
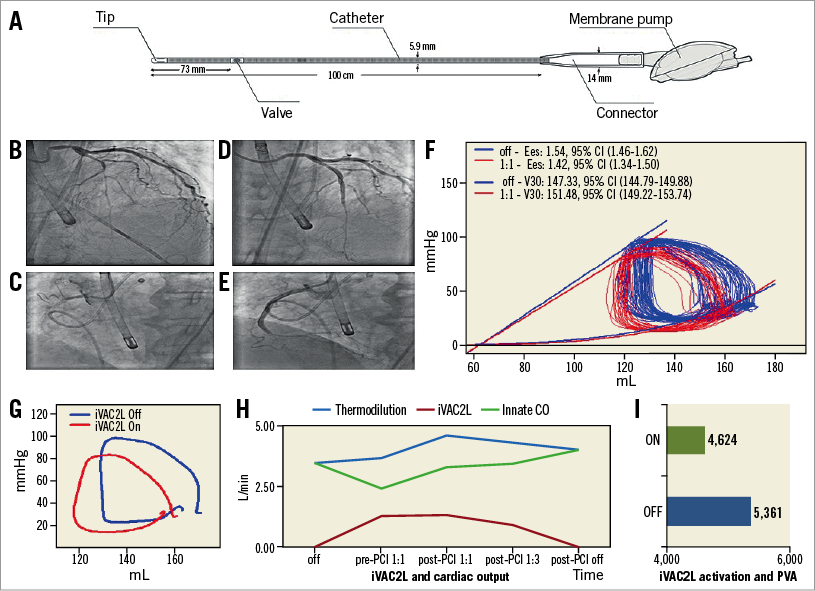
iVAC2L™ (PulseCath BV, Amsterdam, the Netherlands) is a 17 Fr bi-directional catheter with an inlet tip, an integrated two-way valve and a connector to a membrane pump. Transfemoral access requires a 19 Fr expandable or re-collapsible Solopath® (Terumo, Shibuya, Tokyo, Japan) sheath. Driven by a helium-circuited intra-aortic balloon pump (IABP) console, blood is removed from the left ventricle (LV) in systole and ejected in the aorta during diastole. Pulsatile support can reach 2 L/min, depending on heart rate and loading conditions (Panel A). Herein we illustrate LV unloading with the iVAC2L in a 70-year-old patient with an ejection fraction of 20%, severe mitral regurgitation and severe three-vessel disease. The patient initially underwent primary PCI of the left circumflex artery due to lateral ST-elevation myocardial infarction (STEMI). Because of extensive residual ischaemia by single photon emission computed tomography (SPECT), the Heart Team agreed on multivessel percutaneous coronary intervention (PCI) with mechanical circulatory support (MCS). The panels show LAD and RCA lesions before (Panel B, Panel C) and after (Panel D, Panel E) treatment. A 7 Fr conductance catheter provided LV pressure-volume (PV) loops.
Panel F shows baseline and post-activation loop sets. The means of time-matched PV points obtained from multiple loops, demonstrating post-activational left-downwards shifting are shown in Panel G. At 1:1 support, the iVAC2L flow rate was 1.28±0.04 L/min, surpassing contemporary IABP performance. The innate cardiac output (CO) decreased by 33% from its pre-support value of 4.21 L/min. Total systemic resistance fell 17%, end-systolic wall stress (WS) 15% and the pressure-volume area (PVA) 14%.
In coronary disease, oxygen depletion produces ischaemia. LV unloading reduces filling pressures, WS, myocardial oxygen consumption, chamber stiffness and microvascular resistance1. Here, reductions in maximal elastance, end-systolic pressure and +dP/dtmax suggest a decrease in contractility. An increased V30 (estimated compliance at 30 mmHg) suggests a reduction in stiffness. The PVA reduction confirms a decrease in metabolic demand. Lower end-systolic WS and Ea/Ees ratio point to optimisation of ventricular-arterial coupling.
The impact of MCS on survival is controversial. Although the iVAC2L has not been tested in large trials, our case illustrates LV unloading and hence haemodynamic improvement. The consistency of these findings will be assessed in the ongoing PULSE trial (ClinicalTrials.gov NCT03200990).
Conflict of interest statement
N. Van Mieghem is an advisor for PulseCath and has received research grants from PulseCath BV. The other authors have no conflicts of interest to declare.

