
Abstract
Aims: The aim of this study was to evaluate the Impella CP over veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and their impact on left ventricular unloading and end-organ perfusion.
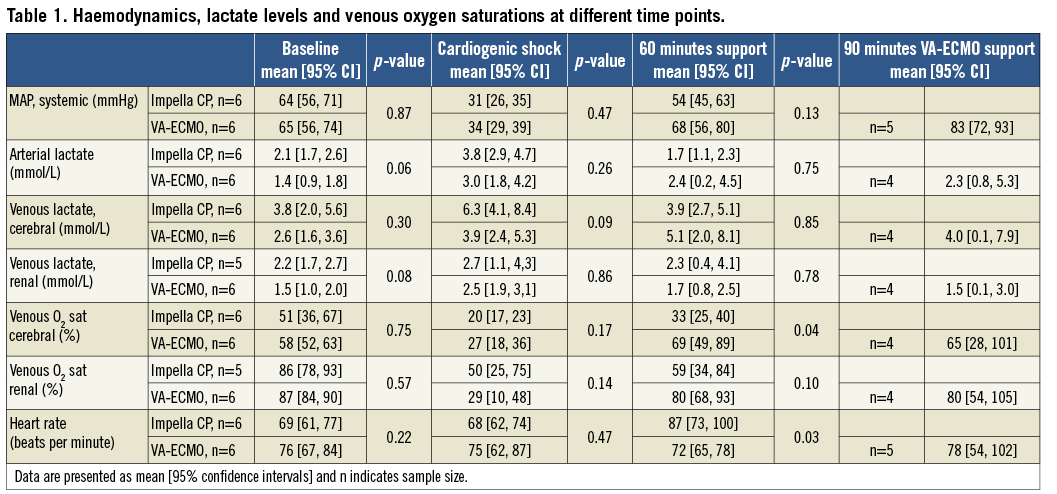
Methods and results: Cardiogenic shock (CS) was induced by injecting microspheres into the left coronary artery in fourteen adult female swine. Impella CP or VA-ECMO was initiated in the presence of CS and evaluated after 60 minutes. Left ventricular pressure-volume area (PVA, total mechanical work) was obtained from a conductance catheter. Results are presented as mean (95% confidence interval) and the rank-sum test was used to assess differences between devices. Compared to the CS state, PVA was unaffected by Impella CP and increased on VA-ECMO (from 2,548 [2,193; 2,904] mmHg x mL during CS to 5,775 [4,451; 7,099], between device p-value=0.02). Arterial lactate increased during CS and decreased on support with no difference between devices. Renal venous oxygen saturation decreased during CS and increased on support with no difference between devices. Cerebral venous oxygen saturation increased to 33% [25, 40] on Impella CP and to 69% [49, 89] on VA-ECMO, p=0.04.
Conclusions: In this porcine model of profound CS, Impella CP unloaded the left ventricle compared to VA-ECMO. Both devices improved end-organ perfusion, with a tendency towards higher venous oxygen saturations on VA-ECMO.
Abbreviations
ESPVR: end-systolic pressure-volume relationship
LV: left ventricle
LVEDP: left ventricular end-diastolic pressure
LVEDV: left ventricular end-diastolic volume
MAP: mean arterial pressure
MCS: mechanical circulatory support
PV: pressure-volume
PVA: pressure-volume area
SW: left ventricular stroke work
VA-ECMO: veno-arterial extracorporeal membrane oxygenation
Introduction
The use of mechanical circulatory support (MCS) in cardiogenic shock (CS) has increased dramatically over the last 15 years. This was in an attempt to improve mortality in CS that continues to be approximately 50%1-3. Use of MCS in CS is currently based on expert opinion; guidelines only recommended MCS to be used in selected patients4,5. Two of the most frequently applied devices for patients admitted with CS are the Impella® CP (Abiomed, Danvers, MA, USA) and veno-arterial extracorporeal membrane oxygenation (VA-ECMO). However, there are no randomised controlled trials to provide evidence on which device or combination of devices, if any, provides optimal support. Current data based on retrospective cohort studies are difficult to interpret, with data suggesting the benefit of left ventricular unloading prior to revascularisation6,7. Further, VA-ECMO on top of an initial unloading device was recently associated with reduced mortality compared to when the two devices were applied simultaneously, emphasising the pertinent issue of improving end-organ perfusion versus ventricular unloading8. Also, patient groups comprise a heterogeneous population in terms of severity of CS and shock progression at the time of admission, impairing the ability to understand the potential benefits and adverse effects of the individual MCS device. This highlights the importance of testing the ability of these devices to provide both organ perfusion and unloading of the left ventricle (LV) under standardised conditions mimicking the clinical scenario of CS. Thus, the primary objective of this study was to compare the Impella CP and VA-ECMO at equal flow rates in a closed-chest large porcine model with profound CS and their effect on the LV as well as the effect on end-organ perfusion. Furthermore, this study also sought to describe the effect of maximising VA-ECMO flow.
