
Abstract
Aims: In this single-centre study, we aimed to evaluate the short- and long-term efficacy and safety outcomes of ultrasound-assisted thrombolysis (USAT) performed in patients with acute pulmonary embolism (PE) at intermediate to high risk and high risk (IHR, HR).
Methods and results: The study group comprised 141 retrospectively evaluated patients with PE who underwent USAT. Tissue-type plasminogen activator (t-PA) dosage was 36.1±15.3 mg, and infusion duration was 24.5±8.1 hours. USAT was associated with improvements in echocardiographic measures of right ventricle systolic function, pulmonary arterial (PA) obstruction score, right to left ventricle diameter ratio (RV/LV), right to left atrial diameter ratio and PA pressures, irrespective of the risk (p<0.0001 for all). In-hospital mortality, major and minor bleeding rates were 5.7%, 7.8% and 11.3%, respectively. Follow-up data (median 752 days) were available in all patients. Absolute and % changes in RV/LV and % changes in PA mean pressure were significantly higher in patients younger than 65 years compared with older patients, whereas bleeding, 30-day and long-term mortality were not related to age, t-PA dosage or infusion duration. HR versus IHR increased 30-day mortality.
Conclusions: USAT was associated with improvements in thrombolysis and stabilisation of haemodynamics along with relatively low rates of complications in patients with PE, regardless of the risk status. However, HR still confers a higher short-term mortality. Increasing the t-PA dosage and prolongation of infusion may not offer benefit in USAT treatments.
Abbreviations
ANOVA: (one-way repeated measures) analysis of variance
CDT: catheter-directed treatment
CT: computed tomography
Echo: echocardiography
ESC/ERS: European Society of Cardiology/European Respiratory Society
FDA: Food and Drug Administration
HR: high risk
IHR: intermediate to high risk
ILR: intermediate to low risk
LR: low risk
MPAP: mean pulmonary artery pressure
PA: pulmonary artery
PE: pulmonary embolism
QS: Qanadli score
RA/LA: right to left atrial diameter ratio
RCT: randomised clinical trial
RV/LV: right to left ventricle diameter ratio
St: systolic tissue velocity
TAPSE: tricuspid annular plane systolic excursion
t-PA: tissue-type plasminogen activator
TT: thrombolytic treatment
USAT: ultrasound-assisted thrombolysis
Introduction
Acute pulmonary embolism (PE) has been documented as being the third most frequent lethal cardiovascular disease in the western world1-4. Haemodynamic instability due to right ventricle (RV) dysfunction may develop within the first two days when 30 to 50% of the pulmonary arterial (PA) vessels are occluded by the clot, and increased RV diameter to left ventricle diameter ratio (RV/LV), assessed either by echocardiography (Echo) or computed tomography (CT), has been documented to predict early and midterm clinical worsening in patients with acute PE, even in the absence of haemodynamic instability2-8. European Society of Cardiology/European Respiratory Society (ESC/ERS) 2014 PE Guidelines have recommended an updated risk stratification based on definitions of high-risk (HR), intermediate- to high-risk (IHR), intermediate- to low-risk (ILR) and low-risk (LR) groups2. The patients at HR require urgent reperfusion therapy2. However, intravenous thrombolytic treatment (TT) has been documented to improve haemodynamic status at the expense of an increased risk of major bleeding2,3,8-12.
Although various catheter-directed treatments (CDT) have been reported in patients with PE10-15, only one of these, a novel ultrasound-assisted thrombolysis (USAT) technology (EkoSonic® Endovascular System; EKOS Corporation, Bothell, WA, USA) was tested in a randomised clinical trial (RCT)16. This technology has been reported to facilitate thrombolysis with reduced lytic dosages which leads to a lowered bleeding risk in PE patients at HR and IHR17-28. We previously published our single-centre results on USAT26 and a meta-analysis29 confirming that USAT significantly reduced mean PA pressure (MPAP), RV/LV and obstruction scores with similar mortality and a lower risk of major bleeding as compared to those with systemic TTs.
