
Abstract
Aims: We aimed to provide a systematic review of catheter-based therapies in acute pulmonary embolism (PE).
Methods and results: Studies published in peer-reviewed journals before February 2017 were included and categorised according to the mechanism of thrombus removal: fragmentation, rheolytic therapy, aspiration or catheter-directed thrombolysis. Strengths, challenges and the level of evidence of each device were evaluated. We found 16 different catheter-based therapies for acute PE, all but one being used off-label. The majority of procedures involve catheter-directed thrombolysis. Aspiration therapy shows promise, but limited data are available. Rheolytic therapy should be used with caution, if at all, due to the high number of associated complications.
Conclusions: Catheter-based therapies show promise as a treatment for acute PE, though evidence is lacking. Further research into the efficacy and safety of devices is needed.
Abbreviations
CDT: catheter-directed thrombolysis
PA: pulmonary artery
PAP: pulmonary arterial pressure
PE: pulmonary embolism
PVR: pulmonary vascular resistance
RV: right ventricle
UFH: unfractionated heparin
USAT: ultrasound-assisted catheter-directed thrombolysis
Introduction
Acute pulmonary embolism (PE) is a common disease with an estimated incidence of 95/100,000/year causing more than 500,000 deaths/year in Europe and a similar number in the USA1. This makes acute PE the third most common cardiovascular cause of death after heart attack and stroke2.
The presentation of acute PE may vary from subtle changes in functional capacity to haemodynamic collapse and death depending on the thromboembolic mass obstructing the pulmonary circulation, the associated vasoconstrictor response of the pulmonary arteries (PA), and the ability of the right ventricle (RV) to overcome the acute pressure overload.
Systemic thrombolysis provides a rapid decrease in thrombus burden, unloading of the RV and, theoretically, prevention of acute RV failure, circulatory collapse and death3-5. Treatment with systemic thrombolysis is associated with an increased risk of major bleeding (13%) and stroke (3%)4. In high-risk PE patients, the beneficial effects of thrombolysis surpass the risk of bleeding5. In intermediate-risk patients, however, the beneficial effects of thrombolysis do not balance the bleeding