Abstract
Background: Intermediate-high risk acute pulmonary embolism (PE) remains associated with substantial mortality despite anticoagulation therapy.
Aims: The aim of this randomised pilot study was to compare catheter-directed thrombolysis to standard anticoagulation therapy.
Methods: Intermediate-high risk acute PE patients were admitted to a tertiary care centre (November 2019 to April 2021) and randomised in a 1:1 ratio to catheter-directed thrombolysis (CDT) or standard anticoagulation. Two catheters were used for the infusion of alteplase (1 mg/hr/catheter; total dose 20 mg) in the CDT group. The primary efficacy endpoint targeted improvement of right ventricular (RV) function, a decrease in pulmonary pressure, and a reduction of thrombus burden.
Results: Twenty-three patients were included (12 in the CDT group and 11 in the standard care group). The primary efficacy endpoint was achieved more frequently in the CDT group than in the standard care group (7 of 12 patients vs 1 of 11 patients, p=0.0004). An RV/left ventricular ratio reduction ≥25% (evident on computed tomography angiography) was achieved in 7 of 12 patients in the CDT group vs 2 of 11 patients in the standard care group (p=0.03). A systolic pulmonary artery pressure decrease of ≥30% or normotension at 24 hrs after randomisation was present in 10 of 12 patients in the CDT group vs 2 of 11 patients in the standard care group (p=0.001). There was no intracranial or life-threatening bleeding (type 5 or 3c bleeding, according to the Bleeding Academic Research Consortium classification).
Conclusions: CDT for intermediate-high risk acute PE appears to be safe and effective. Further research is warranted to assess clinical endpoints.
Introduction
Acute pulmonary embolism (PE) is a common and life-threatening condition (60/100,000 population annually)1 that is associated with high mortality in certain subgroups2. It is essential to identify patients at high risk of early death when selecting the optimal therapeutic management. The current European Society of Cardiology guidelines for the diagnosis and management of acute PE implement risk stratification based on haemodynamic instability and the assessment of other prognostic criteria (principally right ventricular [RV] dysfunction) and other potentially aggravating factors (e.g., comorbidities)3. Intermediate-high risk patients constitute one subgroup of acute PE patients, in which the early mortality rate is 6.0-7.7%4. Previous efforts to decrease mortality in this subgroup via systemic administration of thrombolytic agents have failed; such treatments were associated with an increased rate of extra- and intracranial bleeding56. Thus, systemic thrombolysis is not recommended for such patients, and anticoagulation remains the mainstay of acute PE therapy without any progress over the last several decades.
Catheter-directed thrombolysis: the rationale
Catheter-directed thrombolysis (CDT) in the treatment of patients with acute PE seeks to rapidly reduce the thrombotic load that obstructs the pulmonary arteries, while improving RV function. RV dysfunction is a major predictor of poor prognosis in such patients7. Low-dose thrombolytic therapy does not appear to be associated with an increased risk of intracranial bleeding8910. Ultrasound-assisted local thrombolysis (USAT) was recently introduced; it has yielded promising results but is expensive and, thus, infrequently used91011. Simple CDT (i.e., without ultrasound) is potentially a low-cost alternative12 with similar efficacy, as suggested by the SUNSET sPE Trial13. Data from prospective randomised trials comparing CDT to anticoagulation alone are lacking14.
The main goal of our randomised pilot study was to introduce a safe and simple method of CDT in the treatment of patients with intermediate-high risk acute PE and to compare it to a standard anticoagulation therapy.
Methods
Study population and design
Patients >18 years of age with acute PE, who were admitted to our tertiary care centre from November 2019 to April 2021, were enrolled if they met the inclusion criteria but not the exclusion criteria (Table 1). All patients provided written informed consent to participate. The study was approved by our local ethics committee and adhered to the tenets of the Declaration of Helsinki.
After CDT in five patients (feasibility evaluation), all subsequent patients were randomised using a simple envelope method to CDT and standard care (anticoagulation only) in a 1:1 ratio.
