
Abstract
Aims: The haemodynamic effects of primary implantation of an intra-aortic balloon pump (IABP) versus inotropes in decompensated heart failure and low output (DHF-LO), but without an acute coronary syndrome, have not been investigated. We therefore aimed to investigate the effect of primary IABP implantation as compared to inotropes on haemodynamics in DHF-LO with no acute ischaemia.
Methods and results: Patients (n=32) with DHF-LO despite IV diuretics were randomised to primary 50 mL IABP or inotropes (INO: enoximone or dobutamine). The primary endpoint was the improvement of organ perfusion assessed by ∆ mixed-venous oxygen saturation (SvO2) at 3 hours; secondary endpoints included ∆ cardiac power output (CPO), NT-proBNP proportional change, cumulative fluid balance and ∆ dyspnoea severity score, all at 48 hours. Data are presented as median (IQR). Patients were 60 (48-69) years old and 72% were male. Baseline SvO2 was 44 (39-53)%. ∆SvO2 was higher in the IABP group (+17 [+9; +24] vs. +5 [+2; +9]%, p<0.05). IABP patients had a higher ∆CPO, a greater relative reduction in NT-proBNP, a more negative cumulative fluid balance, and a greater reduction in dyspnoea severity score. There were no IABP-related serious adverse events (SAEs). Thirty-day mortality was 23% (IABP) vs. 44% (INO).
Conclusions: Primary circulatory support by IABP showed a significant increase in improved organ perfusion assessed by SvO2.
Introduction
Decompensated heart failure and low output (DHF-LO) is a severe condition that is characterised by diuretic resistance and high mortality. Use of inotropes in this specific condition, although recommended as first-line therapy, has been associated with no or only temporary improvement or even increased overall mortality1,2. Mechanical circulatory support may be necessary to prevent irreversible end-organ damage. For more than 40 years, the intra-aortic balloon pump (IABP) has been used to improve coronary and peripheral perfusion via diastolic balloon inflation and to augment left ventricular (LV) performance via systolic balloon deflation through a decreased afterload. IABP is the most frequently used LV assist device which has proved to be safe and only minimally invasive. However, use of the IABP failed to improve short- and long-term survival in a post-acute myocardial infarction (AMI) setting and/or in AMI complicated by cardiogenic shock (CS)3,4. The IABP is therefore not routinely recommended in the latest ESC guidelines. Nowadays, advanced mechanical support capable of providing greater output is available, either as a percutaneous ventricular assist device (pVAD) or as extracorporeal membrane oxygenation (ECMO)5,6,7. However, these systems are more invasive, not widely adopted and, so far, there is a lack of compelling evidence supporting their efficacy. Although a frequent cause of DHF-LO is acute ischaemia, a non-ischaemic acute event accounts for at least one fifth of low-output cases8. The haemodynamic needs, as represented by a low mixed-venous oxygen saturation (SvO2), may be different in non-ischaemic DHF, where low output may be caused by biventricular pump failure and volume overload, versus AMI, where myocardial contractile reserve is acutely impaired9,10. We therefore aimed to investigate the effect of primary IABP implantation as compared to inotropes on haemodynamics in DHF-LO with no acute ischaemia.
