
Abstract
Aims: The aim of this study was to evaluate the effectiveness of local low-dose urokinase thrombolysis (LLDUT) in haemodynamically stable pulmonary embolism with right ventricular dysfunction (RVD).
Methods and results: This was a prospective study. LLDUT with a 200,000 IU bolus followed by a 100,000 IU/hr infusion was given. Treatment duration was determined through radiological control performed 48-72 hrs into treatment. A follow-up echocardiogram was performed within seven days after LLDUT completion. Evolution of thrombus burden, pulmonary artery pressures (PAP) and RVD were studied, and haemorrhagic complications and mortality were recorded. Eighty-seven patients were included (62.5±16.5 years). In 67 patients (77%), the baseline echocardiogram showed mild-to-severe RVD, a dilated right ventricle (diameter: 44.4±6.2 mm) and a decreased tricuspid annular plane systolic excursion (14 mm [12-17]). Seventy-six patients (87.4%) experienced radiological improvement. Initially high PAP (mmHg) decreased after LLDUT: systolic 52.4 vs. 35.2 (17.2 [95% CI: 14.5-19.9]; p<0.0001), mean 34.2 vs. 23.5 (10.7 [95% CI: 9.0-12.5]; p<0.0001) and diastolic 23.9 vs. 16.0 (7.9 [95% CI: 6.1-9.7]; p<0.0001). Follow-up echocardiography showed overall improvement of RVD. No life-threatening haemorrhagic complications were reported. Six-month survival was 96.5%.
Conclusions: LLDUT rapidly decreased thrombus burden and PAP, improving right ventricular function, and was not associated with any life-threatening complications or pulmonary embolism (PE)- or treatment-related mortality.
Abbreviations
CDT: catheter-directed therapies
CTPA: computed tomographic pulmonary angiography
LLDUT: local low-dose urokinase thrombolysis
PAP: pulmonary artery pressures
PE: pulmonary embolism
PRBCs: packed red blood cells
RCT: randomised controlled trials
RVD: right ventricular dysfunction
TAPSE: tricuspid annular plane systolic excursion
UK: urokinase
USAT: ultrasound-assisted thrombolysis
Introduction
Venous thromboembolism, a disease that includes deep vein thrombosis and pulmonary embolism (PE), is the third most frequent cardiovascular event, being a major cause of morbidity, mortality and hospitalisation1. In the PE group, the presence of right ventricular dysfunction (RVD), which can be found in at least 25% of haemodynamically stable patients (systolic arterial pressure >90 mmHg), is related to higher rates of mortality, a fact that contributes to increased concern regarding the optimal management of this group of subjects2-4.
Although systemic anticoagulation of the haemodynamically stable PE is considered the treatment of choice, recently published guidelines advise physicians to take into account rescue reperfusion therapies when RVD is present5. In this context, the role of systemic thrombolysis has been evaluated but, to date, no benefits on mortality and a higher risk of major haemorrhage have been demonstrated, leading some authors to dismiss this approach6-9. To avoid haemorrhagic complications, catheter-directed therapies (CDT), such as in situ thrombolytic infusion and ultrasound-assisted thrombolysis (USAT), have demonstrated lower bleeding risks and a rapid improvement of the RVD10-14.
This study investigated the effects of local low-dose urokinase thrombolysis (LLDUT) in haemodynamically stable PE patients with RVD. To achieve this objective, we assessed thrombus evolution and changes in pulmonary artery pressures (PAP) and right ventricular echocardiographic parameters. Haemorrhagic complications, systemic consequences of the treatment and in-hospital and six-month mortality were also recorded.
Methods
STUDY DESIGN
This study was a prospective, single-centre, single-arm study performed in a tertiary hospital (January 2008 to December 2015). The study was approved by the Institutional Review Board (Comité Ético de Investigación Clínica del Hospital Universitario Puerta de Hierro Majadahonda, nº 269) and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients were enrolled after written informed consent had been obtained.