Methods
A detailed description of anaesthesia and instrumentation can be found in Supplementary Appendix 1. Experiments were conducted with approval from and in accordance with guidelines from the Danish Animal Experiments Inspectorate (authorisation number: 2016-15-00951). Fourteen Danish Landrace female swine, approximately 18 weeks old and weighing 74.2±2.9 kg, were used.
LV PRESSURE-VOLUME MEASUREMENTS
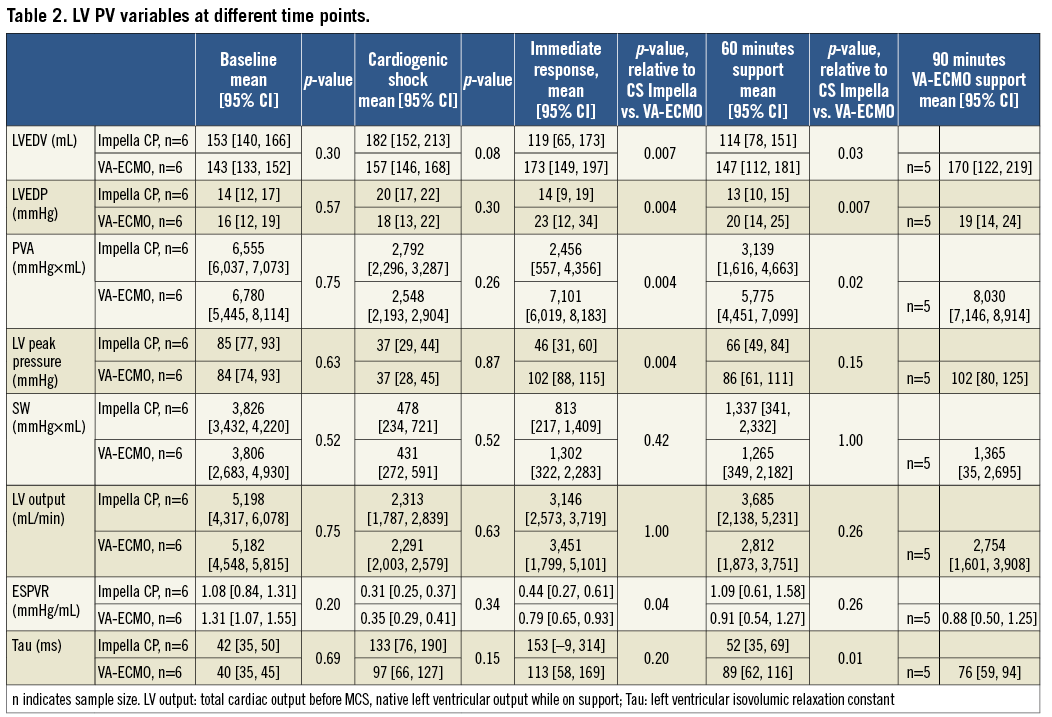
A conductance catheter was inserted through a sheath in the right carotid artery and advanced retrogradely into the LV and connected to an MPVS Ultra® Pressure-Volume (PV) Loop System (Millar Inc., Houston, TX, USA). The MPVS Ultra was connected to a PowerLab 16/35 (ADInstruments, Dunedin, New Zealand) and PV measurements were continuously recorded in LabChart Pro (ADInstruments). Volumes were calibrated using an alpha correctional value9 and parallel wall conductance was determined using the hypertonic saline method. Data from the conductance catheter comprised: pressure-volume area (PVA, mmHg×mL), LV end-diastolic pressure (LVEDP, mmHg), LV end-diastolic volume (LVEDV, mL), LV stroke work (SW, mmHg×mL), LV output (mL/min and representing total cardiac output before and native LV output after MCS is initiated), LV end-systolic pressure-volume relationship (ESPVR, representing contractility), LV peak pressure and the LV isovolumic relaxation constant (Tau, representing ventricular relaxation). The gold standard for estimating ESPVR and PVA is by preload reduction, which was carried out at the study start but not when unsupported profound CS was achieved due to the severely compromised haemodynamics. Further, performing preload reductions during VA-ECMO support would have a major impact on device performance and thereby potentially alter device-derived afterload, making it difficult to extrapolate which component of the heart or device was responsible for changes in ESPVR and PVA. Therefore, baseline preload reductions were performed in each animal and the acquired Vo (the theoretical volume when no pressure is generated) was kept as a constant throughout the study to generate single-beat estimations of ESPVR and PVA (Figure 1)10,11. All other variables were derived automatically from the software.