Methods
PATIENTS
Consecutive patients, aged ≥18 years, admitted with DHF-LO (de novo or acute on chronic) to the intensive cardiac care unit of the Erasmus University Medical Center were eligible for participation. In order to be included in the study, a subject had to meet all of the following characteristics: no signs of acute ischaemia as clinical trigger (defined by dynamic ST-T changes on electrocardiogram [ECG] and typical rise and fall pattern in cardiac enzymes), systolic blood pressure <100 mmHg, fluid retention (elevated central venous pressure, palpable liver, or oedema), at least moderate tricuspid and/or mitral valve regurgitation (MR) and dilated inferior caval vein (>22 mm), high filling pressures and low cardiac output (pulmonary capillary wedge pressure >15 mmHg, central venous pressure >12 mmHg, and SvO2 <55%), neutral or positive fluid balance despite fluid restriction (1.5 L/24 hrs) and administration of high-dose intravenous diuretics, together with dysfunction of at least one other organ. “High-dose” intravenous diuretics was defined as at least 125 mg furosemide per 24 hours (in de novo heart failure) or as the doubled total intravenous dosage equal to the daily oral loop diuretic dosage in furosemide equivalents (in acute on chronic heart failure), for at least 12 hours. “Organ dysfunction” was defined as an arterial oxygen saturation <90% requiring oxygen supplementation aiming at an arterial oxygen saturation of 92-98%, or the presence of a worsening renal function (creatinine clearance <50 mL/min), aspartate transaminase ≥2x upper limit of normal, or lactate levels ≥2.0 mmol/L. Exclusion criteria were moderate-severe aortic valve regurgitation, femoral artery occlusion, and acute myocardial infarction <7 days before inclusion.
TRIAL DESIGN
In this investigator-initiated open-label single-centre parallel randomised controlled trial, patients underwent 1:1 randomisation to primary IABP implantation or treatment with inotropes. Randomisation was performed using random permuted blocks of sizes 4 and 2, using sealed opaque envelopes.
PROCEDURES
Each patient received a pulmonary artery catheter (CCOmbo; Edwards Lifesciences, Irvine, CA, USA). Patients were then randomised to IABP (without inotropes, IABP group) or inotropic therapy (without IABP, INO group). All patients had bed rest for at least three hours after randomisation. Baseline SvO2 was measured twice within an interval of 15 minutes and averaged (with allowed absolute difference <5%) to ensure that the patient was in a stable condition before the trial was started. In the IABP group, an interventional cardiologist implanted the IABP (8 Fr, 50 mL, Sensation® Plus; Getinge, Gothenburg, Sweden) by echo-guided femoral approach. Unfractionated heparin in a prophylactic dose was given, unless the patient had an indication for therapeutic anticoagulation. The IABP catheter remained in situ for at least 48 hours, unless complications occurred or other therapy (long-term left ventricular assist device [LVAD]) was deemed necessary. In the INO group, enoximone was started at a dose of 2 µg/kg/min. Instead of enoximone, dobutamine (3 µg/kg/min) could be administered in case of estimated glomerular filtration rate (eGFR) <30 ml/min and heart rate <90 bpm without the use of a beta-blocker. Before starting the infusion, a bolus injection was given equal to the volume of the central venous line used (=0.5 mL). After three hours, the attending physician was allowed to escalate the dose of the inotrope or to add a second inotrope based on pre-specified targets (cardiac index >2.5 L/min/m2, SvO2 >55%, lactate <2.0 mmol/L, mean arterial pressure ≥60 mmHg and urine output >0.5 mL/kg/hr). Inotropic treatment was given for at least 48 hours. Crossover (after three hours, but within 48 hours) occurred when a patient who received an IABP required inotropes, or when a patient receiving inotropes required IABP. Strict clinical criteria for crossover were recorded within the study protocol and based on prolonged SvO2 <55% and elevated lactate levels in the presence of urine output <0.5 mL/kg/hr. Other escalation of therapy occurred when, after inclusion, patients required norepinephrine (based on a prolonged MAP <60 mmHg in the presence of urine output <0.5 mL/kg/hr) or when a high output circulatory support device (typically veno-arterial extracorporeal membrane oxygenation) was needed (based on progressively worsening SvO2 and lactate measures, despite high dosages of inotropic and vasopressor support and IABP, or in the case of refractory ventricular arrhythmia).