STUDY POPULATION
Patients were considered for LLDUT if they satisfied ALL of the following:
1. PE diagnosed by computed tomographic pulmonary angiography (CTPA; SOMATON Sensation 64 [Siemens Healthineers, Erlangen, Germany])5. CTPA was performed using a bolus tracking technique with a region of interest placed in the pulmonary trunk and after the automatic injection of 80-90 ml of a non-ionic radiopaque contrast (iopromide; Ultravist® 300 mg/ml [Bayer Schering Pharma AG, Berlin, Germany]) followed by the administration of 30 ml of sodium chloride 0.9% at a rate of 3.5-4 ml/sec. A CTPA attenuation of 60 Hounsfield units at the pulmonary trunk triggered image acquisition. AND
2. Haemodynamic stability, defined as spontaneously maintained systolic arterial pressure >90 mmHg5, upon ICU evaluation. AND
3. Suspicion of RVD based on:
a) CTPA findings: bilateral pulmonary emboli occluding the main pulmonary arteries or a right-to-left ventricular diameter ratio >115,16
PLUS
b) Elevated levels of cardiac biomarkers (troponin I >0.05 μg/ml or NT-proBNP >600 pg/ml)17-19.
Patients who met all of the inclusion criteria underwent a formal transthoracic echocardiographic evaluation (EnVisor Ultrasound System; Philips, Eindhoven, the Netherlands) by the Cardiology Department that included parasternal long- and short-axis, apical four-chamber and subcostal views. RVD was diagnosed if at least ONE of the following was demonstrated:
– Subjective alteration of right ventricular contractility20.
– Basal right ventricular diameter in the four-chamber view >40 mm20.
– Estimated systolic PAP >30 mmHg21.
– Tricuspid annular plane systolic excursion (TAPSE) <15 mm22.
Once RVD was confirmed, informed consent was obtained and LLDUT started.
We excluded patients under 18 years of age or who were pregnant, those with a life expectancy of less than six months, ongoing PE symptoms for more than 15 days, an initial platelet count below 100×103 cell/mm3 or non-pharmacological coagulopathy. Patients with a higher risk of bleeding were individually assessed.
CATHETER-DIRECTED UROKINASE (UK) INFUSION
LLDUT was carried out using a 4 Fr 110 cm pigtail catheter (Cordis Corporation, Bridgewater, NJ, USA) inserted through an antecubital vein under direct fluoroscopy by the interventional radiologist. The tip of the catheter was positioned at the pulmonary trunk and, before LLDUT was started, a pulmonary angiogram was obtained with a single bolus automatic injection of 30 ml of a non-ionic radiopaque contrast (iopromide, Ultravist 300 mg/ml; Bayer Schering Pharma AG). Initial invasive PAP were measured and, after evaluating the thrombus distribution, the catheter tip was advanced left or right and positioned in direct contact with the embolus to guarantee intrathrombotic injection23. In patients with similar bilateral occupation, the catheter tip was left in the pulmonary trunk. During the procedure, ECG, O2 saturation and non-invasive arterial pressures were monitored.
Once the catheter was in place, patients were admitted to the ICU where LLDUT using an initial 200,000 IU bolus of UK (MYB, Madrid, Spain) followed by a 100,000 IU/hr local infusion was initiated. Simultaneously, systemic anticoagulation with unfractionated heparin was started and titrated to achieve aPTT levels 1.5-2 times control values. During LLDUT, haemoglobin, platelets and fibrinogen levels were monitored every 12 hrs. It was agreed that treatment would be stopped if platelet or fibrinogen levels fell below 50×103 cell/mm3 or 100 mg/dl, respectively.