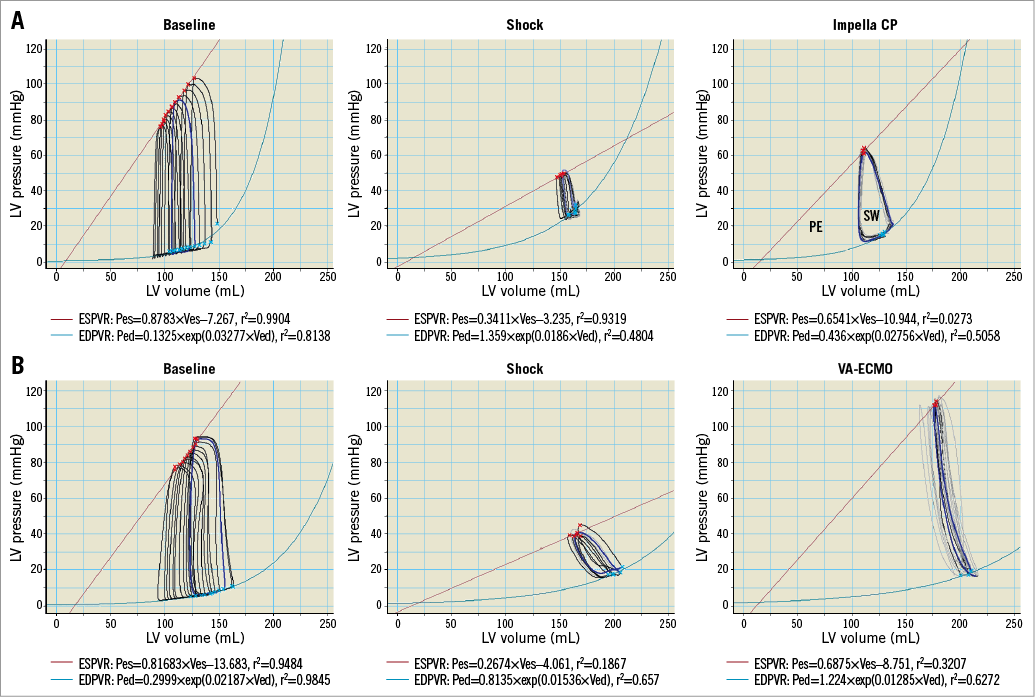
Figure 1. Examples of how the end-systolic (ESPVR, red line) and end-diastolic (EDPVR, light blue line) PV relationships were determined at baseline using preload reduction and during shock and MCS manually selecting loops keeping Vo (theoretical volume when no pressure is generated) attained from baseline as a constant. A) Animal receiving Impella CP. B) Animal receiving VA-ECMO. PE: potential energy, internal energy; SW: stroke work, external energy; PVA is the total mechanical work done by the ventricle per heartbeat and is the sum of PE and SW.