TRIAL ENDPOINTS
The primary endpoint was the change in SvO2 (=∆SvO2) (time point 3 hours [T3h] minus baseline). Secondary endpoints were cardiac power output (CPO, where cardiac output was measured by thermodilution) at time point 48 hours (T48h) (absolute and change vs. baseline), NT-proBNP levels at T48h (absolute and relative change vs. baseline), cumulative fluid balance at T48h, ∆ dyspnoea severity score measured by the visual analogue scale (range 0-10) at T48h, the occurrence of crossover or other escalation of therapy, length of stay in the hospital, and the occurrence of MACE (a combined endpoint of crossover or other escalation of therapy, heart failure rehospitalisation, stroke, or death) at 30 and 90 days. Other clinical, haemodynamic, laboratory, and echocardiographic parameters were all measured at the bedside.
TRIAL OVERVIEW
The institutional review board (IRB) approved the study protocol (MEC-2016-475). The trial was registered online (Dutch Trial Register; www.trialregister.nl; NTR6143). Patients provided written informed consent and, in case of sedated patients, the legal representative provided consent. An independent data safety monitoring board (DSMB) monitored patient safety. The IRB was informed about serious adverse events (SAEs) (within 15 days) and clinical outcomes (halfway, and at the end of the study). An independent monitor checked the case record and informed consent forms after which the trial was officially closed. The trial investigators (C.A. den Uil, N.M. Van Mieghem, L.S. Jewbali, M.J. Lenzen, F. Zijlstra, A.A. Constantinescu) designed the trial. C.A. den Uil collected, analysed and interpreted the data. All authors wrote the manuscript and decided to submit the manuscript for publication. All authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
All data are presented as median (interquartile range) or as number (percentage) when appropriate. Pulmonary artery pulsatility index (PAPi) was calculated as (systolic pulmonary artery pressure – diastolic pulmonary artery pressure)/central venous pressure. CPO was calculated as cardiac output×mean arterial pressure/451. ∆NT-proBNP is presented as percentage change. All patients were followed for at least 90 days after inclusion. All outcomes were reported following the intention-to-treat principle. The Fisher’s exact test or Mann-Whitney U test was used to assess differences, when appropriate. Changes over time were assessed with two-way repeated measures analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test; p<0.05 was considered to indicate statistical significance. A sample of 2×13 patients would provide the trial with at least 80% power to detect a significant difference in SvO2 (+16 [±9]% for the IABP group11 vs. +6 [±9]% for the INO group)12. We increased the sample by 15% for the planned use of a non-parametric test.
Results
PATIENTS
Between March 2017 and November 2018, 32 patients were randomised: 16 received an IABP and 16 received inotropic therapy (median days of support 4 [2-6] vs. 10 [5-12] days, respectively, p=0.002). All patients randomised to IABP were able to undergo the procedure, optionally in a 30° upright position by lying on a pillow. Patients were 60 (48-69) years old and 72% were male (Table 1). Baseline characteristics as well as baseline measurements (Table 2) were not statistically different. Most patients presented with acute on chronic heart failure (75%), of primary non-ischaemic (dilated) aetiology (66%). Twenty-five percent of the patients were mechanically ventilated; these patients received norepinephrine, given to counteract the vasodilatory effects of propofol, at the time of inclusion. Patients presented with high dyspnoea scores (7 [6-8]), elevated filling pressures and low cardiac output. Despite echocardiographic evidence of right-sided heart failure, PAPi was >1.0 in most patients. Most patients had signs of abnormal renal and liver function, high NT-proBNP levels, and elevated markers of systemic inflammation. Baseline SvO2 was 44 [39-53]% (p=0.25 between groups). Baseline administration of furosemide and norepinephrine (in intubated patients only) is shown in Supplementary Table 1. In the INO group, following randomisation, 11 patients received enoximone and five patients were given dobutamine.
