arterial disease and the SoloPath instructions for use had not been followed (the recommended inflation pressure was maintained for less than 60 seconds). This patient experienced intractable pain in the accessed leg and eventually required general anaesthesia. PCI was successfully performed with good iVAC 2L support. However, catheter and sheath removal was difficult, requiring more than normal force. In retrospect, the SoloPath sheath was incompletely expanded. After the procedure, the patient was immediately weaned from the ventilator and subsequently had an uneventful hospital stay. All patients were weaned from iVAC 2L support at the end of the PCI in the catheterisation laboratory. There was a small haemoglobin drop and a temporary fall in haptoglobin levels (Table 5). There was no clinically relevant haemolysis.
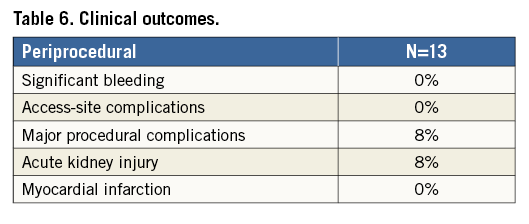
COMPLICATIONS
Clinical outcomes and overall complications are listed in Table 6. Complications at the individual patient level are listed in Table 7. There were no significant bleedings. No patient required red blood cell transfusions. Need for general anaesthesia and intubation occurred in one patient and was listed as a major procedural complication. The same patient developed temporary worsening of renal function (AKIN stage I). There was a periprocedural cardiac enzyme release in terms of high-sensitive troponin T (26 [8-2,829] at baseline vs. 112 [28-1,453] ng/L [peak level]; p=0.03), and CK-MB (3 [1-5] at baseline vs. 7 [2-55] U/L [peak level]; p<0.01). There were no PCI-related myocardial infarctions. No TTE was performed while on support; however, no clinical signs of aortic regurgitation were noted. There was no damage to cardiac structures (aortic valve, left ventricle, pericardial effusion) as assessed by postoperative TTE. All patients were discharged home in good clinical condition with no further complications up to 90 days of follow-up.
Discussion
This single-centre study confirms the feasibility and safety of circulatory support with the iVAC 2L, driven by a standard IABP console, in patients undergoing high-risk PCI. Patients were elderly with a significant operative risk and a challenging coronary anatomy. Two thirds had significant left main stem disease. The patient profile resembled the patient characteristics from the randomised PROTECT II trial comparing IABP with Impella 2.5 L with an STS score of 6% and a SYNTAX score of 294.
The iVAC 2L generated a consistent output of approximately 1.5 L/min and granted haemodynamic stability throughout the procedure, which in our opinion contributed to the 100% haemodynamic success of all PCI. After the procedure, cardiac output remained higher than at baseline level, possibly due to adequate revascularisation, and immediately improved myocardial contraction. However, 38% of patients received intravenous fluids, apart from the contrast dye, during PCI, which might have contributed to the (persistent) increase in cardiac output.
The Balloon-Pump Assisted Coronary Intervention Study (BCIS-1) evaluated intra-aortic balloon pump (IABP) support in 301 elective patients undergoing high-risk PCI3. Use of IABP prevented procedural hypotension and resulted in more stable periprocedural haemodynamics. At long-term follow-up, elective IABP support during PCI was associated with a 34% relative reduction in all-cause mortality compared with unsupported PCI (p=0.04, absolute difference 11%)15. In the PROTECT II study, the Impella 2.5 provided superior haemodynamic support in comparison with IABP4. Severe hypotension occurred in 8.6% of patients with IABP support vs. 4.9% with Impella 2.5 L support. The primary endpoint (a composite of 30-day major adverse events) was not statistically different between the two groups. However, there was a trend for fewer major adverse events associated with Impella support at 90 days. All-cause mortality was not different at one- and three-month follow-up.
PulseCath currently requires a 19 Fr SoloPath sheath. Despite this large profile, we experienced no major access-site or bleeding complications or need for blood transfusions. In the BCIS-1 study, use of an 8 Fr IABP was associated with 3.3% bleeding and access-site complications3. The Impella 2.5 L has a 13 Fr access sheath. The rate of vascular complications was 7.7% and 10.7% in the USpella registry and PROTECT II trial with need for blood transfusions in 11% and 12.5%, respectively4,16. The absence of access-site complications and bleeding in our study may reflect the advanced experience with large bore catheters in our centre.
The iVAC 2L generated a median pulsatile output of 1.4 L/min and therefore outperforms the IABP. The iVAC 2L performance was independent of preload and afterload conditions and was heart rate dependent with an optimum rate of from 70 to 80 bpm. Above 80 bpm, the diastolic phase decreases and the pump will not have sufficient time to eject its maximum volume. Additional factors that control pump performance, such as blood viscosity and the developed driving pressure of the IABP console, may influence pump output and may be the subject of further studies. The theoretical impact of diastolic counterpulsation and flow/pressure augmentation after actively unloading the left ventricle during systole by the iVAC 2L and its potential effect on coronary perfusion are intriguing and also demand additional study. Finally, the percutaneous Impella axial flow device generates an output of 2.5 to 3.5 L/min. How a pulsatile support up to 2 L/min compares with continuous support up to 3.5 L/min is currently unknown and requires further research.
Our data cannot be extrapolated to treatment of patients with cardiogenic shock17,18, as the 19 Fr access sheath may limit antegrade leg perfusion and thus preclude longer-lasting circulatory support. In our current experience, median support time was 67 minutes and the longest support time 149 minutes. Future device iterations may look for a slimmer design profile or means to secure antegrade leg perfusion with dedicated sheath systems.
Limitations
Our data should be considered within the boundaries of a single-centre, uncontrolled observational study and a relatively small sample size. Without a control group, the changes in haemodynamics observed may theoretically be due to factors other than the iVAC 2L support. Access-site management with large bore catheters requires considerable expertise and one should therefore be cautious in generalising the reported absence of access-site and bleeding complications. Our results need confirmation in larger studies including more patients and centres. Also, the comparison of pulsatile vs. axial flow circulatory support should spur further research.
Conclusions
Short-term iVAC 2L support provides significant pulsatile support and is feasible and safe during high-risk PCI. Further research is needed to compare haemodynamic performance with pulsatile vs. axial flow circulatory support.
| Impact on daily practice The iVAC 2L is a novel percutaneous mechanical circulatory support system driven by a standard IABP console. It can generate more net output as compared to a conventional IABP. How its performance compares to continuous flow systems such as the Impella requires further study. |
Guest Editor
This paper was guest edited by Holger Thiele, MD; Medical Clinic II, University Heart Center Lübeck, Lübeck, Germany.
Funding
Except for the costs of the iVAC 2L catheters, we received no external funding.
Conflict of interest statement
N. Van Mieghem is an advisor for PulseCath and has received research grants from PulseCath. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