EXPERIMENTAL PROTOCOL
The method for inducing CS has been described previously: in brief, 0.125 g polyvinyl alcohol microspheres (Contour™; Boston Scientific, Marlborough, MA, USA) mixed with 10 mL saline and 10 mL contrast was injected stepwise into the left main coronary artery through a JL 3.5 guide catheter (Launcher; Medtronic, Minneapolis, MN, USA) until the animal had developed profound CS, defined as cardiac output ≤2.0 L/min and/or a mixed venous oxygen saturation ≤35%, and MCS was initiated immediately thereafter12.
The Impella CP was inserted through an introducer sheath in the left femoral artery, advanced retrogradely and placed with the inlet in the LV and outlet in the ascending aorta. The pump speed was set to P-8 and maintained for 60 minutes before withdrawing support. The venous cannula for the VA-ECMO was placed from the right femoral vein with the inlet just distal to the inferior vena cava ostium, and the arterial cannula was placed from the left femoral artery with the outlet in the left common iliac artery. Pump flow was set at 3.2 L/min for 60 minutes and then increased to maximum flow for 30 minutes before withdrawing support.
DATA ACQUISITION
Arterial as well as cerebral and renal venous blood gases for oxygen saturation and lactate measurements were sampled at baseline, when CS had been induced, and after 60 minutes of circulatory support as well as 30 minutes after maximising VA-ECMO flow. PV relationships were determined at baseline, when CS had developed, immediately after MCS had been initiated and stabilised, every 10 minutes for LVEDP and LVEDV post MCS initiation, after 30 and 60 minutes of circulatory support for PVA, ESPVR, Tau, SW, LV output, and 30 minutes after maximising VA-ECMO flow. Heart rate and systemic blood pressure were recorded before administration of each microsphere bolus, and then every 15 minutes throughout the study.
STATISTICAL ANALYSIS
Data are presented as mean (95% confidence interval) with error bars in the two figures of LVEDV and LVEDP over time representing standard error of the mean. Given the uncertainty of absolute values for ventricular volume using the conductance method, comparison between devices was performed in terms of relative change from the CS state. Given the small sample size, the Wilcoxon rank-sum test was used to assess individual variable differences between the Impella CP and VA-ECMO at different time points. Statistical analyses were performed with Stata/IC 14 (StataCorp, College Station, TX, USA). A p-level ≤0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
CS was successfully induced in all animals. One animal developed intractable ventricular fibrillation two minutes after initiating VA-ECMO and was excluded from the study. Another animal had invalid volume signals from the PV catheter and was also excluded. Two minutes after maximising VA-ECMO flow, one further animal developed irreversible ventricular fibrillation; therefore, data on maximum VA-ECMO flow are reported in four animals.
Animals received 13 [10, 17] mL and 15 [9, 20] mL microsphere solution, p=0.75, in the Impella CP and VA-ECMO group, respectively, and there were no differences between groups in the measured variables at baseline or when CS was induced (Table 1, Table 2). One animal in the VA-ECMO group and all animals in the Impella CP group required norepinephrine 0.01-0.2 µg/kg/min to maintain a mean arterial pressure (MAP) >40 mmHg after CS was established. The one animal in the VA-ECMO group was able to be successfully weaned off norepinephrine shortly after VA-ECMO had been initiated, whereas all animals on Impella CP required norepinephrine throughout the mechanical support. One animal in each group was successfully resuscitated from asystole occurring immediately before initiating circulatory support. Impella CP flow was 3.1 [3.0, 3.2] L/min, borderline significantly lower than the VA-ECMO group: 3.2 [3.2, 3.3] L/min, p=0.05. A flow of 4.6±0.2 L/min was achieved when maximising VA-ECMO flow. Except for one animal in each group, complete circulatory collapse with pulseless electrical activity developed within a few minutes after withdrawal of MCS.
END-ORGAN PERFUSION
Both devices improved MAP although the need for norepinephrine was greater for Impella animals. Markers of end-organ perfusion improved in both groups, with a trend towards greater improvement in regional venous oxygen saturation on VA-ECMO support (Table 1). Maximising VA-ECMO seemed to increase MAP but had no effect on markers of end-organ perfusion.
