Abstract
Aims: The aim of our study was to evaluate the prevalence of left atrial cavity and appendage thrombosis in patients undergoing cardioversion for non-valvular atrial tachyarrhythmias. In persistent atrial tachyarrhythmias, 90% of thromboses are reported to be located inside the left atrial appendage. This prevalence refers to old studies and meta-analysis in a mixed population of valvular and non-valvular atrial fibrillation. Left atrial cavity thrombosis in non-valvular atrial fibrillation has not been investigated recently in large-scale studies.
Methods and results: A total of 1,420 consecutive adult patients with paroxysmal or persistent atrial tachyarrhythmias, candidates to cardioversion, who opted for a transoesophageal echocardiography-guided strategy, were enrolled in the study. Mitral stenosis, rheumatic valve disease and mechanical prostheses were excluded. In total there were 91 thrombi in 87 patients with a prevalence of 6.13% (87/1,420). Patients with left atrial thrombosis had predisposing clinical and echo characteristics (heart failure, lower ventricular function and higher atrial volume). Except for one case in which the thrombus was located in the left atrial cavity (0.07%), and three in the right appendage, all thromboses were detected in the left atrial appendage.
Conclusions: Extra-appendage thrombosis is a very rare finding in non-valvular persistent and paroxysmal atrial tachyarrhythmias and, when present, a left appendage thrombus is usually concomitant.
Introduction
Atrial fibrillation (AF) is the most common tachyarrhythmia and is the cause of the majority of cardiac strokes. The thrombi responsible for these embolic events are located in the body of the left atrium (LA) and very frequently in the left atrial appendage (LAA)1. LAA dysfunction, as demonstrated by low-to-absent LAA emptying velocities, is correlated to thromboembolic risk2,3. LAA velocities are predictors of clot formation, independently from thrombotic risk factors4. However, a significant percentage of left atrial thrombosis (LAT) localises outside the LAA, in the LA cavity (LAC).
Thrombosis localisation inside the LA is an emerging topic due to the increasing use and feasibility of LAA occlusion techniques5,6. In the 1950s, when rheumatic valve disease was the main cause of AF, it was recognised that 50% of thromboses were located in the LAA with a consequent 50% embolic risk reduction after LAA obliteration at the time of the commissurotomy7. Manning et al8, among 233 AF patients, not anticoagulated, found 15% left atrial thrombi, 34 in the LAA and one (3%) located in the LAC. Blackshear9 reviewed studies on rheumatic AF and found 254 LAA and 192 LA cavity thrombi among 3,504 patients, whereas, in non-rheumatic AF, 91% of left atrial thrombi were located in the LAA. This reference, and these frequencies, are still nowadays the most cited, but the populations studied were different, the definition of non-valvular AF was not the current one, and patients were treated with different anticoagulative regimens; therefore, these data need to be updated.
Recent studies have not analysed the frequency of an extra LAA thrombus formation in non-valvular AF10. The populations studied in recent papers are heterogeneous as they include surgical patients, valvular heart disease, candidates to ablation procedures, and patients who experienced stroke or transient ischaemic attack. Moreover, most studies did not distinguish between atrial cavity and appendage localisation of thrombi - even large and well conducted ones - although computed tomography scans were used11.
The changing epidemiology of AF, with a decreased incidence of rheumatic valve disease, the wide use of anticoagulation and the availability of new oral anticoagulants has led to a recent revision of the definition of “non-valvular atrial fibrillation”3. Moreover, new appendage occlusion techniques are increasingly employed in clinical practice in non-valvular heart disease; however, the reported frequency (10%) of a clot in the LA cavity is a non-indifferent percentage, which may limit an extensive use of these techniques. Therefore, a revision of the prevalence of extra-appendage thrombosis in the population with “non-valvular heart disease” (according to the current definition) is necessary.
The aim of our study was to evaluate the relative frequency of atrial cavity and left appendage thrombosis in a large population of consecutive patients with non-valvular AF, defined according to current reappraisal, and to revise the literature on the topic12.