All haemorrhagic events were recorded. They were considered as major if they led to the transfusion of one to three units of packed red blood cells (PRBCs) and as life-threatening if they were associated with haemodynamic instability, the transfusion of ≥4 PRBCs or intracranial bleeding. A haemoglobin level of 7 g/dl was used as the transfusion threshold except for patients with a previous history of cardiovascular disease, where a threshold of 8 g/dl was applied. If haemorrhagic complications appeared, the doctor in charge of the patient made the decision to maintain or stop LLDUT based on the patient’s individual risk.
NT-proBNP values were also assessed 24 hours after the start of LLDUT.
Forty-eight hours into LLDUT, a control angiography or CTPA was performed. Images were evaluated by the interventional radiologist and, if thrombus was still present at the main pulmonary or lobar artery levels (high thrombus burden), LLDUT was prolonged for an additional 24 hrs (up to a maximum treatment time of 72 hrs). If only segmental or subsegmental perfusion defects persisted (low thrombus burden), this was considered favourable radiological evolution and LLDUT was ceased.
Once treatment was concluded, final PAP were recorded and the pigtail catheter was removed. Patients were discharged to the ward 12 to 24 hrs after the end of LLDUT where systemic anticoagulation was continued.
FOLLOW-UP
A new echocardiographic evaluation was performed in the following seven days after the conclusion of the LLDUT. Medical records were reviewed to address in-hospital and six-month mortality.
STATISTICAL ANALYSIS
The analysis was performed on an intention-to-treat basis, and all consecutive patients were evaluated. Continuous data are presented as mean±SD for normal distributions or as median and range (25th-75th percentile) for non-parametric data. Categorical variables are shown as absolute values and frequencies (%). The paired t-test (parametric) or Wilcoxon signed-rank test (non-parametric) was used to compare continuous variables. Results are shown as the mean (before vs. after), mean difference, 95% confidence intervals and p-value. The Stuart-Maxwell test was used to compare qualitative values. A p-value <0.05 was considered statistically significant. All statistics were calculated using Stata, v.14.1 (StataCorp LP, College Station, TX, USA).
Results
Of the 5,037 patients admitted to the ICU during the study period, 87 were eligible for LLDUT and all signed the informed consent (Figure 1). Baseline demographics, clinical characteristics and initial findings are listed in Table 1.
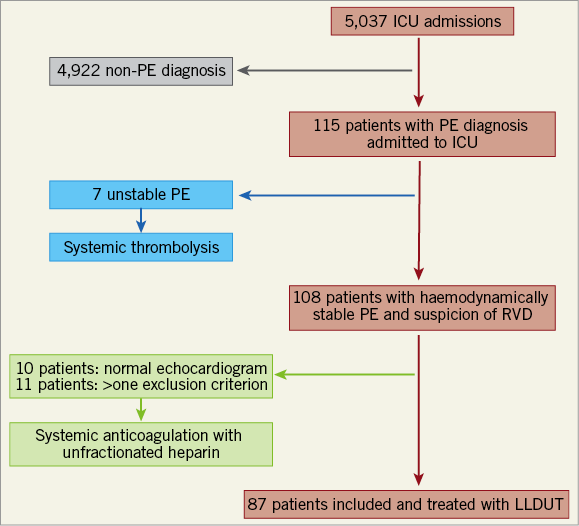
Figure 1. Flow chart of patient enrolment.