CDT
All procedures were performed by experienced interventional cardiologists (V. Kocka, J. Kroupa). All procedures were performed as soon as possible, but within normal working hours. After the exclusion of deep vein thrombosis via ultrasound or computed tomography (CT), venous access was obtained under ultrasound guidance (via the common femoral vein) and a double-lumen 8 Fr introducer was inserted (Fast-Cath Duo Hemostasis Introducer 8 Fr; St. Jude Medical/Abbott). The pulmonary thrombus was crossed using a standard diagnostic catheter (Radifocus Optitorque TIG 4 Fr; Terumo) by employing either a standard soft J-wire (EMERALD Guidewire, Exchange J-Tip 0.81 mm, 260 cm; Cordis) or a hydrophilic wire (Radifocus Guidewire M, Stiff type [angled]; Terumo). Thrombolytic catheters (Cragg-McNamara Valved Infusion Catheter 4 Fr; Medtronic) were placed in each of the right and left interlobar pulmonary arteries with a short overlap in the main pulmonary artery. The catheters featured a 10 cm infusion zone; thus, thrombolytic concentrations were high. Fused computed tomography angiography (CTA) and real-time fluoroscopy were used to guide the catheter placement; no contrast was employed. After the catheter placement, a continuous infusion of alteplase (Actilyse; Boehringer Ingelheim) at 1 mg/hr/catheter was commenced and continued for 10 hrs (total dose 20 mg). Intravenous unfractionated heparin was continued to a target activated partial thromboplastin time (aPTT) of 50-60 secs. After the local thrombolysis was complete, the catheters were removed and anticoagulation with unfractionated heparin (without a bolus) was continued to a target aPTT of 70-90 secs. The 8 Fr sheath was removed from the femoral vein 60 mins after the alteplase infusion had ended, and the access site was manually compressed for 10 min.
Anticoagulation
Before randomisation, all patients received intravenous unfractionated heparin (to a target aPTT of 70-90 secs) or subcutaneous low-molecular-weight heparin (LMWH; the full therapeutic dose). For patients in the CDT group, the anticoagulation treatment was as described above; among CDT patients who received LMWH, the procedure was postponed for 8 hrs after the last dose of LMWH. Patients in the standard care group continued therapeutic anticoagulation with either unfractionated heparin or LMWH.
CTA, echocardiography and laboratory tests
Diagnostic CTA was a component of routine clinical care. A second CTA was performed 48 hrs after randomisation in both groups. All CTA were performed in the Department of Radiology, University Hospital Královské Vinohrady, using a Somatom Drive and a Somatom Definition AS (both Siemens). CT scans were acquired using a standardised protocol that employed 60-100 ml of contrast fluid (Iomeron 400; Bracco). Acquisition was not electrocardiogram (ECG)-gated. All CT measurements were performed by experienced radiologists and a biomedical engineer (J. Weichet, M. Buk, L. Bartova) who had been blinded to group assignment, using proprietary Siemens software and Fluoro-CT software (Circle Cardiovascular Imaging, version 3.2).
RV and LV diameter measurement
Using Fluoro-CT 3D reconstruction software, the standard four-chamber view was identified. The distance between the atrioventricular groove and the left ventricular apex was measured; the basal third of the interventricular septum was identified (Figure 1A). At this point, the LV and RV diameters were measured in the plane perpendicular to the mid-septum in both the short-axis and four-chamber views (Figure 1B). The severity of the pulmonary artery tree obstruction was scored using the Qanadli method15.
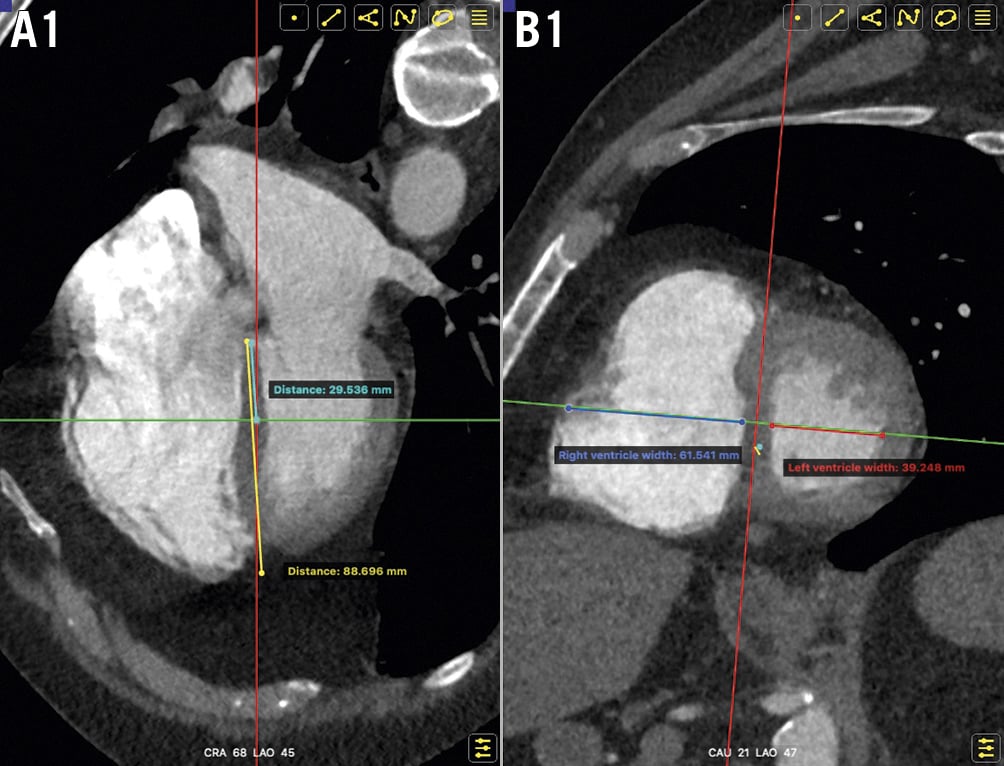
Figure 1. RV and LV measurements via CTA. A. Identification of the basal one-third of the interventricular septum in the four-chamber view. B. Measurement of RV and LV diameters in the plane perpendicular to the mid-septum on both the short-axis and four-chamber views. CTA: computed tomography angiography; LV: left ventricle; RV: right ventricle
Echocardiography was performed at the time of randomisation, then repeated 24 hrs later. Standard measurements were performed in accordance with the European Society of Cardiology recommendations1617. The subannular RV/LV ratio was derived as suggested by the ULTIMA study10; in brief, end-diastolic dimensions of the RV and LV were measured 1 cm above the tricuspid annular plane. All measurements were performed in our local echo lab by experienced cardiologists (H. Linkova, O. Ionita) who had been blinded to group assignment.