In this single-centre study, we evaluated the short- and long-term efficacy and safety outcomes following USAT performed in patients with PE at HR or IHR.
Methods
Our study population comprised 141 retrospectively evaluated patients (male 59, female 82, age 61.8±16.2 years) out of the 420 patients who were referred to our tertiary cardiovascular centre with a diagnosis of acute PE and who underwent USAT treatment from October 2012 to January 2018. The diagnosis of acute PE, definitions of the risk groups and provoked or unprovoked PE were based on the criteria as recommended by the ESC/ERS 2014 PE Guidelines2.
Inclusion criteria are given in Supplementary Appendix 1. Baseline risk status was HR or IHR in all included patients. The status was clinically stable IHR without significant PA obstruction, ILR or LR in the remaining 243 patients in whom only anticoagulant treatments were utilised. The initial TT with tissue-type plasminogen activator (t-PA) (mean 42.8±28.8 mg) prior to USAT was performed in 38 patients for several reasons (Supplementary Appendix 1). The use of USAT as a treatment option in acute PE was approved by the National Ministry of Health and reimbursed by the National Social Security Authority. Moreover, the eligibility criteria for USAT were defined by the Institutional Review Board, and the appropriateness of this treatment for each patient was discussed by a multidisciplinary team (Supplementary Appendix 2).
EFFICACY MEASURES
As compared to baseline, improvements in the measures of pressure overload and RV function assessed by Echo, RV/LV, PA obstruction severity and PA diameters quantitated by CT, and invasively assessed PA pressures were used as the surrogates for the acute efficacy of USAT.
CHEST CT (Supplementary Appendix 3)
Images were acquired before and after the USAT procedure using a 64-slice helical CT scanner (Aquilion 64™; Toshiba Medical Systems Corp., Tokyo, Japan) with angiographic contrast material (Omnipaque 350; GE Healthcare, Chicago, IL, USA). The stored images recorded at the time of diagnosis and following the USAT were retrospectively evaluated. For evaluation of PA obstruction, the CT score proposed by Qanadli et al (QS) was used30. The methods for assessment of RV/LV and right to left atrial diameter ratio (RA/LA) have been defined previously2,3,7.
RIGHT HEART CATHETERISATION, PULMONARY ANGIOGRAPHY AND USAT PROCEDURES
Only the femoral venous route with a 6 Fr sheath was used; arterial puncture was avoided. Details of these procedures and management principles are provided in Supplementary Appendix 4.
SAFETY MEASURES
Safety outcomes included minor and non-fatal major bleeding and all-cause mortality, during the in-hospital period and 30 days after USAT. Long-term follow-up data were also included in the analysis for mortality and bleeding outcomes. Definitions of bleeding events are given in Supplementary Appendix 1.
STATISTICAL ANALYSIS (Supplementary Appendix 5)
Continuous variables were expressed as mean and standard deviation, and categorical variables as percentages. The outliers were assessed by box plot and the distribution of variables was assessed by the Shapiro-Wilk test. A one-way repeated measures analysis of variance (ANOVA) was used to determine the temporal sequential changes in RV/LV, MPAP and QS before and after USAT over the course of time. The details of repeated measures multivariate analysis of covariance are also given in Supplementary Appendix 5. All statistical analysis was performed using SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA). All the statistical analyses were performed by a blinded analyst.
RESULTS (Supplementary Appendix 6)
The USAT group represented 33.5% of the overall 420 patients with acute PE. Patient and treatment characteristics are summarised in Table 1. Age was over 80 years in 10.6% of patients. Echo, CT and invasive measures before and after USAT are summarised in Table 2. The mean time delay from baseline CT to post-procedural CT acquisition was 4.7±3 days.