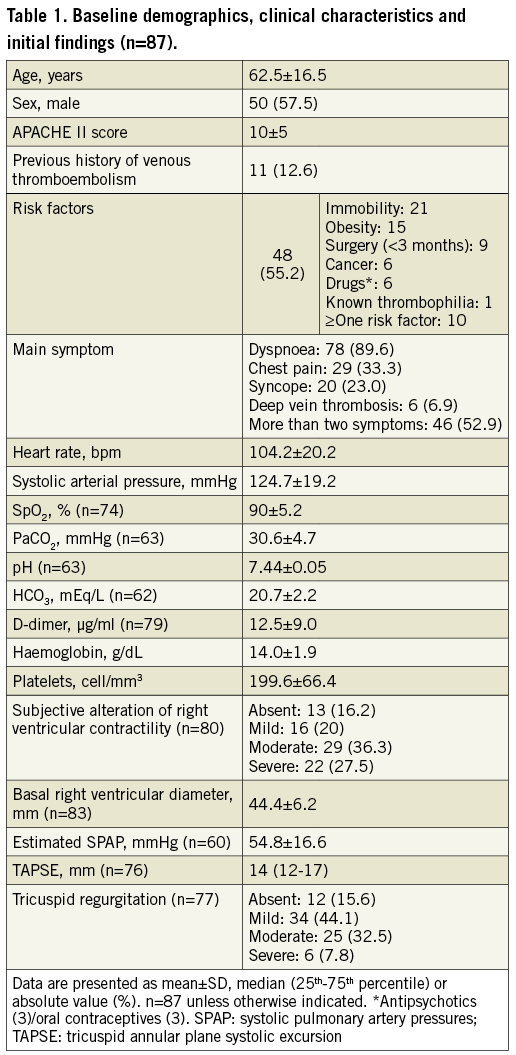
The CTPA showed bilateral pulmonary emboli in all 87 patients, with 83 (95.4%) having thrombus in the main pulmonary arteries. A right-to-left ventricular diameter ratio >1 was described in 67 (77%). Initial troponin I and NT-proBNP values were high, reaching 0.225 ng/ml (0.07-0.47; n=86) and 3,083 pg/ml (1,300-6,020; n=65), respectively. The findings of the first echocardiographic evaluation are shown in Table 1. Initial invasive PAP readings showed pulmonary hypertension (Figure 2).
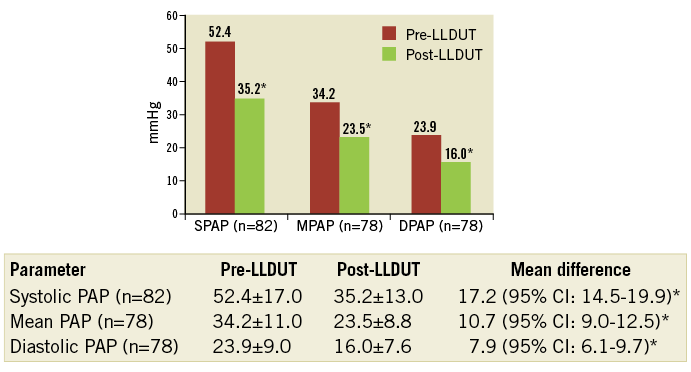
Figure 2. Evolution of pulmonary artery pressures. *p<0.0001. DPAP: diastolic pulmonary artery pressure; MPAP: mean pulmonary artery pressure; SPAP: systolic pulmonary artery pressure
Twenty-four hours into treatment, there was an increase in systolic arterial pressure (124.7 vs. 130.4 mmHg; 5.7 [95% CI: 0.8-10.6]; p=0.02) and a decrease in both heart rate (104.2 vs. 77.1 bpm; 27 [95% CI: 22.7-31.4; p<0.0001) and NT-proBNP (3,083 pg/ml [1,300-6,020] vs. 949 pg/ml [442-2,538]; p<0.0001; n=57).
Mean LLDUT time was 56.3±15.5 hrs. Treatment was stopped in the first 48 hrs in six patients (6.9%). In four of them (4.6%), this was due to haemorrhagic complications (two haematomas, one haematuria and one case of lower gastrointestinal bleeding), and in two (2.3%) due to a drop in platelet or fibrinogen values below the established levels.
After 48-72 hrs of LLDUT, a radiological evaluation was performed to determine the duration of treatment. This was not technically possible in six cases (6.9%). In the other 81 (93.1%), it was done using pulmonary angiography (68) and CTPA (13). Seventy-six (87.4%) patients presented favourable radiological evolution (Figure 3).