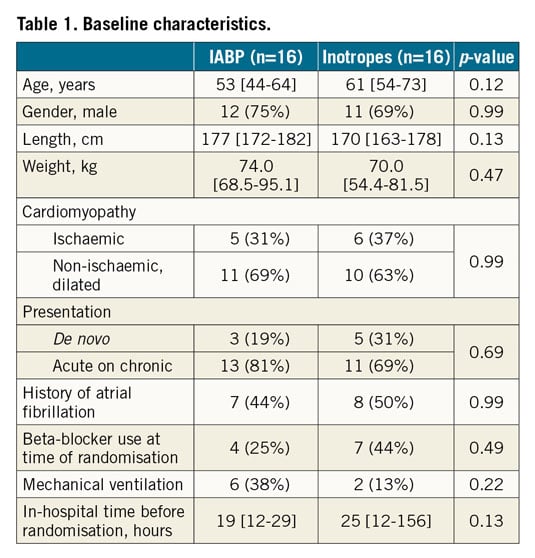
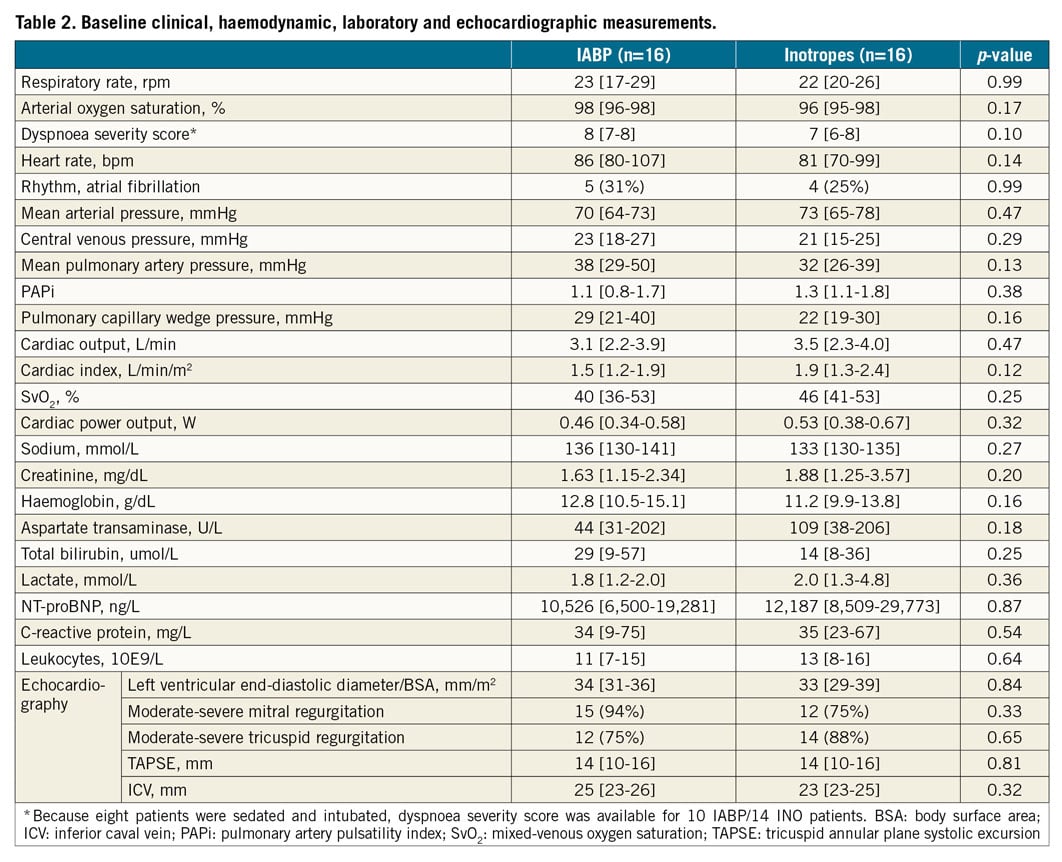
ENDPOINTS
PRIMARY ENDPOINT
∆SvO2 was higher in the IABP group (+17 [+9; +24] vs. +5 [+2; +9]%, p<0.001) (Table 3). Figure 1 demonstrates that most IABP patients, in contrast to INO patients, immediately and persistently achieved an SvO2 >60%.
