
Abstract
Aims: Mechanical right ventricular (RV) support offers a treatment option for critically ill patients with RV failure (RVF). We developed an assist device for rapid percutaneous implantation. The aim of the present study was to investigate the implantation procedure, haemodynamic performance and possible side effects of the novel right ventricular assist device - PERKAT RV - in an animal model.
Methods and results: The PERkutane KATheterpumptechnologie RV (PERKAT RV) device consists of a nitinol chamber covered by foil containing inflow valves. An outlet tube is attached to its distal part. The system is designed for 18 Fr percutaneous implantation. The chamber is unfolded in the inferior vena cava while the outlet tube bypasses the right heart with the tip in the pulmonary trunk. An IABP balloon is placed inside. Balloon deflation generates blood flow into the chamber; during inflation, blood is guided into the pulmonary arteries. Acute RVF was induced by venous injection of Sephadex in seven sheep for evaluation of the device. The PERKAT RV was able to improve haemodynamics immediately generating a median increase in cardiac output of 59%. Longer pump support was evaluated in a second study. Four sheep were supported for eight hours without any problems.
Conclusions: The percutaneous implantation and explantation of the PERKAT RV device was possible in the designed way. The sheep studies proved beneficial haemodynamic effects in acute RVF. The system offers easy and safe treatment in acute RVF.
Abbreviations
ACT: activated clotting time
ECG: electrocardiogram
ECMO: extracorporeal membrane oxygenation
Fr: French
IABP : intra-aortic balloon pump
IVC: inferior vena cava
PiCCO: pulse contour cardiac output
RP: right percutaneous
RV: right ventricle
RVAD: right ventricular assist device
RVF: right ventricular failure
Introduction
Right ventricular failure (RVF) is still a clinically unsolved problem. The predominant causes of RVF are acute pulmonary embolism, left-sided heart failure, and myocardial infarction. The in-hospital mortality of patients with RVF ranges from 6-17%1,2.
The clinical management of RVF is based on reversal of the underlying cause, volume optimisation to increase RV preload, vasopressor and inotropic support to stabilise haemodynamics, and pulmonary vasodilatation to reduce RV afterload. Acute mechanical circulatory support of the right ventricle should be discussed in cases of profound RVF3. Besides surgically implantable right ventricular assist devices (RVADs), percutaneously delivered circulatory support in RVF is an increasing field with only a few devices presently available. The well-established systems such as veno-arterial extracorporeal membrane oxygenation (ECMO), the TandemHeart (CardiacAssist, Inc., Pittsburgh, PA, USA)4, or the Impella RP® (Abiomed Inc., Danvers, MA, USA)5 show certain limitations. We therefore developed a new percutaneously implantable RVAD - PERKAT® RV. The technical dossier and in vitro performance were published recently6,7. The system is based on a nitinol stent which has to be implanted into the inferior vena cava via an 18 Fr approach and uses the already established intra-aortic balloon pump (IABP) system as its drive unit. In standardised in vitro evaluation, the PERKAT RV device was able to provide circulatory support of up to 3.9 L/min.
The aim of the present study was to investigate the implantation procedure, haemodynamic performance and possible side effects of the novel right ventricular assist device - the PERKAT RV - in an animal model.
Methods
PERKAT RV
The concept, technical dossier, and implantation technique of the PERKAT RV system were published recently6,7.
In brief, the 18 Fr PERKAT RV system consists of a 220 mm nitinol stent cage which is covered by flexible membranes containing foil valves. A flexible outlet trunk with a pigtail tip with outflow valves is attached to the distal part of the stent cage. A standard IABP balloon is placed inside the stent and connected to a standard IABP console as the power unit for the PERKAT RV. Deflation of the IABP generates a slight negative pressure causing blood flow into the stent cage through the foil valves. During balloon inflation, the foil valves will be closed due to the pressure created by the helium-filled IABP balloon. This creates a pulsatile blood flow through the flexible outlet tube towards the pigtail ending. The novel foil valve concept enables a given direction of blood flow. The PERKAT RV is designed for percutaneous sheathless implantation (Figure 1).