LV UNLOADING
PV data are summarised in Table 2 and the repeated measurements of LVEDV and LVEDP throughout the study period can be found in Figure 2 and Figure 3, respectively. After 60 minutes of MCS, heart rate was significantly higher in the Impella CP group (Table 2). MCS resulted in an immediate decrease in LVEDV and LVEDP in the Impella CP group compared to a tendency to increased LVEDV and an increase in LVEDP in the VA-ECMO group (Figure 4), resulting in a significantly lower PVA in the Impella CP group. These findings were maintained during the initial 60 minutes of circulatory support, during which the diastolic relaxation constant, Tau, only improved in the Impella group. Active SW and native LV volume output showed a tendency towards being increased in both groups after initiating MCS with no statistical difference between groups.
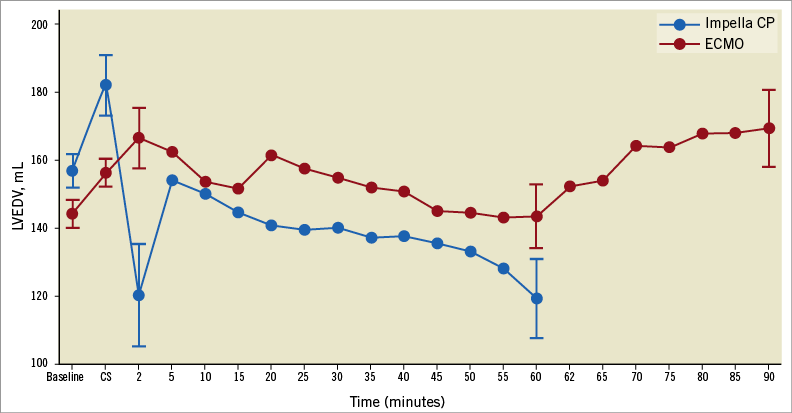
Figure 2. LVEDV over time. Dots represent mean values and error bars standard error of the mean.
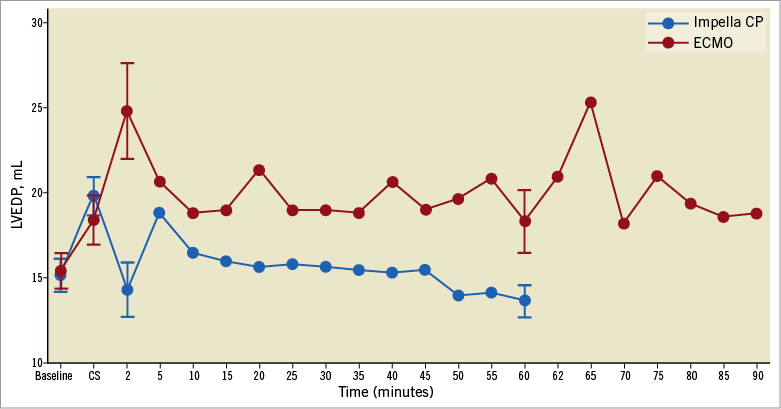
Figure 3. LVEDP over time. Dots represent mean values and error bars standard error of the mean.
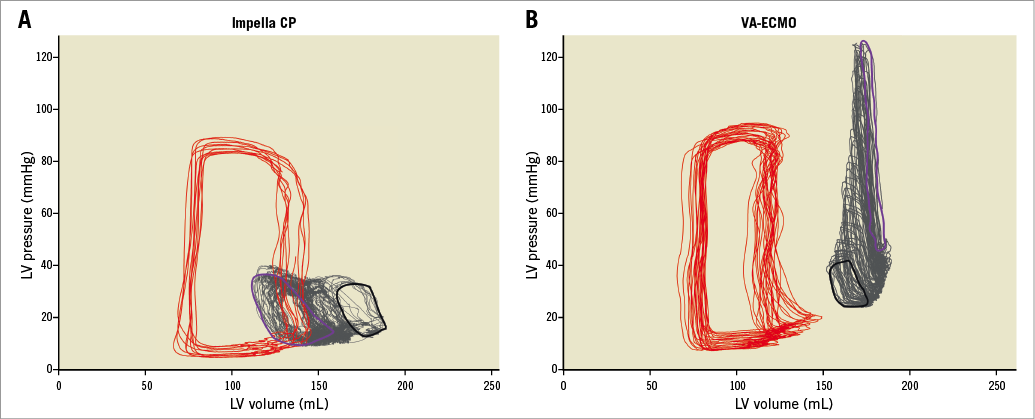
Figure 4. LV PV loop examples of two animals receiving either Impella CP (A) or VA-ECMO (B). Red is a baseline loop, black is CS, grey represents beat-by-beat changes to the immediate response (purple).