risk6. While 1/3 of PE patients have contraindications to thrombolysis, even in patients eligible for thrombolysis up to 2/3 do not receive the treatment3. Surgical embolectomy may be an option for a fraction of these patients. The poor preoperative state together with a high degree of comorbidity, however, make the majority poor candidates for surgery. This leaves a substantial proportion of PE patients undertreated.
Novel catheter-based approaches may provide effective thrombus removal without the risks associated with systemic thrombolytic or surgical treatment7. Numerous new techniques have been developed and refined during the last decade. Unfortunately, randomised clinical trials remain rare, so off-label use is common and case series dominate the literature. In this systematic review we provide an overview of all catheter-based therapies available to treat acute PE, outlining the strengths and challenges as well as the evidence behind each device.
Methods
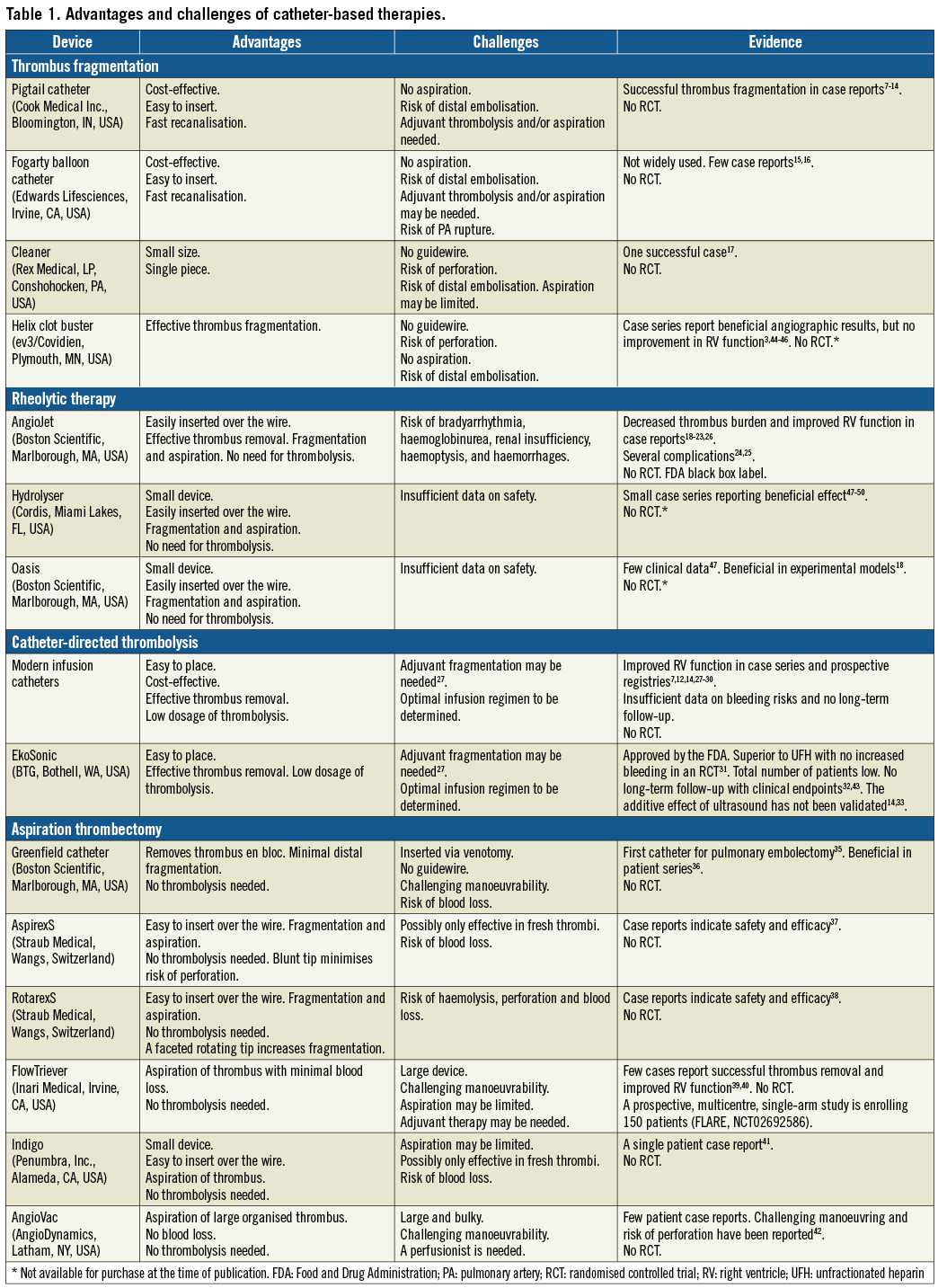
We performed a systematic literature search of the scientific databases PubMed and Embase using both Mesh and free search with the following key words: “pulmonary embolism”, “catheter”, “device”, “catheter-directed”, “catheter-based”, “trans-catheter”. Abstracts were evaluated and studies included if they met the following criteria: (1) studied acute PE, (2) studied catheter-based therapies. We included both clinical and experimental studies published in peer-reviewed journals before February 2017. No language restrictions were applied. References from review articles, not found by the initial search, were also included. A secondary search was performed combining device names with “pulmonary embolism”. We categorised studies according to the device investigated and the mechanism of thrombus removal: mechanical fragmentation, rheolytic therapy, catheter-directed thrombolysis (CDT) and aspiration (Figure 1, Figure 2). All studies were evaluated to determine the strengths and challenges of each device and the evidence supporting its use in acute PE (Table 1).
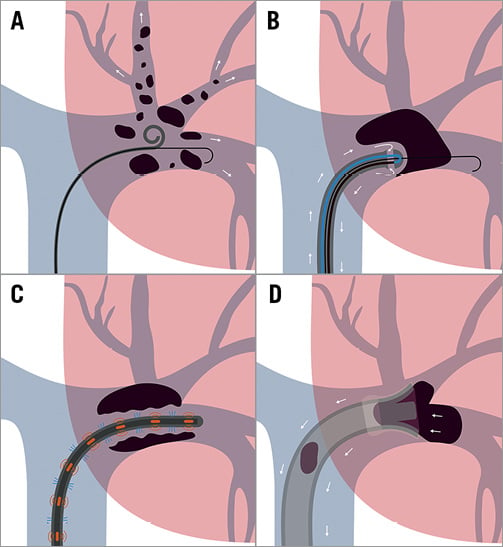
Figure 1. Mechanisms of catheter-based therapies for acute PE. Schematic illustration of the four mechanisms of catheter-directed therapy for acute PE. A) Fragmentation. B) Rheolytic therapy. C) Catheter-directed thrombolysis. D) Aspiration.