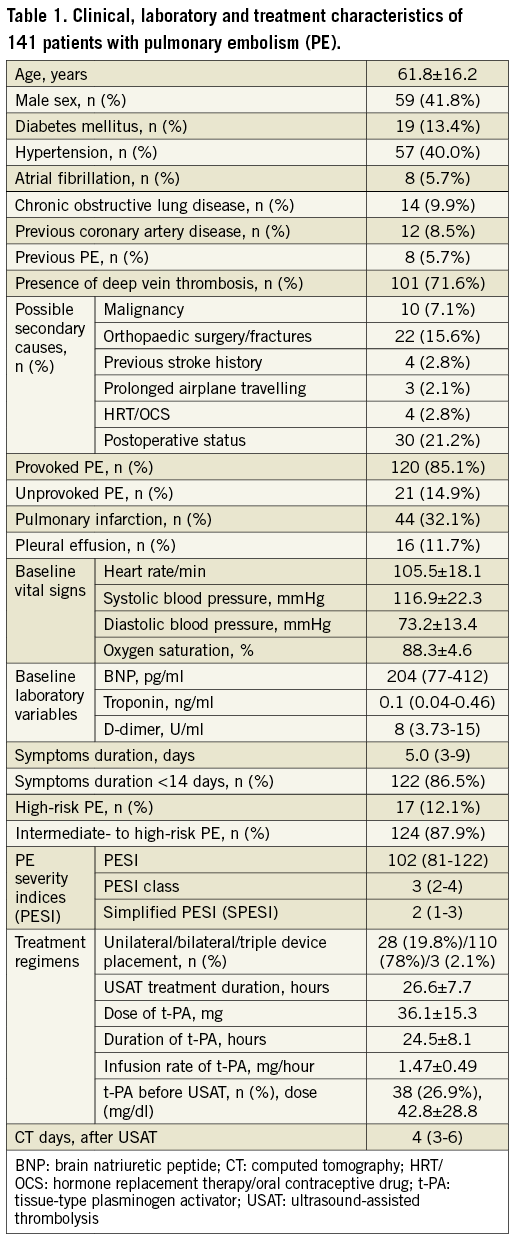

PROCEDURAL CHARACTERISTICS
Bilateral devices were used in 94.1% of patients at HR and in 78.2% of patients at IHR. For unilateral and bilateral treatment, the t-PA dosage (27.1±8.3 vs. 38.4±15.9 mg) and the duration of infusion (22.6±4.7 vs. 25±8.7 hrs) were comparable. Similarly, t-PA dosage (31.4±21 vs. 36.8±14.4 mg) and infusion duration (21±10.2 vs. 25±7.7 hrs) were not different between risk groups (p>0.05).
EFFICACY MEASURES
There were significant differences between pre- and post-USAT Echo, CT and invasive measures (Table 2, Figure 1A-Figure 1C). Absolute and % changes in MPAP, RV/LV and QS after USAT were significant (p<0.001 for all) (Table 2). Repeated measures multivariate analysis of covariance (MANCOVA) for changes in RV/LV, QS and MPAP are shown in Supplementary Table 1.

Figure 1. Changes in mean pulmonary artery pressure (MPAP) before and after USAT. A) Right heart catheterisation. B) Qanadli score (QS). C) Right to left ventricle diameter ratio (RV/LV). All changes were significant (p<0.001 for all).
SHORT- AND LONG-TERM OUTCOMES
In-hospital mortality and bleeding data are summarised in Table 3. During a median six days of hospital stay, eight (5.7%) patients died, and in 3, 4 and 1 of these mortality was due to fatal bleeding, unresolved PE and nosocomial infection, respectively. Bleeding was noted in 27 (19.1%) patients during in-hospital stay: 16 and 11 of these were minor and major bleeding, respectively. Post-discharge follow-up (median 752 [8-1,803] days) data were available in all patients, and 11 (7.8%) deaths were documented during follow-up.