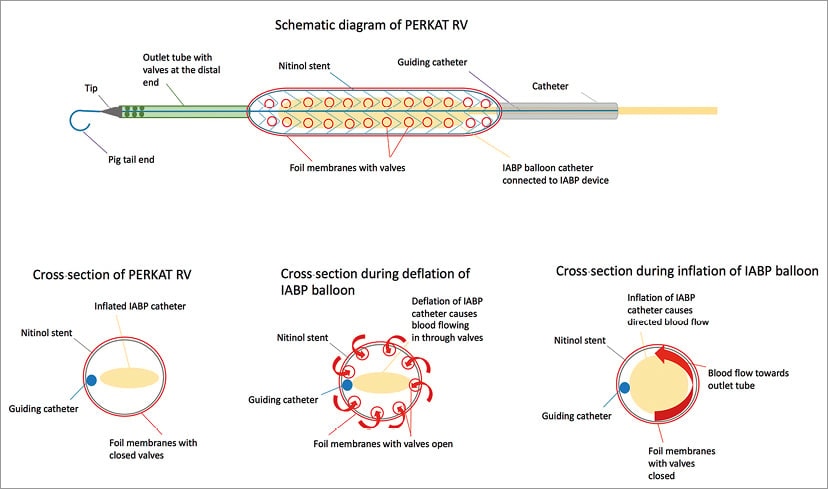
Figure 1. Schematic drawing of the PERKAT RV.
ANIMALS
Adult female sheep (15 Merino animals; two to six years old [3.64±0.34 years, mean±SEM]; 55-85 kg body weight [69.33±2.55 kg]) were used. Permission for the animal experiments was obtained from the governmental commission for animal protection, Free State of Thuringia, Germany (registration number: 02-048/14). All animals received appropriate care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996).
ANAESTHESIA AND SURGICAL TECHNIQUE
After food withdrawal for 24 hrs, anaesthesia was induced by intramuscular injection of 10-15 mg·kg−1 ketamine (Ketavet®; CP-Pharma, Burgdorf, Germany) and 0.2 mg·kg−1 midazolam (Midazolam-Hameln®; Hameln Pharmaceuticals, Hamelin, Germany). Following orotracheal intubation, an inhalation anaesthesia was induced (isofluorane 1.5-1.8 vol% [Isofluran-CP®; CP-Pharma], propofol 0.2 mg/kg/hr i.v. [Narcofol 10 mg/ml; CP-Pharma]). Perioperative analgesia was induced by intravenous injection of 0.1 mg/kg fentanyl before the operation (Fentadon®; Albrecht GmbH, Aulendorf, Germany). A venous catheter was implanted in both jugular veins for intraoperative administration of analgesics and volume substitution, which was performed by supply of Ringer’s lactate solution (10 ml/kg/hr) (Fresenius Kabi Deutschland GmbH, Langenhagen, Germany). Analgesics (0.1 mg/kg fentanyl [Fentadon]) were administered upon need or hourly.
All sheep were additionally instrumented with vascular sheaths inserted into both femoral arteries for blood sampling and blood pressure measurement and into both femoral veins (Radifocus® Introducer II, 6 Fr/7 Fr; Terumo, Tokyo, Japan) for implantation of the PERKAT RV. Arterial and venous blood pressure was recorded using transducers (Combitrans Transducer®; Braun, Melsungen, Germany).
Electrocardiograms (ECG) were derived using cutaneous adhesive electrodes. Heparin (70 IU/kg bw; Rotexmedica GmbH, Trittau, Germany) was administered intravenously before starting the procedure.
Blood gases and lactate were measured once per hour on a standard clinical blood gas analyser (ABL 600; Radiometer GmbH, Willich, Germany).
After the experiment, animals were euthanised by intravenous injection of pentobarbital sodium (Narcoren®; Merial, Hallbergmoos, Germany).