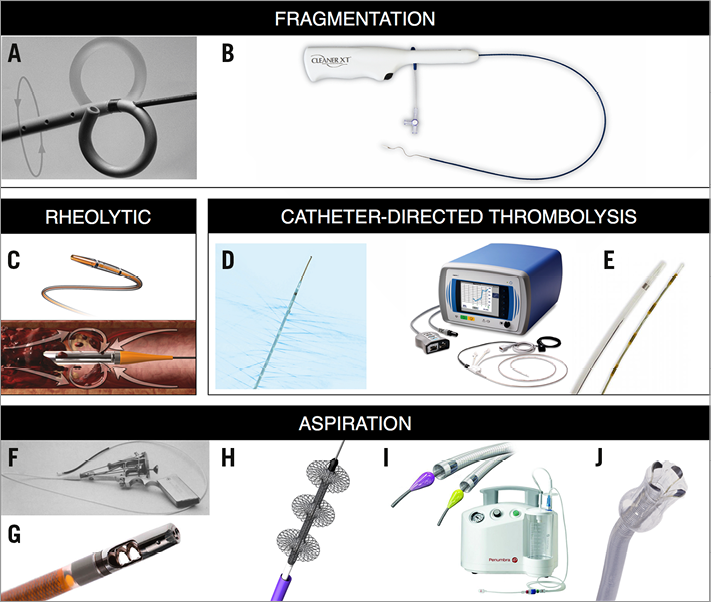
Figure 2. Images of catheter-based therapies for acute PE. A) Rotational pigtail catheter. B) CleanerXT. C) AngioJet. D) Uni*Fuse. E) EkoSonic. F) Greenfield. G) AspirexS. H) FlowTriever. I) Indigo. J) AngioVac. The manufacturers and copyright holders of all devices mentioned in this article, except Edwards Lifesciences (Fogarty catheter), have given permission to reproduce the images of their devices.
Results
MECHANICAL FRAGMENTATION
Mechanical fragmentation is based on the principle that haemodynamics can be improved by converting a large embolic mass into smaller emboli, which then travel distally to smaller pulmonary vessels (Figure 1A). Theoretically, this decreases mechanical obstruction and makes the emboli more accessible for endogenous fibrinolysis. A challenge is that distal embolisation may cause an acute, paradoxical elevation of pulmonary vascular resistance (PVR) and actually increase strain on the RV8.
ROTATIONAL PIGTAIL CATHETER®
The rotational pigtail catheter (Cook Medical, Bloomington, IN, USA) was modified by adding a side hole allowing the passage of a guidewire9. The guidewire serves as the central axis around which the pigtail catheter can be rotated manually thereby fragmenting the embolus. Studies have shown successful recanalisation and decreased pulmonary artery pressures (PAP) and shock index7,9-14. The strategy is cost-effective, easy to insert and is readily available in most centres, making it one of the most commonly used catheter-based therapies for acute PE7.
FOGARTY ARTERIAL BALLOON EMBOLECTOMY CATHETER®
The Fogarty arterial balloon embolectomy catheter (Edwards Lifesciences, Irvine, CA, USA) is placed in the embolus and the balloon is inflated, leading to recanalisation of the PA15,16. To minimise the risk of PA rupture, an undersized balloon is recommended. However, concerns regarding residual thrombus and distal embolisation remain, which is why balloon angioplasty should generally be combined with either aspiration or CDT.
CLEANERtm
The Cleaner rotational thrombectomy system (Rex Medical, Conshohocken, PA, USA) is placed in the thrombus and the flexible sinusoid tip can be deployed and rotated by a battery-driven motor. The catheter is then slowly withdrawn and removed through the sheath. The design may, however, have a few challenges. Introducing without a guidewire could pose a risk of vessel perforation. Furthermore, it is uncertain whether aspiration of thrombus through the small sheath is effective as it causes a risk of distal fragmentation. Only one case has been reported using the Cleaner off-label in a patient with PE17.
RHEOLYTIC THERAPY
Rheolytic catheters have a small lumen that takes a hairpin loop at the tip of the catheter. Through this lumen, an agent, usually heparinised saline, is injected at a high flow rate into a larger exhaust lumen, creating a vortex. When passing the catheter through a thrombus, the vacuum created by the Venturi effect may disrupt and aspirate thrombus (Figure 1B).