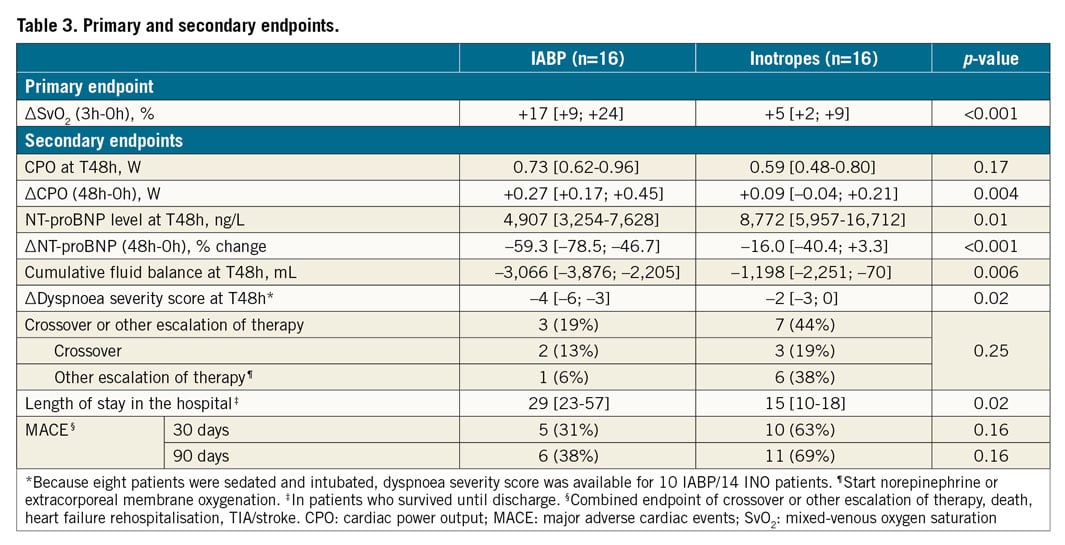
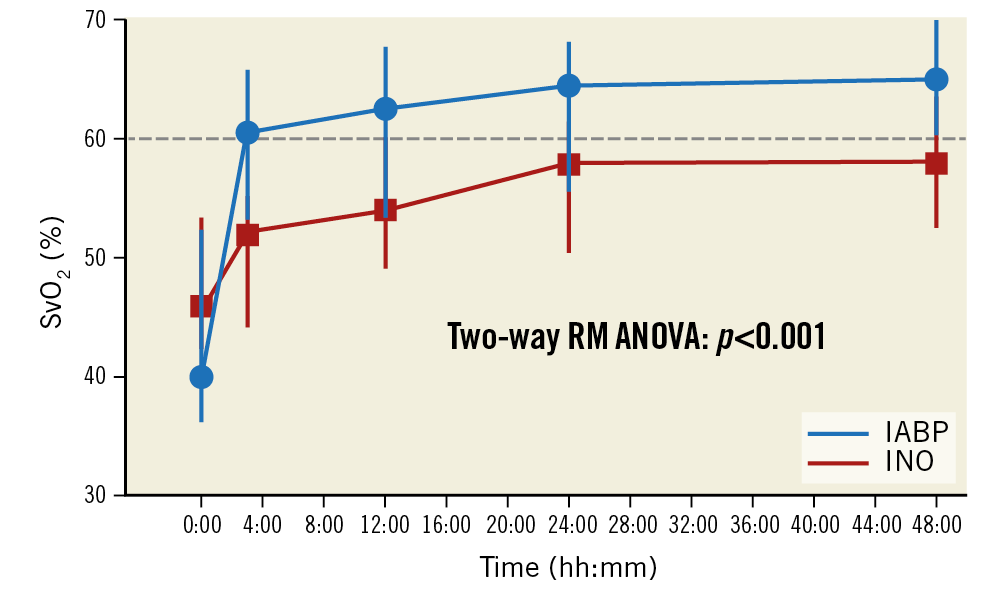
Figure 1. Mixed-venous oxygen saturation over time. Data represent median (interquartile range). Changes over time between the two groups were tested with two-way repeated measures (RM) ANOVA, where Sidak’s multiple comparisons test indicated statistical significance between the two groups for time points T3h, T24h, and T48h. The reference line was set at SvO2=60% to indicate the therapeutic target.