Discussion
Circulatory support with Impella CP and norepinephrine more efficiently unloaded the LV compared to peripheral VA-ECMO in a large closed-chest porcine model of profound CS. Both strategies improved organ perfusion by lactate clearance and increased cerebral/renal venous oxygen saturation, with a tendency towards higher venous oxygen saturation on VA-ECMO support.
LV UNLOADING
Theoretically, Impella CP decreases and VA-ECMO increases LV loading and the loading by VA-ECMO is expected to be greater with higher flow, which is why the study was designed to compare the devices at equal flow rates. PVA was chosen as the primary outcome, representing total mechanical (pressure and volume) work per heartbeat and linearly correlated with myocardial oxygen consumption independent of heart rate13. PVA is the sum of all mechanical energy performed by the ventricle per heartbeat and comprises external active forces, stroke work (SW) and internal passive forces, potential energy (PE) as shown in Figure 1A within the Impella CP PV example. Comparing systemic cardiac output during MCS is a challenge, as standard monitoring using a Swan-Ganz catheter in the pulmonary artery is not applicable when VA-ECMO almost completely bypasses the pulmonary circulation. For this reason, organ perfusion was evaluated using lactate levels given their relationship with oxygenation14 and regional venous oxygen saturations given their relationship with blood flow applying the Fick principle.
After an immediate increase in LVEDP and LVEDV in the VA-ECMO group, the LV seemed to adapt as LVEDP and LVEDV gradually decreased over the 60 min (Figure 1). Interestingly, when maximising VA-ECMO flow, LVEDV did not adapt to the same degree as during less flow (Figure 1), and PVA was markedly increased without any real changes in markers of tissue perfusion, meaning that the main effect of maximising VA-ECMO flow was an increased PVA. In accordance with our findings, a porcine study of CS reported that increasing VA-ECMO flow showed tendencies towards increased LVEDV15, but the study did not investigate changes in LVEDV over time as they increased VA-ECMO flow every five minutes. It is difficult to say whether the observed LVEDV adaption during low VA-ECMO flow was due to reductions in systemic vascular resistance, reduction in preload or better contractility in non-ischaemic myocardium. The lack of adaption during full flow speaks against a reduction in preload. The systemic vascular resistance component of afterload is difficult to calculate as total cardiac output during VA-ECMO is difficult to determine accurately and it is likely that the device-related component of afterload was constant since device flow was constant throughout the study. However, trends in LV contractility (ESPVR, Table 2) follow the LVEDV changes in the VA-ECMO animals, indicating that LVEDV adaption during low flow could be due to revived non-ischaemic myocardium.
COMPARING STUDIES OF IMPELLA AND VA-ECMO
To our knowledge, only two studies have compared MCS with the Impella and VA-ECMO head to head16,17 and our study is the first to compare the devices during CS due to LV failure. Kawashima et al compared the Impella LD® (Abiomed), requiring surgical insertion with a maximal flow of 5 L/min, to VA-ECMO at comparable device flow rates in dogs weighing 20 to 24 kg16. They created acute LV failure after sternotomy in dogs with stepwise total LAD occlusion starting distally and moving proximally, testing the devices at each stage. Their study showed that the Impella LD unloads LV more than VA-ECMO, while maintaining systemic blood pressure. Ostadal et al compared the Impella 2.5® (Abiomed) to VA-ECMO at different pump speeds targeting a MAP >70 mmHg in adult female swine weighing 50 to 60 kg17. They created different degrees of LV dysfunction by ventricular pacing from 200 beats/min to ventricular fibrillation. Their study showed that VA-ECMO was superior to the Impella 2.5 during the most severe heart failure condition in terms of maintaining adequate MAP, and that norepinephrine had to be initiated in the Impella 2.5 group to keep MAP ≥40 mmHg during ventricular fibrillation. In agreement with these studies we found that the Impella CP unloads the LV compared to VA-ECMO and that VA-ECMO generates a higher MAP. As opposed to the study by Kawashima et al16 where cardiac output only decreased from 2.46 to 2.08 L/min, the present study tested the devices in a situation of severe shock with a reduction in cardiac output from 5.2 L/min to 2.3 L/min in animals weighing on average 75 kg. This could explain why Kawashima et al16 found no difference between the Impella LD and VA-ECMO in terms of systemic blood pressure. Another explanation could be that the Impella LD is powerful enough to maintain systemic blood pressure in severe heart failure, especially in smaller animals.