Laboratory tests were performed at the time of randomisation (creatinine, troponin hsTnl, and NT-proBNP; a blood count; and the aPTT and international normalised ratio [INR] coagulation tests) and 48 hrs after randomisation. In CDT patients, additional tests (blood count, coagulation parameters) were performed 6 hrs after the commencement of local thrombolysis.
Endpoints
Primary endpoints
- 1) Effectiveness of CDT, defined as improved RV function (where at least two of the following criteria were met):
a. Reduction of the RV/LV ratio by 25% between admission and 48 hrs post-randomisation, as revealed by CTA.
b. Reduction of the systolic pulmonary artery pressure (sPAP) by 30% from baseline or attainment of normotension (≤35 mmHg sPAP), as revealed by echocardiography at 24 hrs post-randomisation.
c. Reduction of the Qanadli score15 by 30% between admission and 48 hrs post-randomisation, as revealed by CTA.
- 2) Safety of CDT, defined as the absence of intracranial or life-threatening bleeding according to the Bleeding Academic Research Consortium (BARC) classification18 (i.e., type 5 or 3c bleeding) within 72 hrs post-randomisation.
Secondary endpoints
- 1) Technical success of CDT: successful catheter placement followed by continuous infusion of alteplase.
- 2) All bleeding complications (scored using the BARC classification).
- 3) Haemodynamic instability during hospitalisation.
- 4) Length of hospitalisation.
- 5) In-hospital mortality.
Statistical analysis
Statistical analysis was performed using IBM SPSS software version 23.0 (IBM). Independent samples t-tests were used to compare the CDT and standard care groups. P-values <0.05 were considered statistically significant. Continuous variables are expressed as means (± standard deviations [SD]) and categorical variables are expressed as numbers (with percentages).
Results
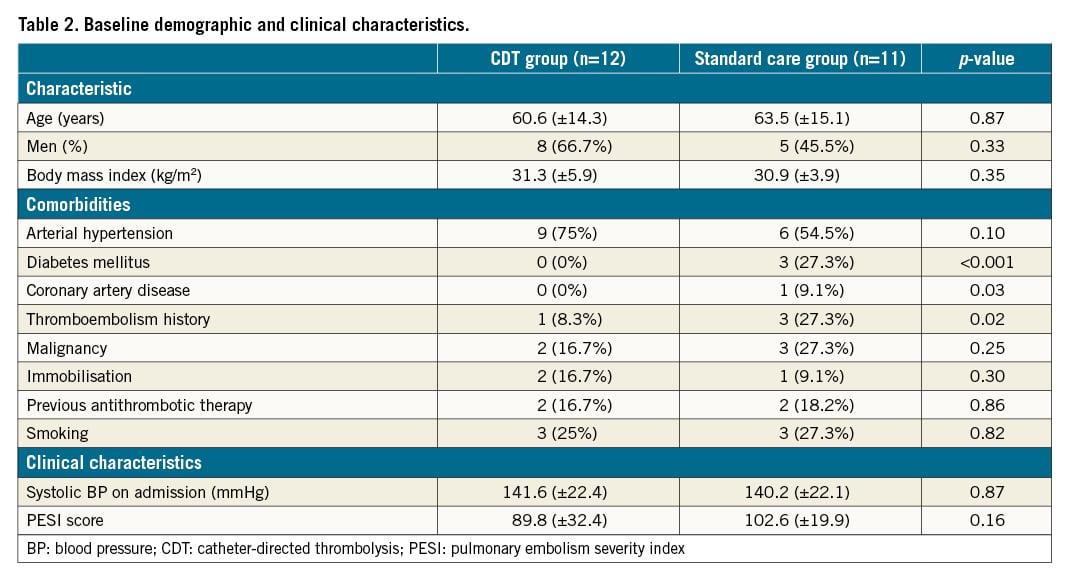
Twenty-three patients with an intermediate-high risk acute PE were randomised (12 into the CDT group and 11 into the standard care group). The baseline demographic and clinical characteristics are summarised in Table 2.