ANGIOJET®
The AngioJet (Boston Scientific, Marlborough, MA, USA) is a rheolytic catheter designed for thrombus removal in peripheral vessels. Case reports describe decreased thrombus burden and shock index18-23. Bradyarrhythmia, haemoglobinurea, renal insufficiency, haemoptysis, haemorrhages and procedure-related deaths have been reported24,25. The FDA has therefore labelled the AngioJet with a black box warning for use in the pulmonary circulation. Despite this, it is still being used in some European centres26. A temporary pacemaker and close monitoring of haemodynamics, renal function and electrolyte status are however pivotal.
CATHETER-DIRECTED THROMBOLYSIS
CDT involves direct delivery of a low-dose thrombolytic drug into the PA at the site of the thrombus (Figure 1C). Due to altered flow patterns, it is important that the thrombolytic agent is injected inside the thrombus or that the thrombus is fragmented before injection27. The majority of published case reports describe CDT combined with some degree of fragmentation, most frequently using the pigtail catheter (69%)7,12,28,29. Thrombolysis may be administered by standard infusion catheters, or by newly designed catheters such as the Uni*FuseTM or Pulse*Spray® (both AngioDynamics, Latham, NY, USA) which have special side holes for optimal intra-thrombus drug delivery. Dosing regimens vary between centres and may involve infusion of 0.5-1.0 mg/h alteplase in one or both main PA for up to 24 hours. The total dosage is typically kept under 30 mg, significantly lower than the systemic dose of 100 mg.
A recent prospective multicentre registry of patients with intermediate- and high-risk PE found that CDT improved haemodynamics in most cases without major bleeding14, but the findings were not reproduced in a larger retrospective study30.
ULTRASOUND-ASSISTED CATHETER-DIRECTED THROMBOLYSIS
Ultrasound-assisted catheter-directed thrombolysis (USAT) uses ultrasonic waves purportedly to disrupt the fibrin structure of the thrombus, allowing more binding sites for the thrombolytic agent.
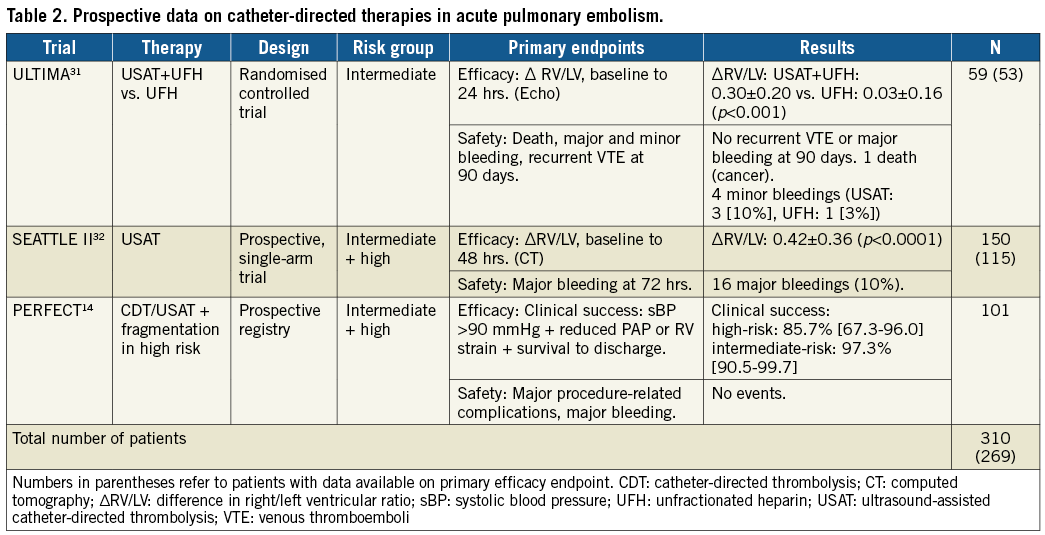
The EkoSonic® Endovascular System (BTG, Bothell, WA, USA) is the only device evaluated in a randomised controlled trial. The ULTIMA trial randomised 59 patients with intermediate-risk PE to unfractionated heparin (UFH) plus a low-dose USAT infusion of alteplase for 15 hours or UFH alone31. This study found USAT to be superior to UFH alone in reducing RV/LV ratio after 24 hours with no increase in bleeding complications. However, at the 90-day follow-up, the RV/LV ratio was equalised between the two groups. These findings were supported by the SEATTLE II study32, a prospective, multicentre, single-arm trial of 150 intermediate- and high-risk patients. The study found that USAT was associated with decreased RV dilatation, PAP and Miller index and a low rate of bleeding complications. A randomised trial (SUNSET sPE, NCT02758574) will investigate whether ultrasound improves outcome compared to non-ultrasound-assisted CDT, which is currently unknown. In patients suffering from deep vein thrombosis, there are no additional beneficial effects of USAT33.