IMPLANTATION PROCEDURE
After the animals were anaesthetised, the femoral vessels on both sides were provided with sheaths (6 Fr for the arteries and 7 Fr for the veins) using the percutaneous Seldinger technique. Then, 5,000 IU of heparin were intravenously administered. Via both femoral veins, two 5 Fr standard pigtail catheters (Boston Scientific, Marlborough, MA, USA) were placed in the pulmonary trunk using a standard 0.035-inch J-tip wire (EMERALD® PTFE guidewire; Cordis, Cardinal Health, Milpitas, CA, USA). The first catheter was used for monitoring pulmonary artery pressure and application of Sephadex® (if indicated) (Sigma-Aldrich, St. Louis, MS, USA). The second pigtail catheter was used to insert a super stiff 0.035-inch wire (Amplatz Super Stiff™; Boston Scientific). After the placement of the super stiff wire, the sheath was removed and the femoral vein access dilated using 16, 18, 20, and 22 Fr dilators (Cook Medical, Bloomington, IN, USA). The flushed PERKAT RV device was then inserted, and the pumping chamber deployed in the inferior vena cava using fluoroscopy guidance (Figure 2). After removing the super stiff wire, a standard IABP balloon (34 cc Sensation; Maquet Getinge Group, Rastatt, Germany) was inserted into the pumping chamber of the PERKAT RV pump and connected to a standard IABP console (Datascope System 97; Maquet Getinge Group) and the pump support was launched. Another 5,000 IU of heparin were administered and a 500 ml infusion of sodium chloride 0.9% with 7,500 IU of heparin was connected to the side port of the system and started with a speed of 125 ml/hr. We aimed for an activated clotting time (ACT) of more than 250 seconds.
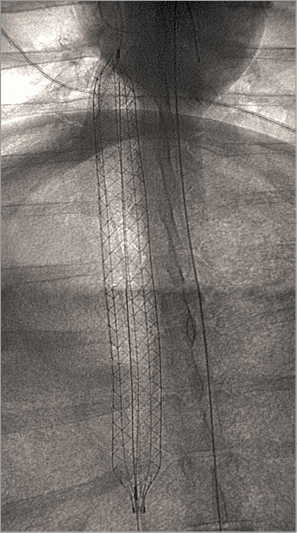
Figure 2. Positioning of the PERKAT RV in the inferior vena cava of a sheep.
Resting haemodynamics were measured before PERKAT RV implantation and then every 30 to 60 minutes.
INDUCTION OF PULMONARY EMBOLISM
In order to investigate the PERKAT RV in haemodynamically compromised animals, a pulmonary embolism was induced. Macroscopic beads synthetically derived from the polysaccharide dextran (Sephadex, size 100 µm) were dissolved in sodium chloride 0.9% (0.5 g Sephadex in 50 ml sodium chloride 0.9%); 10 ml of this solution were injected into the pulmonary trunk via a pigtail catheter. After each application, haemodynamics were measured. We defined “haemodynamically compromised” as sheep with a cardiac output <2.5 L/min and/or with a reduction of at least 50% in comparison to the initial value.
8-HOUR EXPERIMENT
To study the device for a longer time period, it was implanted in healthy sheep without induction of a pulmonary embolism. Haemodynamics were again monitored before PERKAT RV implantation and then every 60 minutes. After eight hours the device was explanted and screened for possible thrombus formation. The animals were then sacrificed.
HAEMODYNAMICS
In order to study the haemodynamic effects of the PERKAT RV, detailed haemodynamic measurements were performed. We recorded invasive blood pressure, heart rate, mean pulmonary pressure, and cardiac output at the start, after each Sephadex injection, and at the end of each experiment. In the eight-hour evaluation of the PERKAT RV, these parameters were recorded at the beginning, after device implantation, and every 60 minutes. Cardiac output was measured using the pulse contour cardiac output (PiCCO) method. For each parameter we registered three consecutive values.
LABORATORY EVALUATION
To evaluate the haemolytic potential of the PERKAT RV, blood was drawn before implantation of the device and at the end of every experiment. We measured the following parameters: haemoglobin, free haemoglobin, lactate, lactate dehydrogenase (LDH), potassium, and thrombocytes.
Results
SHEEP EXPERIMENT
The first three animals were used to study the handling, usability and functionality as well as the implantation procedure of the PERKAT RV and to establish the artificial induction of the pulmonary embolism.