Methods
Adult patient candidates to cardioversion for paroxysmal or persistent AF or atrial flutter (AFL), referred to the Cardiological Imaging Department, Misericordia Hospital, Grosseto, Italy, were consecutively enrolled in the study. When the arrhythmia onset time was longer than 48 hours, patients were informed of the risks and benefits of the two strategies - conventional, with at least three-week oral anticoagulation, or transoesophageal (TEE)-guided cardioversion. Patients who chose the conventional strategy, and therefore did not give consent for the TEE exam, were excluded from the study. Patients included in the study were candidates to a TEE-guided strategy of cardioversion and were anticoagulated according to current guidelines: if not chronically anticoagulated they were treated with intravenous sodium heparin, low molecular weight heparin or novel anticoagulants (NOAC); if they were already anticoagulated with vitamin K antagonists, they were included in the study if the international normalised ratio (INR) was below the therapeutic doses or in cases where they had been anticoagulated for less than three weeks. Exclusion criteria were: acute coronary syndrome, acute pulmonary embolism, acute stroke or transient ischaemic attack, history of rheumatic valve disease, mitral stenosis, mechanical prosthesis. Heart failure was defined by the presence of typical symptoms of decompensated heart disease (e.g., breathlessness, pulmonary crackles, peripheral oedema) with demonstration of instrumental signs (such as signs of pulmonary hypertension at chest X-ray, ultrasound finding of liver congestion, elevated proBNP). The definition of “valvular AF” was derived from De Caterina et al12. Atrial tachyarrhythmias were defined as “persistent” when non self-terminating and requiring cardioversion, in accordance with current guidelines13.
ECHOCARDIOGRAPHIC EXAMINATION
All patients underwent transthoracic echocardiography (TTE). Two-dimensional measurements were obtained by TTE according to the standards of the American Society of Echocardiography14. Left ventricular volumes and ejection fraction (EF) were measured by the Simpson’s biplane method.
All patients underwent multiplane TEE within 24 hours of the scheduled cardioversion. Echocardiographic exams were performed in our institutional single referral centre by three experienced operators with a level III competence (in accordance with the European Society of Cardiovascular Imaging)15. Studies were digitally recorded and analysed by two different operators and, in case of inter-observer discrepancy, images were reviewed by another well-trained observer not involved in the study and an agreement was reached. LAA images were acquired from the upper and middle oesophagus positions at different degrees of multiplane examination (generally from 0° to 135° with slight or more pronounced counterclockwise rotation of the probe) to explore comprehensively the left and right atrium and all the LAA lobes. The presence or absence of LAT and LAA flow velocity (the highest positive velocities within five consecutive cycles were measured in the LAA and then averaged) was analysed. A cut-off value of 20 cm/sec was used to define low emptying velocities16.
Thrombi were defined as echo-reflecting masses in the atrial body or in the LAA, distinct from the underlying endocardium, observed in more than one imaging plane, and not related to pectinate muscles. The presence of spontaneous echo contrast was classified according to Fatkin17. Dense persistent spontaneous echo contrast class 4 (according to Fatkin’s classification) was differentiated from true thrombosis, diagnosed as a separate mass with a clear, well-defined “non-smoking” edge.
STATISTICAL ANALYSIS
For anatomic and functional comparisons between groups, continuous variables of mean LAA and right atrial appendage (RAA) data were compared by using independent samples Student’s t-tests. Categorical variables between groups were compared by using χ2 tests, particularly for differences between patients with and without atrial thrombosis. All results were considered significant with p-values <0.05. In order to evaluate thrombosis predictors, univariate and multivariate analyses were performed on several clinical and echographic parameters including age, sex, presence of hypertension, coronary heart disease, heart failure at admission, CHA2DS2-VASc risk score18, EF, left atrial volume, and anticoagulant/antithrombotic therapy. Statistical analysis was performed using SPSS, Version 23 (IBM Corp., Armonk, NY, USA).
RETROSPECTIVE ANALYSIS OF ATRIAL CAVITY THROMBOSIS
A retrospective analysis was performed on the entire institutional TEE database searching for atrial cavity thrombosis, in order to review previous cases of extra-appendage thrombosis. This review included a total of 5,195 consecutive TEE studies performed for several reasons, most of them in sinus rhythm.
Results
A total of 1,420 patients were enrolled. The main clinical features are described in Table 1. Sixty-five percent of the patients were male, 78.2% were affected by AF and 21.8% by AFL. Mean age was 70±10 years. Ten percent of the patients had a history of previous cardioversion for the same types of arrhythmia.
The total incidence of atrial thrombosis was 6.13% (87/1,420). The estimated arrhythmia onset, available in 978 patients, was 14 days. The characteristics of patients affected by LAT are reported in Table 2.
In total, 91 atrial thrombi were detected in 87 patients (80 AF and 7 AFL). An LAA thrombosis was present in all these cases. In three cases, a thrombus was found in the RAA (in one case, two distinct right atrial thrombi) and in one case in the LAC (Figure 1, Moving image 1-Moving image 3). The prevalence of extra-LAA thrombosis was 4/1,420 (0.28%) including three cases (0.21%) of right appendage thrombosis. Only one case of left atrial thrombus was detected, accounting for 1.15% of all patients with thrombi (1/87). Therefore, the prevalence of atrial thrombosis located outside the LAA in this large population of non-valvular AF/AFL was 0.07% (1/1,420). The main results are represented in Figure 2.