Procedural characteristics
CDT was successful (i.e., no periprocedural complications) in all 12 patients. The mean time from admission to intervention was 25.1 (±12.1) hrs; the mean procedural duration was 44.5 (±8.8) mins (commencing with the venous puncture). The mean fluoroscopy time was 12.8 (±8.4) mins and the mean dose area product was 26.5 (±16.1) Gy·cm2. Two catheters were used in all patients - one in each of the right and left interlobar pulmonary arteries for bilateral thrombolysis.
CTA, echocardiography and laboratory results
All data are shown in Table 3.
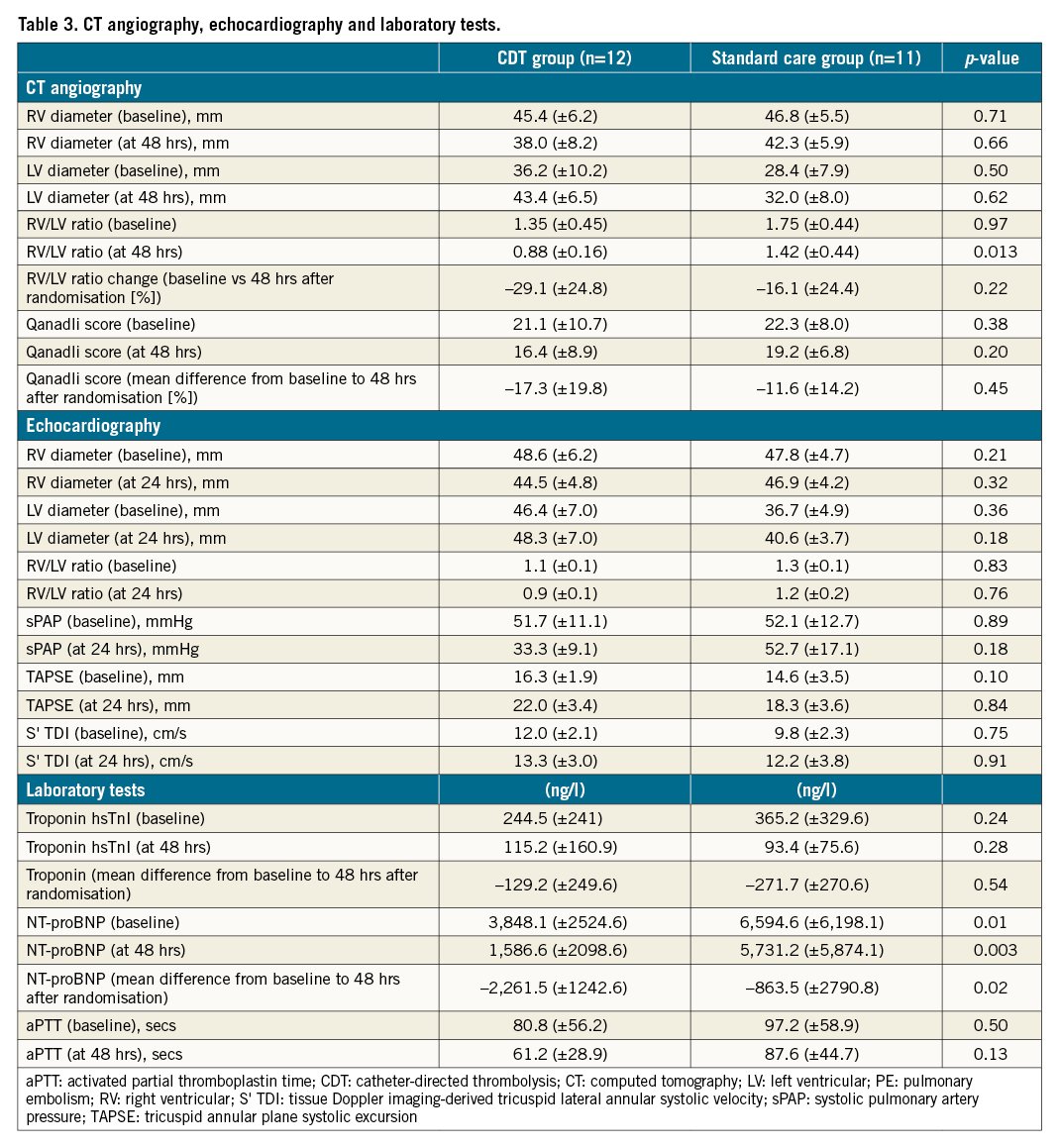
CTA proceeded without complications in all patients; the standard dose of contrast agent was 80 ml. At baseline, the RV/LV ratios were 1.35 (±0.45) in the CDT group and 1.75 (±0.44) in the standard care group (p=0.97). Forty-eight hours after randomisation, the RV/LV ratio was significantly lower in the CDT group than in the standard care group (0.88±0.16 vs 1.42±0.44, p=0.013). The Qanadli index fell 17.3% in the CDT group and 11.6% in the standard care group (p=0.45).