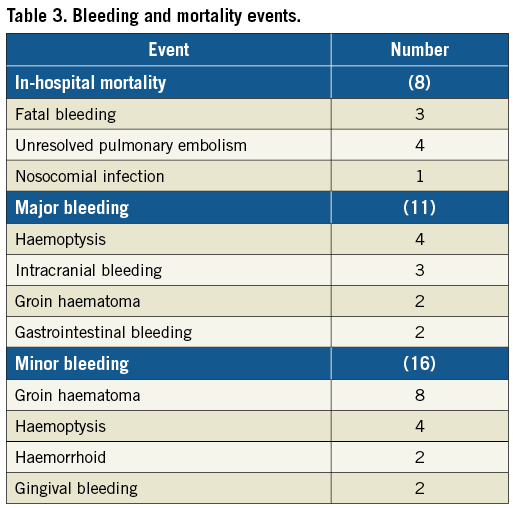
EFFICACY AND SAFETY ACCORDING TO THE AGE AND BASELINE RISK
The absolute and % changes in RV/LV and % change in MPAP, but not the change in QS, were significantly higher in patients younger than 65 years compared with older patients (Table 4). However, improvements in RV/LV, MPAP and QS were similar between risk groups. The younger and older patients had comparable 30-day and long-term mortality (Figure 2), and minor and major bleeding rates (Figure 3). The HR versus IHR status conferred a significantly higher 30-day mortality and a non-significant trend for higher long-term mortality (Figure 4).

Figure 2. Comparisons of post-procedural 30-day and long-term survival between patients younger than 65 years and those older than 65 years.
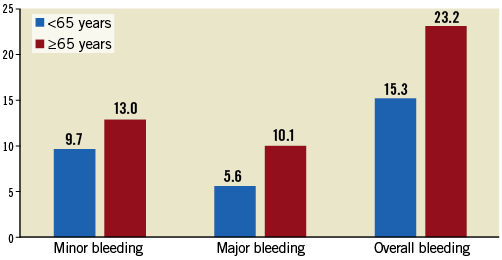
Figure 3. Comparisons of 30-day and long-term minor, major and overall bleeding rates between patients younger than 65 years and those older than 65 years.
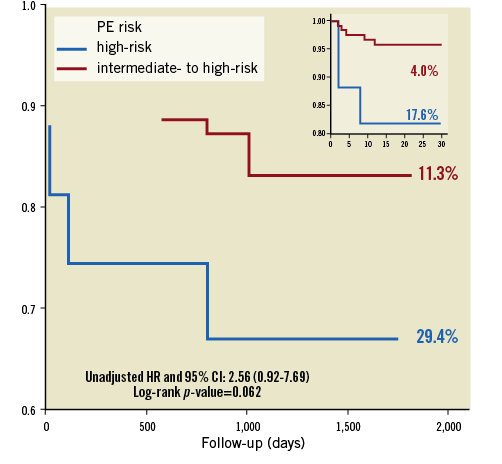
Figure 4. Comparisons between high-risk and intermediate to high-risk groups for 30-day and long-term survival. PE risk: pulmonary embolism risk group
LYTIC PROTOCOLS IN RELATION TO BLEEDING AND MORTALITY
In the ANOVA test, t-PA dosages (36.5±14.6 vs. 32.8±13.4 vs. 37.7±24.1 mg, p=0.63), infusion durations (25.2±7.4 vs. 23.1±10.4 vs. 19.6±9.5 hrs, p=0.066), and infusion rates (1.44±0.5 vs. 1.47±0.41 vs. 1.82±0.40 mg/hr, p=0.051) were comparable among patients without bleeding and patients with minor and major bleeding episodes, respectively. The infusion rate was numerically higher in patients with bleeding despite the absence of statistical significance. Survivors compared with non-survivors also had similar t-PA dosages (35.8±15.3 vs. 38.7±16 mg, p=0.46), t-PA infusion durations (24.4±7.8 vs. 25.7±10.2 hrs, p=0.62) and t-PA infusion rates (1.53±0.35 vs. 1.46±0.51 mg/hr, p=0.502), respectively. The mortality and bleeding rates were similiar between patients with or without previous t-PA treatment (p=0.396 and 0.322, respectively).