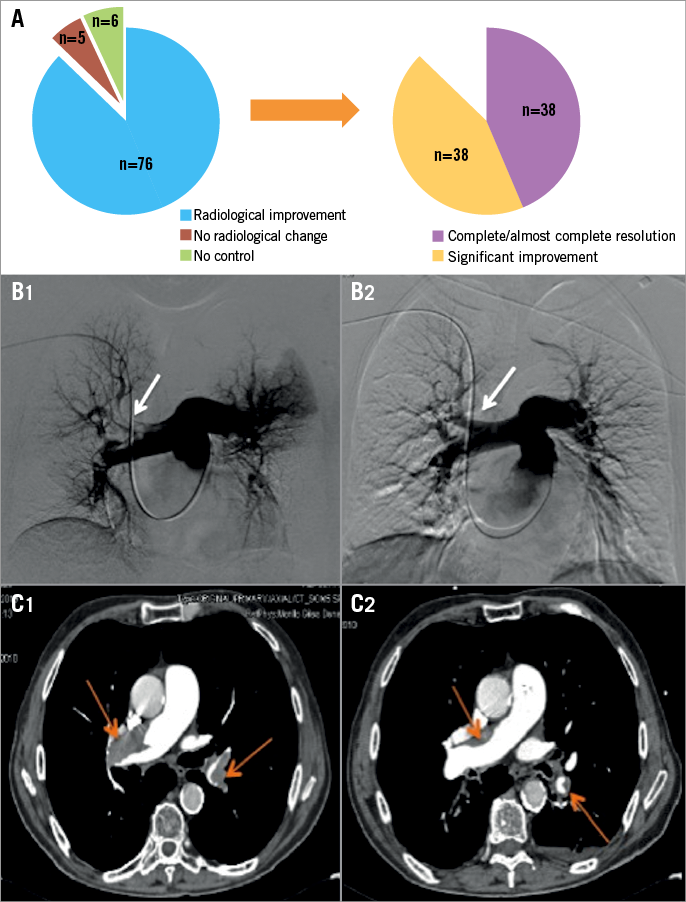
Figure 3. Radiological findings and evolution. A) Radiological changes after treatment (n=81). B1) Baseline pulmonary angiography showing a right pulmonary artery thrombus (arrow). B2) Follow-up angiography showing a reduction in thrombus burden (arrow). C1) Diagnostic CTPA demonstrating PE affecting both main pulmonary arteries (arrows). C2) Control CTPA after treatment (arrows).
PAP measured before catheter removal almost reached normal values (p<0.0001) (Figure 2).
During LLDUT, we experienced 18 (20.7%) bleeding complications, of which 13 were puncture site haematomas. There were no life-threatening haemorrhagic events. Although LLDUT was associated with a drop in haemoglobin values (14.0 vs. 11.6 g/dl; 2.4 [95% CI: 2.2-2.7]; p<0.0001), only three patients (3.4%) required the transfusion of two units or fewer of PRBCs. Minimum mean platelet and fibrinogen levels remained above the previously established lower limits (134.2×103±44.7×103 cells/mm3 and 251.9±91.8 mg/dl, respectively). Mean ICU length of stay was 4±1.5 days.
Follow-up echocardiograms were performed in 80 patients (92%), demonstrating an improvement in the RVD (Figure 4). A decrease in the incidence and severity of tricuspid regurgitation was also noted (p<0.0001; n=64).