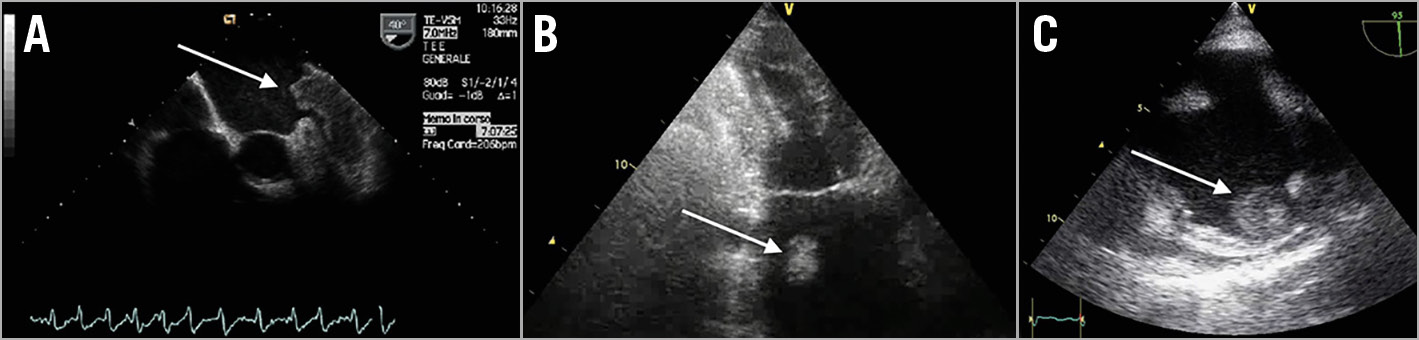
Figure 1. Echographic examples of intra- and extra-appendage thrombosis. A) A large left atrial appendage (LAA) thrombus protruding in the left atrium (still frame of Moving image 1). B) A left atrial cavity (LAC) thrombus is clearly visible in a transthoracic two-chamber view (still frame of Moving image 2). C) A right atrial appendage (RAA) thrombus in a transoesophageal bicaval view (still frame of Moving image 3).
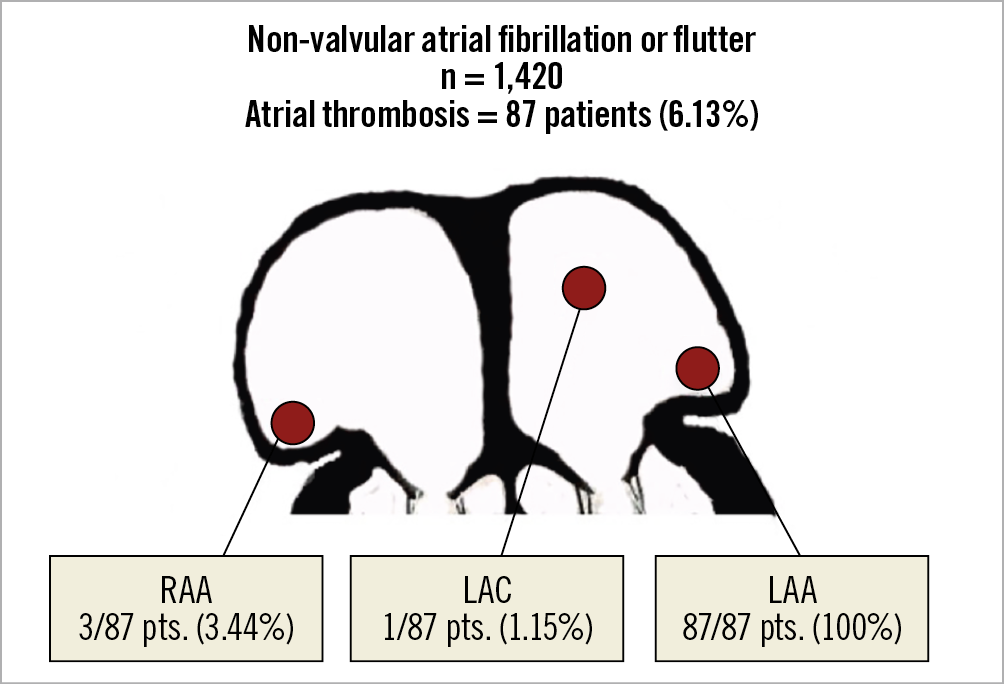
Figure 2. The localisation of atrial thrombi in the population study is represented in the schema.