Echocardiography did not reveal any significant between-group differences. The sPAP tended to be lower 24 hrs after randomisation in the CDT group than in the standard care group (33.3±9.1 vs 52.7±17.1 mmHg, p=0.18).
The baseline levels of hsTnl and NT-proBNP were elevated in both groups (Table 3). The NT-proBNP levels were higher in the standard care group than in the CDT group at baseline (6,594.6±6,198.1 vs 3,848.1±2,524.6 ng/l, p=0.01) and 48 hrs after randomisation (5,731.2±5,874.1 vs 1,586.6±2,098.6 ng/l, p=0.003). The NT-proBNP level improved to a greater extent 48 hrs after randomisation in the CDT group than in the standard care group (–2,261.5±1,242.6 vs –863.5±2,790.8 ng/l, p=0.02) (Figure 2). There were no between-group differences in any other laboratory parameters (creatinine, haemoglobin, platelet, or fibrinogen level; the aPTT; or the INR)
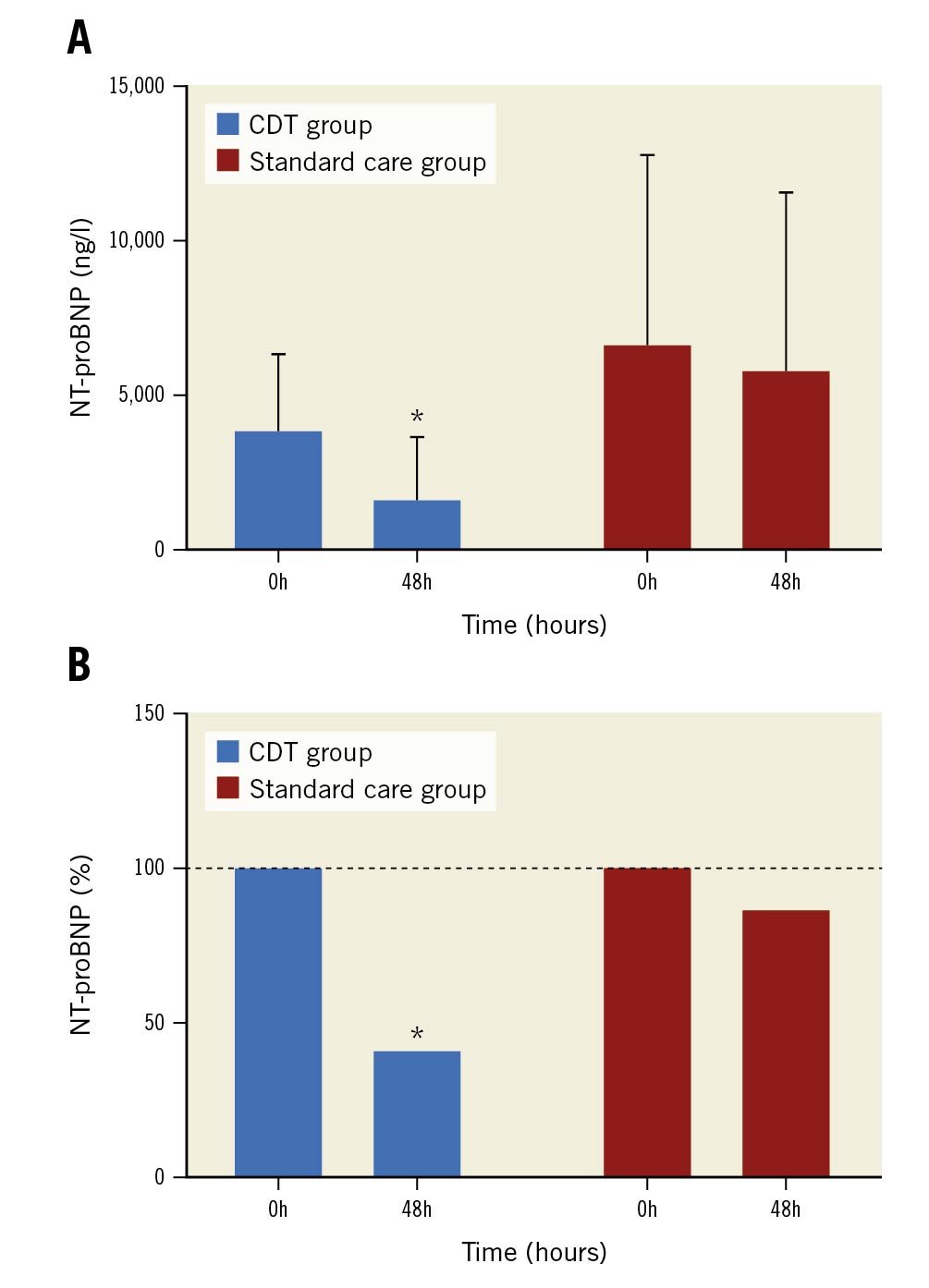
Figure 2. NT-proBNP levels in the CDT and standard care groups at baseline and 48 hrs post-randomisation. A. Absolute values; B. Relative reductions. *: significant difference with p-value <0.05. CDT: catheter-directed thrombolysis
Primary endpoint analysis
The combined endpoint “effectiveness of catheter-directed thrombolysis” was better in the CDT group than in the standard care group (7 of 12 patients vs 1 of 11 patients, p=0.0004). An RV/LV reduction ≥25% (on CTA) was achieved in 7 of 12 patients in the CDT group vs 2 of 11 patients in the standard care group (p=0.03). An sPAP decrease of ≥30% or the attainment of pulmonary artery normotension at 24 hrs after randomisation was evident in 10 of 12 CDT patients vs 2 of 11 standard care patients (p=0.001). Qanadli score reductions ≥30% were achieved by 3 of 12 patients in the CDT group vs 2 of 11 patients in the standard care group (p=0.45) (Central illustration). The “safety of CDT” endpoint was achieved in all patients; no intracranial or life-threatening bleeding (BARC type 5 or 3c) was observed in any study participant.
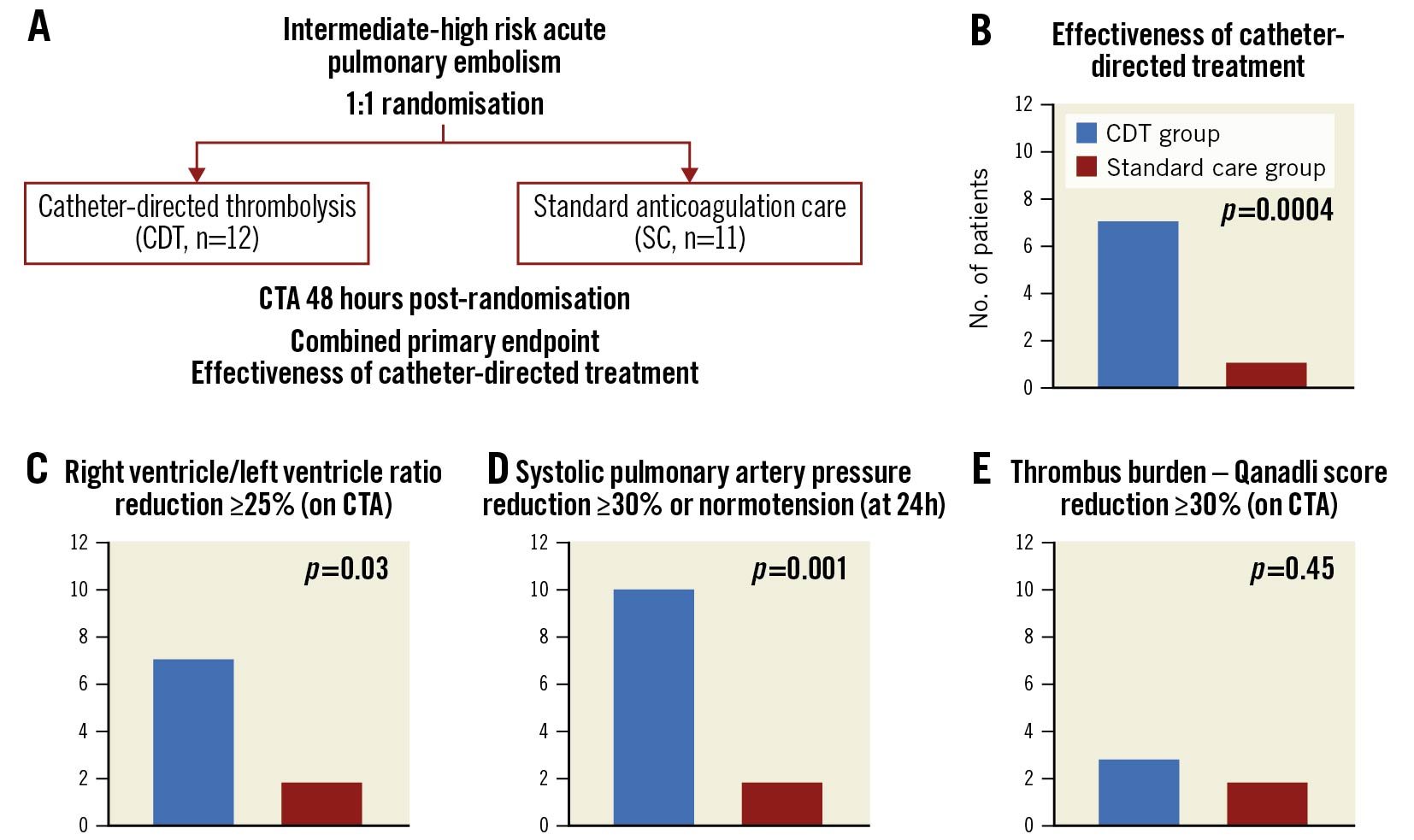