PULMONARY EMBOLISM
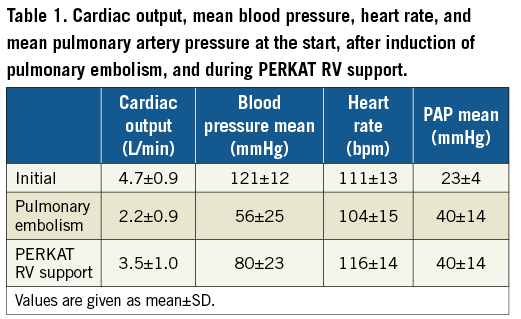
The PERKAT RV was studied in eight sheep with acute right ventricular failure due to severe pulmonary embolism. One animal died after a Sephadex injection due to ongoing ventricular fibrillation. The PERKAT RV was therefore not implanted in this sheep. In the other seven animals, the system could be implanted without any difficulties within a few minutes. The initial cardiac output in these sheep was 4.7 L/min (±0.9 L/min); after the induction of pulmonary embolism, cardiac output decreased to 2.2 L/min (±0.9 L/min). The PERKAT RV was able to stabilise the animals with an increase of 59% to a mean cardiac output of 3.5 L/min (±1.0 L/min) (Figure 3). The other haemodynamic results are summarised in Table 1.
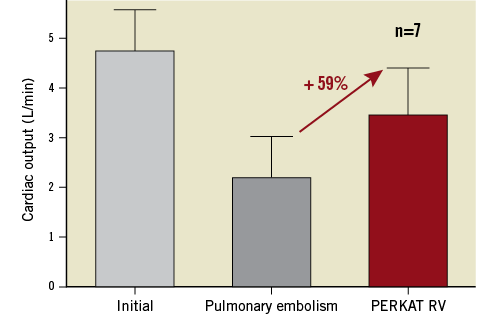
Figure 3. Cardiac output (L/min) of seven sheep at baseline, after induction of pulmonary embolism, and after stabilisation with the PERKAT RV. Values are given as mean±SD.
8-HOUR EXPERIMENT
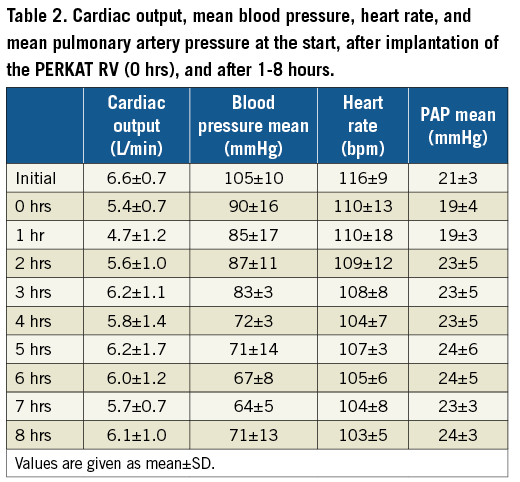
We were able to implant the PERKAT RV in all four animals without any complications. Haemodynamics remained stable over the whole period of eight hours (Table 2). The slight drop in mean blood pressure was caused by the intensification of the anaesthesia by the veterinary surgeon. The other parameters (cardiac output, heart rate, mean PA pressure) remained stable.
At the end of the experiment, the system was removed without any complications. Only very small thrombi were detected on the surface of the PERKAT RV (Figure 4).

Figure 4. The PERKAT RV stent system after an in vivo pumping period of 8 hours.
LABORATORY EVALUATION
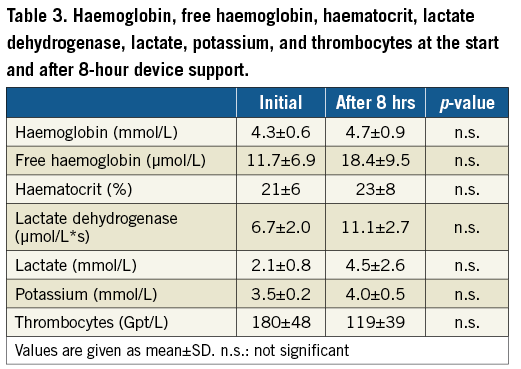
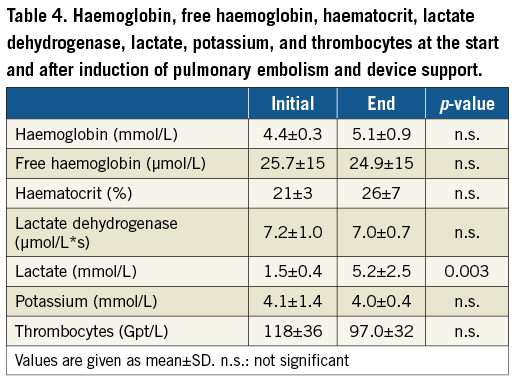
Laboratory values of the acute and 8-hour experiment are shown in Table 3 and Table 4. In the acute setting, lactate was significantly higher at the end of the experiment. The other parameters were comparable between the two measurements. There was a trend towards higher LDH and lower platelets during circulatory support with the PERKAT RV in the 8-hour setting.