The EkoSonic is currently the only device with an FDA approval for treatment of acute PE34. A randomised trial aiming to determine the optimal dose and time of infusion has just been completed (OPTALYSE PE, NCT02396758). Results are pending, but presentations suggest improvement of RV function with as low as 4 mg infusion over 2 hrs.
ASPIRATION
The goal of aspiration thrombectomy is to decrease thrombus burden without distal embolisation (Figure 1D).
THE GREENFIELD SUCTION CATHETER®
Developed in 1969, the Greenfield suction catheter (Boston Scientific) has a plastic cup at its tip35. By advancing the cup into the PA and manually applying suction with a syringe, the cup can attach to a centrally located thrombus and the thrombus can be removed en bloc with minimal distal embolisation. The device is inserted by a surgical venotomy as insertion is not possible through conventional sheath access. The stiff cup with a 7 mm diameter is difficult to manoeuvre in the PA, especially as it is not compatible with a guidewire. Despite reports of a success rate of 76%36, the catheter has not achieved broad use in clinical practice.
ASPIREX®S AND ROTAREX®S
The AspirexS and RotarexS (Straub Medical, Wangs, Switzerland) have a metal helix that can be rotated inside the catheter to create a vacuum at the tip of the catheter based on the principle of Archimedes’ screw. Thrombus material is thus pulled towards the tip, fragmented and transported via side holes through the catheter. The tip of the AspirexS is fixed and blunt, so fragmentation is limited. The device is therefore only indicated for use with fresh thrombus. The tip of the RotarexS is faceted and rotates together with the helix which enables fragmentation of more rigid thrombus material, but with a risk of damaging the vessel. Both catheters have been used off-label for PE37,38. A drawback of the devices is the potential for large-volume blood loss (130 ml/min with the largest catheter).
FLOWTRIEVERTM
The FlowTriever system (Inari Medical, Irvine, CA, USA) consists of an aspiration catheter and a flow restoration catheter. The aspiration catheter is advanced into the thrombus. The flow restoration catheter is then advanced through the aspiration catheter and three self-expanding nitinol wire discs are deployed into the thrombus. Thrombus is caught in and between the discs, allowing aspiration by retracting the catheter. Challenges may be limited thrombus removal and manoeuvrability. A few patient cases have demonstrated successful thrombus removal without adverse effects39,40. A prospective, multicentre, single-arm study is now enrolling patients. The FlowTriever Pulmonary Embolectomy Clinical Study (FLARE) aims to evaluate the safety and effectiveness of the FlowTriever in 150 patients with acute PE from 20 sites in the USA (clinicaltrials.gov, NCT02692586).
INDIGO®
The Indigo mechanical thrombectomy system (Penumbra, Inc., Alameda, CA, USA) consists of an aspiration catheter, a pump providing suction and a separator wire. When the aspiration catheter is located at the thrombus, the pump creates a vacuum to aspirate thrombus. The catheter can be advanced through the thrombus repeatedly to allow more thrombus removal. A separator wire can be introduced into the aspiration catheter to clear it of obstructing material. The catheter is available with three different tips ranging from straight to angled to improve manoeuvrability. The small diameter, which makes the system easy to insert and manoeuvre, may also be its limitation as aspiration may be limited. To limit blood loss, it is critical that the pump is turned off when the catheter is outside the thrombus. Only a few cases of PE treatment have been reported with this device41.
ANGIOVAC®
The AngioVac (AngioDynamics) is a cannula designed for en bloc removal of venous thrombi during extracorporeal venous-venous bypass, allowing filtration of aspirated blood from thrombi before re-introduction to the systemic circulation. The catheter has a tulip-shaped distal tip that can be expanded by a balloon when situated proximal to the thrombus. The size and shape of the catheter allow aspiration of larger thrombi without clotting the catheter. It has been used off-label in small case series of patients with PE with some success42. The bulkiness of the system does however make it difficult to manoeuvre and may increase the risk of perforation42.