Central illustration. Combined primary endpoint effectiveness of catheter-directed thrombolysis. A. Study design. B. Combined primary endpoint effectiveness of catheter-directed treatment – at least two of three predefined criteria - per C), D), E) had to be met. CDT: catheter-directed thrombolysis; CTA: computed tomography angiography; SC: standard care
Secondary endpoint analysis
One local bleeding complication developed in the CDT group (an access site haematoma of BARC type 2). There were two bleeding complications in the standard care group (haematuria in one patient [BARC type 2] and renal parenchymal haemorrhage [BARC type 3a] in one patient with acute kidney ischaemia caused by a paradoxical renal artery embolism during hospitalisation).
No haemodynamic instability was observed in any patient. Positive trends toward a shorter intensive care unit stay (99.3±61.1 vs 153.5±96.0 hrs; p=0.29) and hospitalisation time (189.8±65.3 vs 234.6±98.6 hrs; p=0.16) were more apparent in the CDT group, compared to the standard care group. Two patients in the standard care group underwent rescue local thrombolysis on clinical grounds at 72 and 53 hrs post-randomisation; this led to clinical improvement and reduction of the RV size in both patients. There was zero mortality and no hospital readmission at 30-day follow-up.
Discussion
Both primary endpoints were achieved. The combined endpoint “effectiveness of catheter-directed thrombolysis” was more common in the CDT group than in the standard care group, and there were no safety concerns, with no occurrence of intracranial or life-threatening bleeding according to the BARC classification in our study.
Patients included in our study appear to be similar to patients in the non-randomised PERFECT and USAT CDT studies891011, despite differences in the high-risk stratifications between the European Society of Cardiology and the American College of Cardiology/American Heart Association. Our study was conducted in a large tertiary care centre, as were the cited works. Patients treated in high-volume centres represent approximately half of all patients with acute PE19. The remaining patients (including patients at intermediate-high risk) are usually treated (via anticoagulation therapy) in low-volume centres. Patients at intermediate-high risk might benefit from transfers to large tertiary care centres where other treatment options are available.