SECONDARY ENDPOINTS
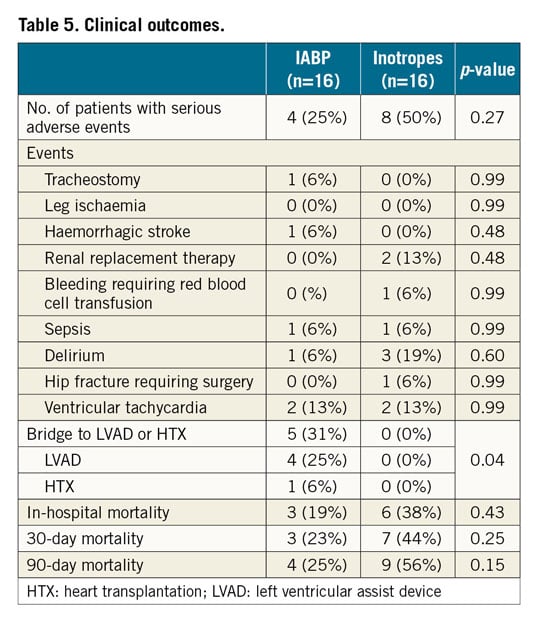
All patients survived the first 48 hours after randomisation. All patients had all measurements taken unless otherwise indicated (Table 1-Table 5). IABP patients had a similar CPO at T48h, but had a higher ∆CPO, lower NT-proBNP levels, a greater relative reduction in NT-proBNP levels, a more negative cumulative fluid balance and a greater reduction in dyspnoea severity score. Other secondary endpoints (crossover or other escalation of therapy, and the occurrence of MACE at 30 and 90 days) did not differ significantly between the groups of patients. Two and three patients crossed over from IABP to INO and vice versa, respectively.
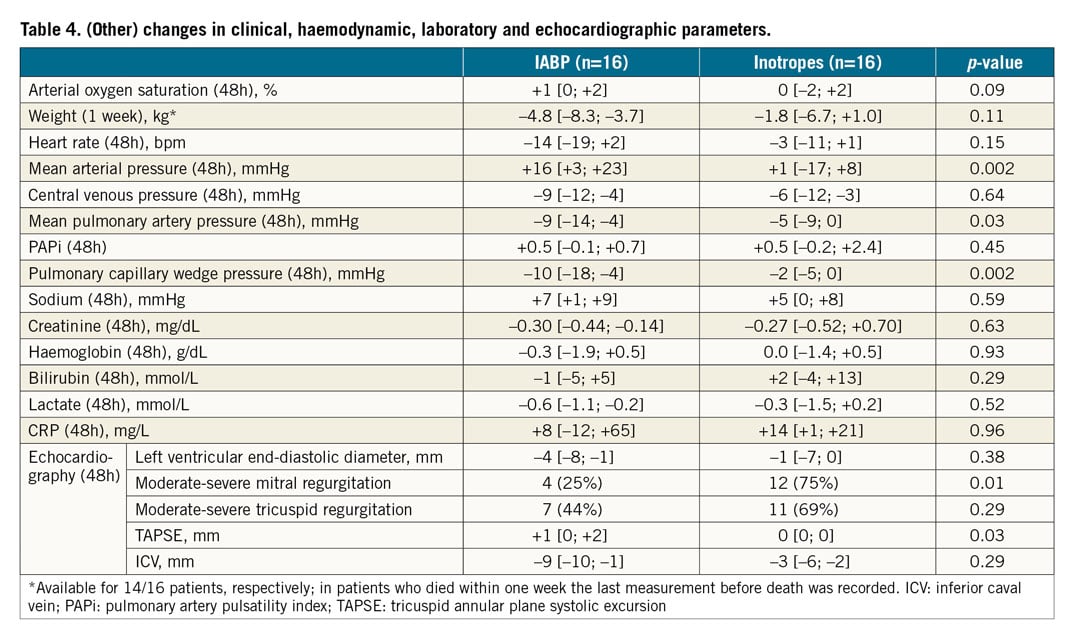
OTHER OUTCOMES
Other changes are listed in Table 4. IABP patients had a greater decrease in mean pulmonary artery and wedge pressure. The prevalence of moderate to severe mitral regurgitation after 48 hours of treatment was lower in IABP patients; right ventricular function (as measured by tricuspid annular plane systolic excursion [TAPSE] and PAPi) did not change significantly. There were no IABP-related SAEs and the total number of patients with SAEs was 4 (25%) in the IABP group vs. 8 (50%) in the INO group (p=0.27) (Table 5). More IABP patients were bridged to durable LVAD or heart transplant (5 [31%] vs. 0 [0%], p<0.05), which was the main reason for the increased length of hospital stay. Thirty-day mortality was 23% (IABP) vs. 44% (INO, p=0.25).