Discussion and limitations
The use of catheter-based therapy for PE is increasing, but the exact number of acute PE patients receiving catheter-based therapy is unknown. In one recent longitudinal report, 9% of patients with acute PE received catheter-based therapy34, but in the majority of hospitals anticoagulation and systemic thrombolysis are the only available treatment options.
We found 16 different catheter-based therapies used in acute PE (Table 1, Figure 2). Prior to 2008, 70% of catheter-based therapies involved mechanical fragmentation by a pigtail catheter7. However, with the FDA approval of the EkoSonic, USAT has surpassed mechanical fragmentation as the preferred catheter-based therapy34,43. Rheolytic catheters were used in greater numbers, but with the black box warning from the FDA the current use is limited7. Aspiration therapy is promising, but devices are still in the early stages of development and data are lacking. It is worth noting that CDT typically requires more than 12 hours of therapy, which may be too long in high-risk patients, suggesting that aspiration therapy with faster thrombus removal may provide a valid treatment option. Assist devices such as the Impella RP® (Abiomed, Danvers, MA, USA) or ECMO may also have a place in the treatment of high-risk patients by serving as a bridge to other treatment options or endogenous thrombolysis.
European guidelines recommend catheter-based therapies as an alternative treatment in patients with high-risk PE and contraindications to or inadequate effect of systemic thrombolysis (Class IIa, level C). In intermediate-risk patients, catheter-based therapies are recommended in patients with a high bleeding risk (Class IIb, level B), with the ULTIMA trial as the only reference emphasising the lack of evidence.
The literature supporting catheter-based therapies in acute PE is mainly from case reports and retrospective patient series, yielding a risk of publication bias. Furthermore, the patient population in these reports may be highly selected. Therefore, the promising success rates and low complication rates reported should be interpreted with care7. The ULTIMA trial is the only randomised controlled trial investigating catheter-based therapies for acute PE31. Together with the uncontrolled SEATTLE II study and the prospective PERFECT registry, the totality of prospective data is limited to around 300 patients (Table 2). Furthermore, these studies all investigated the effects of CDT so, while the evidence supporting this approach is growing, evidence for other techniques is sparse.
The high bleeding risk of systemic thrombolysis limits the clinical use to patients at highest risk of early mortality. If large randomised clinical trials prove that novel catheter-directed therapies provide effective reduction in thrombus burden with a complication rate lower than systemic thrombolysis, it could cause a paradigm shift equal to that of acute myocardial infarction and, in time, render systemic thrombolysis obsolete in the treatment of acute PE. The potential of catheter-based therapies might even be greater in intermediate-risk patients. Currently, there is a gap between the high morbidity and mortality of intermediate-risk PE treated with anticoagulation alone, and the lack of overall benefit of systemic thrombolysis in this patient subgroup6.
We advocate a more systematic approach where clinicians and researchers take the leading role of driving this promising field forward using rigorous scientific inquiry. Ideally, thorough preclinical testing, preferably in large, in vivo animal models, will precede better-designed clinical safety trials, which will then be followed by randomised clinical trials with long follow-up and clinical endpoints.
Another concern is the cost of catheter-based therapies for PE. Pricing of devices varies greatly and can be very high. Due to the few efficacy data and lack of long-term follow-up, it is currently not possible to determine the cost-effectiveness of these novel catheter-directed therapies.
Conclusions
Catheter-based therapies are promising alternatives in patients suffering from acute PE. Currently, there is only one FDA-approved device for the catheter-based treatment of PE, while all other devices are used off-label. The majority of procedures are performed using CDT, either ultrasound-assisted or in combination with mechanical fragmentation. Aspiration therapy shows promise, but limited data are available. Rheolytic therapy should be used with caution, if at all, due to the high number of associated complications. Much more research into the efficacy, safety and cost-effectiveness of these devices is needed.
| Impact on daily practice We provide an overview of catheter-based therapies of acute PE categorised according to the mechanism of thrombus removal: fragmentation, rheolytic therapy, aspiration or catheter-directed thrombolysis. We found 16 different catheter-based therapies for acute PE, all but one being used off-label. Though catheter-based therapies show promise as a treatment for acute PE, further research into the efficacy, safety and cost-effectiveness of devices is needed. |
Funding
Aarhus University Graduate School of Health.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The references can be found in the online version of this article.
To read the full content of this article, please download the PDF.