Our CDT philosophy was the simplest possible approach. The introduction of two 4 Fr catheters via a single dual-lumen introducer was safe and patient-friendly. The fusion of CTA and real-time fluoroscopy eliminated any need for contrast, reducing the risk of kidney injury. In contrast to the previously published papers8911, we have described the standardised methodology of CDT. Radiation time and dose were non-negligible, but a large field of view was required; the exposure is reasonable considering the clinical severity of intermediate-high risk acute PE.
To our knowledge, no prospective randomised comparison of CDT (local thrombolysis alone) with standard anticoagulation has been published14. In our pilot study, patients undergoing CDT exhibited a greater RV/LV ratio reduction and a greater sPAP decrease than patients in the standard care group, without any serious bleeding. The dose of the thrombolytic agent and infusion duration varied across the studies891011. Our thrombolytic choice was alteplase; the use of one 20 mg vial per patient was practical and economical. It may be useful to individualise the dose and duration of local thrombolytic therapy (based on treatment effects) in future studies.
The study was not powered nor prospectively designed to analyse cost-effectiveness, but a simple financial assessment is possible. The cost of a standard thrombolytic catheter (approximately 120 USD) is significantly lower than the cost of an ultrasound-assisted thrombolytic EKOS catheter (Boston Scientific). Furthermore, the intensive care unit and total hospitalisation times tended to be shorter in the CDT group. The cost savings afforded by two fewer days in the intensive care unit would compensate for the cost of 20 mg alteplase (approximately 260 USD). Future CDT studies will need to include formal cost-effectiveness analysis with quality-of-life evaluation.