END-ORGAN PERFUSION
Successful treatment of CS involves breaking the vicious cycle of myocardial ischaemia, myocardial dysfunction, hypotension and low cardiac output18, and restoring organ perfusion, evident by improved survival in patients clearing arterial lactate19. In our study, LV contractility, defined as ESPVR, improved during MCS in both groups whereas the diastolic parameter Tau only improved in the Impella CP group. Systemic blood pressure and organ perfusion improved in both groups but at the expense of LV loading by higher LVEDV, LVEDP and PVA and thereby oxygen consumption in the VA-ECMO group. This indicates that the Impella CP with norepinephrine and VA-ECMO can improve organ perfusion and LV functions even during severe ischaemia, and that a combination of the Impella CP and norepinephrine does so without increasing myocardial oxygen consumption, which might protect the myocardium against further ischaemic injury20. The fact that all animals treated with the Impella CP required norepinephrine to maintain a MAP >40 mmHg is probably due to the Impella CP being an axial pump that, by design, increases flow but has limited impact on pressure, whereas VA-ECMO is a centrifugal pump that, by design, increases both flow and pressure.
Limitations
A limitation to our study is that we infused microspheres without a proximal occluding balloon, introducing the possibility that some microspheres affected end organs although a minimal effect can be assumed as oxygenation and lactate levels improved during support. Also, the model does not allow the assessment of device impact after reperfusion, and is unsuitable to assess infarct size, as the microcirculation is obstructed. Another limitation is the short ascending aorta of pigs, resulting in the Impella CP outflow being positioned in the aortic arch/descending aorta in at least one animal, possibly limiting device-related perfusion of the cerebrum in affected animals when using a femoral access approach. The seemingly higher arterial and cerebral venous lactate level on VA-ECMO support was driven by a single outlier with coherent low oxygen saturations, possibly due to hypocapnia as arterial pCO2 ranged between 12 and 18 mmHg during the first 60 minutes of MCS in that animal. This is a study on the immediate short-term effect of the Impella CP and VA-ECMO and it is unknown if the loading effects would become exaggerated with long-term MCS. Further, norepinephrine was used in this study, and it is unknown if other vasoactive agents have the same or a different impact on LV loading and end-organ perfusion in combination with the Impella CP. Also, Impella and VA-ECMO are sometimes used in combination when treating CS patients21, but so far it is unknown how the devices in combination affect the LV and end-organ perfusion, indicating the need for future studies.
Conclusions
In this closed-chest porcine model with profound CS due to microsphere injections into the left main coronary artery, a combination of the Impella CP and norepinephrine provided unloading of the LV compared to VA-ECMO. Both devices improved markers of end-organ perfusion, with a trend towards higher local venous oxygen saturations in the VA-ECMO group.
| Impact on daily practice Increasing end-organ perfusion without increasing myocardial oxygen demand is a key element in the treatment of CS but is often a double-edged sword. We demonstrated that both the Impella CP and VA-ECMO are capable of improving end-organ perfusion and the Impella CP does so without increasing myocardial oxygen demand. VA-ECMO might be more powerful in improving organ perfusion, but at the expense of a substantial increase in myocardial oxygen demand. These considerations should be taken into account when MCS is required. |
Acknowledgements
We thank the skilled staff at the Biomedical Laboratory, Odense, Denmark.
Funding
The study was funded by Abiomed and the Danish Heart Foundation.
Conflict of interest statement
J.E. Møller has received research grants from Abiomed. L.O. Jensen has received research grants to her institution from Terumo, Biotronik, St. Jude Medical and Biosensors. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Anaesthesia.
To read the full content of this article, please download the PDF.