Discussion
In the present study we could prove that the percutaneous implantation and explantation procedure of the PERKAT RV was successful in terms of functionality and usability. Furthermore, the device was able to stabilise haemodynamically impaired sheep in a setting of acute pulmonary embolism. This was measured as an increase in cardiac output of 59%. In addition, the PERKAT RV was used for eight hours without any complications in four sheep.
Severe RVF is an increasing clinical problem and leads to a rise in morbidity and mortality. In the case of profound RVF, surgically implantable assist devices have been used as a last resort therapy. However, these devices involve the risks of haemolysis, thromboembolic events, and infection. In addition, outcome results of surgical RVADs are inconclusive8. Percutaneously implantable RVADs are therefore a reasonable treatment option. There are only a few systems on the market at the moment such as the Impella RP9 and the TandemHeart10. In comparison to the TandemHeart, the PERKAT RV is implantable using only one femoral vein without the need for arterial access. The PERKAT RV requires an 18 Fr access whereas the TandemHeart needs two 21 Fr cannulas10 and the Impella RP5 is designed to be implanted via a 23 Fr sheath. After induction of the pulmonary embolism in sheep, cardiac output went down to 2.2 L/min. The PERKAT RV system was able to stabilise these severely impaired animals haemodynamically and the cardiac output increased to 3.5 L/min. This is comparable to the findings of the RECOVER RIGHT study where the Impella RP system improved haemodynamics in a range from 1.8-3.3 L/min/m² 9. The TandemHeart system was able to increase the cardiac index by 0.5 L/min/m2 10. Besides the benefits in haemodynamics, the safety issues of RVADs have to be studied. We saw one mild groin bleeding but could not detect any severe bleedings. The haemoglobin values remained stable over the whole experiment. In contrast to our results, the Impella RP was associated with 60% major bleeding events9. Kapur et al10 reported a statistically significant drop in haemoglobin of 1.4 g/dL and a major bleeding rate of 41% in the percutaneous group. However, due to the fact that all sheep in our study were sacrificed at the end of the experiment, these data have to be compared with caution. We cannot exclude that bleeding would have occurred after device explantation to some degree. Furthermore, we measured a trend towards a slight decrease in thrombocytes in the 11 reported animals. This is in concordance with the TandemHeart system where a significant drop in thrombocytes was reported10. The authors of the RECOVER RIGHT study reported a haemolysis rate of 13.3%9. The clinical relevance of this issue is still unclear. Again, the comparability of our data to both clinical trials is limited. The coagulation system of sheep differs significantly from that of humans11.
Study limitations
The data reported in this study were collected in a small number of animals, seven sheep in the acute and four sheep in the so-called “8-hour” experiments. Further evaluation of the system in sheep is ongoing. In addition, there are several reasons for severe RVF, as discussed above. We evaluated the PERKAT RV system in this reported study in the context of acute pulmonary embolism.
Conclusions
In this paper we present the first in vivo evaluation of the PERKAT RV system. The handling and usability of the PERKAT RV during percutaneous implantation and explantation were demonstrated. After initiation of pump support, the PERKAT RV led to a significant increase in cardiac output in haemodynamically severely impaired sheep. Based on these findings, further research and work should now focus on implementing the results for application in human patients in the future.
| Impact on daily practice The PERKAT RV is a novel percutaneous right ventricular assist device. It offers sufficient haemodynamic support in haemodynamically impaired sheep. We therefore believe that the PERKAT RV could be a potential treatment option for haemodynamically compromised patients with right heart failure. |
Acknowledgements
The PERKAT RV device was developed and designed in co-operation with NovaPump GmbH (Jena, Germany). We thank Ronald Reich, Joerg Pfeifer, Patrick Patzer, and Yu-Jin Heinekamp. The team from NovaPump has contributed significantly to the in vivo testing of the PERKAT RV and has been working closely with the authors to implement the suggestions for improved handling of the device.
Funding
This study was supported by the German Ministry of Education and Research (BMBF-PERKAT-13GW0013C).
Conflict of interest statement
M.W. Ferrari is co-founder and a shareholder in NovaPump. The other authors have no conflicts of interest to declare.