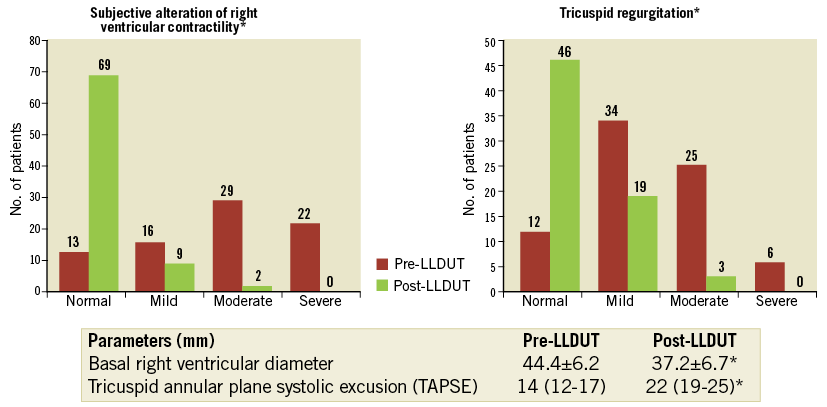
Figure 4. Echocardiographic evolution of the right ventricle after LLDUT. *p<0.0001.
Hospital length of stay was 11.5 days (10-16). Six-month survival was 95.4% (83 patients alive) and all deaths recorded were related to cancer. One patient was lost to follow-up.
Discussion and limitations
Management of PE in haemodynamically stable patients with RVD, also referred to as submassive PE, is controversial. The ICOPER registry showed, more than 15 years ago, that the presence of RVD led to an increase in mortality that almost reached 20% at three months, doubling the mortality expected for haemodynamically stable PE patients without RVD2.
The higher mortality recorded for this group of patients raises the question of the suitability of heparinisation alone as an efficient treatment. For this reason, different therapeutic approaches that prompt a rapid recovery of the RVD have been studied2-5. One of the first strategies evaluated was systemic thrombolysis. Konstantinides et al, in a registry-type study, compared systemic thrombolysis vs. heparinisation alone in PE patients with documented RVD but without cardiogenic shock. They found a decrease in 30-day mortality in the thrombolysis group (4.7% vs. 11.1%; p=0.016) together with an elevated risk of major bleeding episodes (21.9% vs. 7.8%; p<0.001) but without increased rates of intracranial haemorrhage24. However, subsequent randomised controlled trials (RCT) and meta-analyses have not been able to confirm these findings, demonstrating, in fact, no reduction in mortality and an increase in major bleeding events in the systemic thrombolysis group6-9,25-27.
To avoid these haemorrhagic complications, CDT are being considered as an alternative approach. In the last couple of years, the first RCT and large case series for the treatment of submassive PE using local infusion of thrombolytics (rtPA or UK), in some situations combined with USAT, have been published10-12. Previously, only small series and case reports had been documented28-31.
The only RCT to date is the ULTIMA trial, in which submassive PE patients were randomised to USAT using an rtPA infusion of 10-20 mg over 15 hrs (n=30) or to unfractionated heparin (n=29). They concluded that patients in the USAT group had a rapid reversal of right ventricular dilatation at 24 hrs (mean difference in right-to-left ventricular ratio from baseline to 24 hrs: 0.30±0.20 vs. 0.03±0.16; p<0.001) with no episodes of major bleeding and a 10% incidence of minor haemorrhagic events10.
In the PERFECT study, 101 patients suffering from either massive (n=28) or submassive (n=73) PE were reviewed. Treatment was provided using a low-dose local infusion of rtPA (28±11 mg; n=76) or UK (2,697,101±936,287 IU; n=23) combined in 29 cases with USAT. After treatment, there was a significant drop in systolic PAP (51.17±14.06 mmHg vs. 37.23±15.81 mmHg; p<0.0001) and an improvement in right ventricular performance. The study also demonstrated that the application of USAT was not associated with differences in pre-treatment and post-treatment PAP changes, average thrombolytic doses or average infusion times. There were no major haemorrhagic complications, and 13% of patients suffered a minor bleeding event12.
Finally, SEATTLE II, a single-arm prospective study evaluating the role of USAT using a 24-hr infusion of 24 mg of rtPA for the treatment of massive (n=31) and submassive PE (n=119), revealed a decrease in right ventricle dimensions (right-to-left ventricular diameter ratio 48 hrs post procedure: 1.55 vs. 1.13; mean difference, 0.42; p<0.0001), systolic PAP (51.4 mmHg vs. 36.9 mmHg; p<0.0001) and thrombus burden (Miller Index score 22.5 vs. 15.8; p<0.0001). During this trial, there was one severe non-intracranial bleeding event and, as in the ULTIMA trial, a 10% incidence of minor haemorrhagic events11.