Discussion
This study compared IABP use and inotropes as first-line treatment for non-ischaemic DHF-LO. IABP patients, in contrast to INO patients, immediately achieved an SvO2 >60% which is regarded as an important therapeutic target13. This benefit was sustained beyond three hours, despite the fact that clinicians made titrations in the INO group. IABP-treated patients could be better diuresed and had a more negative fluid balance. Other endpoints and changes in clinical, haemodynamic and laboratory parameters were in favour of primary IABP implantation.
DESIGN AND OUTCOME
All patients had DHF and a low cardiac output, and did not respond well to high-dose intravenous diuretics, a condition where inotropes may be considered2. Patients such as these may receive prolonged inotropic support, preferably low-dose phosphodiesterase inhibition, used as a bridge to (re)introduce chronic heart failure therapy including beta-blockers. This strategy is not evidence-based but was justified in a previous publication14. Although randomised controlled trials in patients with shock from AMI failed to demonstrate improvement in haemodynamic parameters or clinical outcome4,15, a number of uncontrolled studies reported on the use of the IABP as a first-line rescue therapy, after failure of inotropes, in non-ischaemic DHF-LO or CS10,11,16,17,18,19,20,21. However, none of the latter studies was a randomised trial and their results thus indicate current clinical practice. Given the observational findings that early use of mechanical circulatory support may be beneficial in low-output and CS patients22, we designed the current study, in which we decided to start mechanical circulatory support as first-choice therapy (before inotropes were administered) in the IABP arm. The control group received a single inotrope for at least three hours and two control patients were given norepinephrine from the start. We chose SvO2 as the primary outcome measure, since (restoring) the balance between oxygen delivery and consumption may be a more important and better reproducible target than, for example, cardiac index in DHF23,24. Since we reported a greater reduction in dyspnoea scores at T48h in IABP patients, the IABP was generally well tolerated. The trial was not designed to test for differences in clinical outcomes, including MACE. Beforehand, we were convinced that crossover or escalation of therapy would be of major clinical relevance, particularly in view of the lack of evidence that IABP has a benefit in the patients whom we described; we therefore included these items in the definition of MACE.
PROCEDURES
We used 50 cc balloon pump catheters, since Kapur et al showed that the haemodynamic support was greater as compared to the previously used 30-40 cc balloon catheters25. In INO patients, we primarily used a relatively low continuous infusion rate of enoximone. Enoximone has a better safety profile than dobutamine26, and is more effective in patients pre-treated with beta-blockers. In addition, enoximone has a fast onset of action of one hour after starting a maintenance infusion without a loading bolus, where the peak effect is observed after two to three hours27. According to our local practice, we did not give a loading dose or a higher infusion rate, because we wanted to prevent drops in mean arterial pressure that may have necessitated administration of norepinephrine.