Discussion
This study based on the five-year, single-centre USAT experience in patients with PE at HR or IHR has revealed statistically significant and clinically relevant improvements in the measures of PA occlusive burden, pressure overload and right heart functions with relatively low rates of complications regardless of the baseline risk. Although changes in RV/LV and MPAP were significantly higher in patients younger than 65 years versus older patients, overall bleeding, 30-day and long-term mortality rates were not different. The IHR compared with HR status conferred a lower 30-day mortality.
The need for ideal risk-adjusted PE treatment strategies has remained unmet for at least four decades2-5. Although TTs compared with anticoagulants were found to be associated with significant reductions in all-cause mortality both in overall PE patients and in the IHR group, this benefit was obtained at the expense of increased risks for major bleeding11. Therefore, current strategies in PE have been targeted to facilitate the resolution of PA obstruction, pressure overload and haemodynamic instability and to prevent evolution to pulmonary hypertension without an increased bleeding risk2-4,8-12. Although various CDT systems have been developed to infuse the lytic agents directly into the PA thrombus, none of these has provided robust evidence in terms of superiority to other treatment modalities4,13-15,31. In a meta-analysis, utilisation of CDT in massive PE was associated with a high clinical success with minor and major complication rates of 8% and 2.4%, respectively31. In a multicentre registry, success rates of CDT including fragmentation, embolectomy and catheter thrombolysis techniques in massive PE and submassive PE groups were reported to be 85.7% and 97.3%, respectively32. Morever, in a non-randomised PE study performed in HR patients, t-PA infusions via CDT combined with fragmentation versus systemic TT showed a non-significant trend for a lower mortality and significant reductions in the risks of clinically significant bleeding and evolution to chronic pulmonary hypertension33.
Because USAT technology integrates CDT with a high-frequency and low-power acoustic streaming, this treatment has been documented to facilitate recovery of RV function and haemodynamic status with a lower dose of lytics which may reduce the risk of bleeding compared with other CDT modalities17-36. In an RCT, USAT, but not heparin alone treatment, was associated with a significant reduction in RV/LV at 24 hours and a greater benefit in RV/LV at 90 days with comparable bleeding outcomes16. Furthermore, in a multicentre, prospective trial, the fixed-dose regimen of t-PA of 24 mg for both unilateral and bilateral treatments provided a significant improvement in RV/LV, PA pressures, and modified Miller score from baseline to 48 hours, irrespective of the submassive or massive PE status, with a 2% in-hospital mortality and a 2.7% 30-day mortality rate27. Major bleeding within 30 days was 10% while no patients experienced intracranial bleeding27. In reference to these results, the FDA approved this USAT system for the treatment of PE four decades after the approval of the Greenfield catheter13. Our first single-centre study based on initial USAT experience in 75 patients with PE at HR and IHR showed significant improvements in Echo and CT measures of right heart functions, QS and PA pressures with rates of 5.3% in-hospital mortality, and 6.6% and 2.6% major and minor bleeding events, respectively26. Moreover, our meta-analysis revealed satisfactory efficacy and safety outcomes with USAT utilising the t-PA dosages as low as one third of those in systemic TT trials29. USAT resulted in similar mortality as compared to the TT arms of three pivotal RCTs, while the major bleeding risk was significantly reduced with USAT in comparison to two of these RCTs9-12,29. As compared to those in the TT arms of two meta-analyses, USAT was found to provide a similar pooled rate of mortality with a significantly reduced pooled rate of major bleeding11,12,29.
Despite the fact that the majority of evidence has been derived from patients at IHR, recommendations in PE Guidelines have been directed to patients at HR in whom the data for efficacy of CDT were inconclusive at that time4.