To our knowledge, this paper describes the largest existing study evaluating the use of LLDUT exclusively in patients with haemodynamically stable PE and concomitant RVD. Our data demonstrate that this technique rapidly reduces thrombus burden and PAP, improving the RVD, which is consistent with previously published results10-12. This prompt reduction in RVD is supported not only by radiological (CTPA or angiography), echocardiographic and haemodynamic determinations, but also by the clinical (heart rate) and biochemical (NT-proBNP) evolution32,33.
Even though rtPA can be delivered in shorter infusion times and UK may not be widely used in some countries, we decided to employ UK because, to date, there is no clear evidence to favour the use of one fibrinolytic over another, because our centre had previous experience managing limb deep vein thrombosis with LLDUT and also due to a more favourable cost profile34. One of the advantages of our approach is that LLDUT was performed with a regular 4 Fr pigtail catheter inserted through an antecubital vein. This approach simplifies the treatment and makes it feasible for most interventional radiology services because it avoids the use of specific equipment, such as ultrasound-assisted or mechanical thrombectomy devices, which may not always be available and can be related to complications, and because it prevents the cannulation of central veins, which can result in more severe puncture site haematomas12,35. Also, the use of a peripheral insertion site, as compared with jugular or femoral insertion points, is related to fewer movement limitations and better patient comfort.
Even though the incidence of minor haemorrhagic complications in our series was higher than that previously described (20% vs. 10-15%)10-12, only in four patients (4.6%) did treatment have to be stopped due to bleeding, with three of them requiring (3.4%) two units or fewer of PRBCs. Whether bleeding was related to LLDUT itself or to systemic heparinisation remains unclear, but we believe that the fact that mean fibrinogen values remained at normal levels during treatment might reflect a lack of systemic effects of this technique. When compared to systemic thrombolysis, the incidence of intracranial bleeding and major haemorrhagic events in our study was similar to previous data for CDT6-8,10-13,25.
Six-month survival in our series was 95.4%, with none of the deaths being related to PE or complications of LLDUT. This survival rate is comparable to previously reported data and supports the hypothesis that, overall, CDT are associated with a lower mortality rate than would be expected for submassive PE2-4,10-12.
It remains unclear whether the outcome achieved with CDT would differ from that achieved by anticoagulation alone in the long term, and if the rapid improvement of right ventricular function will cause better survival rates. The ULTIMA study is, to our knowledge, the only RCT that compared both strategies. Even though it showed a rapid decrease in the right-to-left ventricular ratio in the USAT plus rtPA group, it was unable to demonstrate differences in mortality, haemodynamics or major bleeding complications at 90 days when compared to the heparin group10. A recent retrospective study evaluating patients with submassive PE receiving anticoagulation vs. CDT found that CDT led to a faster restoration of right ventricular function and shorter ICU stays, while being associated with higher complication rates and similar outcomes at up to eight months36. Because the impact of CDT, when compared to systemic anticoagulation, is not yet clear, further RCTs assessing both strategies are still needed before establishing the best therapeutic approach for haemodynamically stable PE patients with RVD.
Conclusions
In our group of patients, LLDUT rapidly decreased thrombus burden and improved right ventricular function while being associated with no major complications.
| Impact on daily practice CDT should be considered when treating haemodynamically stable PE with RVD. They are associated with a rapid decrease of PAP and a prompt improvement of the RVD, with a lower rate of major complications when compared to systemic thrombolysis. |
Acknowledgements
We would like to thank Dr Hergen Buscher and Dr Ellen Corrigan for their revision and input on the text and Dr Domínguez de Villota for his advice during the study and endless reviews of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.