IABP - MECHANISMS OF ACTION
This manuscript shows the benefit of IABP over inotropes in reducing pulmonary capillary wedge pressure, mean pulmonary artery pressure and the prevalence of severe MR after 48 hours. Studies on ventricular dynamics during counterpulsation report that LV unloading occurs more extensively at lower ejection fractions28,29. Starting support immediately reduces stroke work, possibly decreasing myocardial oxygen consumption. Counterpulsation decreases LV afterload, preload and intraventricular dyssynchrony. Stroke volume may rise 18% and there is an increment in cardiac output ranging up to 1.0 L/min, together with an acute increase in LV compliance. Aortic counterpulsation may also improve renal blood flow30. This may be of interest when treating DHF-LO, which is often characterised by insidious onset and sliding progression, triggered by biventricular heart failure and volume overload. One may wonder if the effects, attributed to the IABP in our trial, may in fact be explained by other treatment differences, particularly fluid management. However, this interpretation is unlikely for the following reasons. 1) We included patients who had a neutral or positive fluid balance despite fluid restriction and administration of high-dose intravenous diuretics. This means that our patients had diuretic-resistant DHF, together with a low cardiac output. 2) Although diuretic dosages were down-titrated in both study arms, a more negative fluid balance was reached in the IABP arm at T48h. The primary endpoint (∆SvO2), however, was already assessed at T3h. We therefore cannot imagine that the acute improvement in haemodynamics was caused by inadequate fluid management. The correlation between increased cardiac output, improved renal perfusion and (time-dependent changes in) renal function is, however, complex and should be evaluated in further studies, focusing not only on renal filtration function but also on other components such as renal tubular avidity for fluid and sodium.
CLINICAL OUTCOMES
Interestingly, 30% of the IABP patients in our study were bridged to heart transplant (HTX) or long-term surgical LVAD, in line with other studies31, and several patients may be bridged to recovery11. Three of six INO patients who died in the hospital could not be bridged due to multi-organ failure. No SAEs directly related to the use of the IABP catheter were observed. This observation is in line with the results from the IABP-SHOCK trial4. One haemorrhagic stroke was recorded in the IABP group, in a patient who received only a prophylactic dose of heparin. One hip fracture was recorded in the INO group, due to a fall out of bed from delirium.
Limitations
First, this study was a single-centre open-label study with a small sample size. The study should therefore be considered as a pilot study; the data need to be confirmed in a larger trial, for which the present study provides a good rationale. Second, one could argue that IABP patients seemed sicker up front, given the (non-significant) worse haemodynamic profile at baseline, and thus had more relative benefit to gain. Given the critical nature of our patients and the small sample size, some other variables (including furosemide dosage) were indeed not equal at baseline, although not statistically different. However, the overall finding of improved haemodynamics with the IABP was supported by several secondary endpoints, underlining the validity of both the primary endpoint and the control group. Finally, the results of echocardiography were not validated by an echo core lab.
Conclusions
We demonstrated the safety and haemodynamic efficacy of a strategy of early mechanical circulatory support by IABP. An appropriately powered pivotal trial comparing primary mechanical circulatory support to the current standard of care is required.
|
Impact on daily practice This study highlights a “forgotten” indication for IABP usage. As non-ischaemic DHF-LO was not covered by the IABP-SHOCK II trial, this field lacks the appropriate evidence. Primary IABP implantation caused a clear haemodynamic benefit relative to administration of inotropes, which warrants further investigation by a confirmatory study. |
Acknowledgements
Eric A. Dubois (cardiologist), Ard Struijs (intensivist) and Robert J. van Thiel (intensivist) who critically reviewed the manuscript’s content. DSMB: K. Martijn Akkerhuis, cardiologist (Department of Cardiology) and Sanne E. Hoeks, statistician-epidemiologist (Department of Anesthesiology), both from the Erasmus Medical Center, Rotterdam, the Netherlands.
Conflict of interest statement
The authors have no conflicts of interest to declare.