Several important issues remain open to further research. A large randomised comparison of CDT and anticoagulation therapy alone, with well-defined clinical endpoints, is required to verify the effectiveness of our approach. The optimal thrombolytic dose and infusion duration should be clarified. Formal cost-effectiveness analysis of different treatment modalities (anticoagulation alone vs CDT local thrombolysis vs ultrasound-assisted local thrombolysis) should be performed.
Limitations
Our sample size was obviously insufficient to allow us to draw definitive conclusions including long-term outcomes (quality of life, mortality, etc.). However, the study was randomised, so the data can facilitate sample size calculation for subsequent larger studies with clinical endpoints. The minor differences in baseline characteristics are likely attributable to chance; they are unlikely to influence our (principally image-based) endpoints. The study was performed in a tertiary care cardiac centre; we lacked information concerning the need for patient transfer. The timing of CDT might have had an impact on the study outcome; the average delay of 25 hrs might lead to selection bias. We sought to be maximally practical; thus, we did not invasively measure pulmonary pressures after local thrombolysis was concluded. However, transthoracic echocardiography with an estimation of sPAP was performed at 24 hrs post-randomisation. Our data appear similar to the findings in previous studies8910 (Figure 3). No formal interobserver comparisons of CT or echocardiography measurements were performed; difficulties were resolved by discussion among observers.
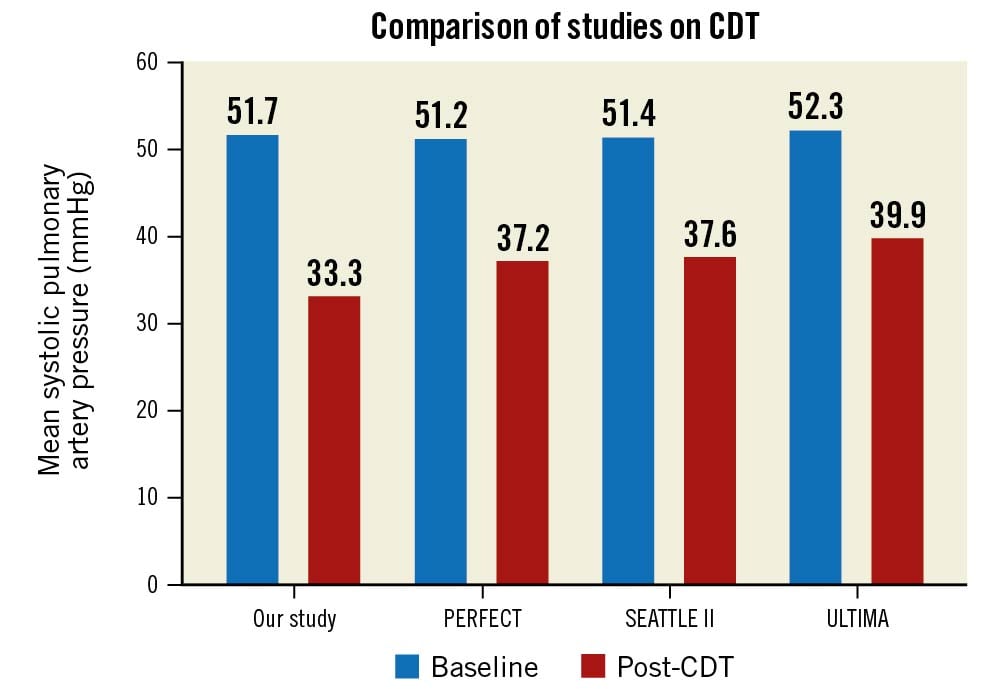
Figure 3. Comparison of studies on CDT. Comparison of our study with previous studies on CDT for patients with acute PE: mean systolic pulmonary artery pressures at baseline and post-CDT. PERFECT8, SEATTLE II9, ULTIMA10. CDT: catheter-directed thrombolysis
Conclusions
CDT for intermediate-high risk acute PE patients appears simple and safe; it improves known predictors of poor patient prognosis. Further research featuring carefully chosen clinical endpoints is warranted.
Impact on daily practice
Simple catheter-directed local thrombolysis might offer a safe and effective treatment option for patients with intermediate-high risk acute PE.
Acknowledgements
This work was supported by the Charles University Research Program Q 38 and the Interventional Treatment of Life-Threatening Cardiovascular Diseases (INTERCARDIS) EU project CZ.02.1.01/0.0/0.0/16_026/0008388. All authors agree with the submission of the manuscript. Data are available from the corresponding author on request.
Funding
This work was funded by the Charles University Research Program Q 38 and the Interventional Treatment of Life-Threatening Cardiovascular Diseases (INTERCARDIS) EU project CZ.02.1.01/0.0/0.0/16_026/0008388.
Conflict of interest statement
V. Kocka received consultation fees from Medtronic related to work on another structural intervention (TAVI). J. Kroupa received consultation fees from B. Braun for work in a different area (related to coronary interventions). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