The present study represents the largest single-centre USAT series ever reported, and has confirmed that the acoustic pulse with low-dose and slow-infusion t-PA protocol in patients with PE is associated with improvements in thrombolysis and haemodynamics with relatively low rates of complications regardless of the HR or IHR status. The mortality rate in the study presented (5.7%) seems somewhat but not significantly higher than those ranging from 2.16% to 7.3% reported in our meta-analysis on USAT series, the SEATTLE trial, TT arms of two meta-analyses on RCTs and of two of three pivotal RCTs comparing TT versus anticoagulants9,11,12,27. The major bleeding rate of this study (7.8%) was lower than those reported (between 9.2% and 11.5%) in SEATTLE II, the TT arms of two meta-analyses and the PEITHO trial9,11,12,27. Bleeding risks and 30-day or long-term mortality were also not significantly related with age and t-PA regimen. There was a non-significant trend in favour of prolonged infusions for minimising the bleeding events. Although HR versus IHR conferred a significantly higher 30-day mortality, this trend seemed to attenuate over time. “Shades of grey” might be present between IHR and HR status as defined by initial assessments which may coincide with clinically silent phases of a vicious circle, and these subgroups may deteriorate to HR from IHR status with the unmasking of RV failure and potentially lethal haemodynamic compromise within days.
A recently reported RCT seems to raise the bar in USAT strategies by allowing the very low-dose and short-duration regimens for efficacious and safe treatments in patients with PE at IHR28. The reductions in RV/LV were comparable among the four cohorts of low-dose and short-duration regimens (23 to 26%) and appear to be as acutely effective as the regimens in other USAT studies. These % reductions in RV/LV were also comparable to those achieved in our study. Although t-PA dosages were higher and infusion durations were longer in our study compared with those in this RCT, absence of a relation between both the t-PA dosage and infusion duration with a % reduction in RV/LV, bleeding and mortality rates in our study seems to be not inconsistent with a low-dose hypothesis.
Study limitations
Absence of a control cohort, the retrospective nature of the analysis, and operator-driven selection of the t-PA regimen were the main limitations. Therefore, the risk of selection bias could not be totally eliminated. However, a tailored approach for t-PA dosage and infusion durations depending on the individual risks of PE progression and bleeding seem to be more appropriate than standardised protocols in USAT, and may provide an important clinical impact in this setting. The comparisons with a heparin alone arm in the IHR group and with a fixed dose intravenous t-PA alone arm in the HR group might provide more relevant outcome data. Moreover, randomisation among the different t-PA protocols might provide valuable data for new USAT strategies.
Conclusions
Our single-centre study revealed that USAT was associated with improved lysis of PA clot and stabilisation of haemodynamics with relatively low risks of complications in patients with PE regardless of the baseline risk. The HR versus IHR status, but not age, conferred a higher 30-day mortality risk. Moreover, increasing the t-PA dosage and prolongation of infusion duration seemed not to provide additional benefit in USAT treatments.
| Impact on daily practice USAT should be considered as an efficacious and safe treatment strategy in patients with acute PE at HR and IHR who are prone to an increased bleeding risk with systemic TT or are candidates for progression to more severe obstruction with anticoagulants. However, even with USAT, HR increases the 30-day mortality. |
Acknowledgements
Acknowledgements are listed in Supplementary Appendix 7.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Structure of the institutional multidisciplinary team, decision protocols and informed consent for USAT.
Supplementary Appendix 3. Chest CT.
Supplementary Appendix 4. Right heart catheterisation, pulmonary angiography and USAT procedures.
Supplementary Appendix 5. Statistical analysis.
Supplementary Appendix 6. Results.
Supplementary Appendix 7. Acknowledgements.
Supplementary Table 1. Repeated measures MANCOVA for changes in RV/LV, Qanadli score, and MPAP.
To read the full content of this article, please download the PDF.