High CHA2DS2-VASc risk score, higher age, heart failure, higher atrial size, and lower ventricular function were thrombosis risk factors at the univariate analysis, but at the multivariate analysis only the presence of heart failure, low EF and higher atrial volume index correlated with the thrombosis risk. In our study, among patients with atrial thrombosis a decompensated heart failure was present in 51 of them (51/87=59%). The presence of atrial thrombosis was correlated to a higher prevalence of heart failure (59% vs. 23%, p<0.001). All patients with left extra-appendage thrombosis were affected by heart failure and had a significantly lower EF (31.2% vs. 52%, p:<0.001). Patients with a thrombus had higher atrial volumes (53 vs. 40 ml/m2, p=0.03) (Table 2).
Discussion
In this study we demonstrated that, in a large population of consecutive patients affected by non-valvular paroxysmal or persistent AF/AFL, the prevalence of thrombosis not localised inside the left appendage was 4/1,420 (0.28%) including three cases of right appendage thrombosis. Only one case of left atrial cavity clot was detected, accounting for 1.15% of patients with thrombi (1/87). In particular, in all cases of extra-LAA thrombosis the patients presented even an LAA thrombus. These data are different from previous literature reporting cases of atrial thrombosis outside the LAA amounting to 9%9,10. Mahajan et al10 performed a systematic review of studies reporting the location of thrombus in the left atrium. Among 1,206 papers on the topic, 34 describing the location were selected, seven in a mixed population of valvular and non-valvular AF and only ten in a population of non-valvular AF. In this review, the proportion of thrombosis located outside the LAA (inside the left atrial cavity) was 56% in valvular, 22% in the mixed cohort studies and 11% in non-valvular AF, respectively - similar to Blackshear’s data9. It is noteworthy that study populations and methods were different among the reviewed studies: three were autoptic, nine intraoperative for mitral stenosis or mitral valve disease. TEE studies were mostly performed preoperatively for mitral valve disease; therefore, the results are not completely comparable to our data and to the real daily practice of non-valvular AF.
Not all the results of these recent studies can be compared to our data. The reasons are: a) most of them focused the research of atrial thrombosis on patients who would undergo ablation procedures, a population in which anticoagulation was mandatory with a consequently lower incidence of thrombosis; b) in three studies, cardiac computed tomography was used for the assessment of atrial thrombosis and none of them reported data on right appendage thrombosis; c) single studies were significantly less numerous than ours.
A retrospective analysis of our entire institutional TEE database was performed in order to review the other cases of extra-appendage thrombosis. This retrospective review revealed that, among 5,195 consecutive TEE studies, an atrial cavity thrombosis had been found in eight patients - six affected by permanent AF (one malignant pulmonary tumour, two mitral stenosis, one mechanical mitral prosthesis, two sepsis) and two in sinus rhythm (one malignant pulmonary tumour and one affected by a vasculitis). Therefore, our data confirm that the presence of a thrombus in the LAC is associated, in most cases, with mitral stenosis, a mechanical prosthesis, or, in non-valvular AF, with a thrombophilic condition and therefore they could not be candidates to appendage occlusion.
Right appendage thrombosis is another localisation which had already been analysed by our group19. Although probably under-recognised in clinical practice, its prevalence is low (0.2% in the present series of non-valvular AF), the clinical impact of embolisation is probably less critical, and yet never observed in our series.
The main differences of our study, compared to previous literature, are: a) it is a recent and homogeneous population of consecutive patients with persistent atrial tachyarrhythmias with the exclusion of rheumatic disease, mitral stenosis and prosthetic mechanical valves, according to the current definition of “non-valvular AF”, representing the largest series of patients ever published on the topic; b) patients were anticoagulated according to current guidelines, most of them started within a few hours from arrhythmia onset; c) a research of right atrial appendage thrombosis was routinely performed; d) CHADS2-VASc2 risk score, presence of heart failure (HF), EF and indexed atrial volumes were available for all patients and have been evaluated in multivariate analysis; e) a large number of patients with AFL were included who, nowadays, are believed to have a similar thrombotic risk to AF20.
Limitations
Our patients were affected by non-valvular AF and candidates to cardioversion; therefore, the findings are not extensible to all cases of atrial tachyarrhythmia, in particular to permanent forms. Most of our patients were anticoagulated according to current guidelines, thus reflecting the daily practice population. Further prospective studies confirming our results should be performed.
Conclusions
In this large-scale series, patients with non-valvular AF/AFL had a prevalence of extra-LAA thrombosis of 0.28% (4/1,420). Among 87 patients in whom an atrial thrombosis was demonstrated, three (3.45%) presented both LAA and RAA thrombi and only one (1.15%) showed both LAA and LAC thrombosis. Therefore, the prevalence of atrial clots outside the LAA in the population of non-valvular AF is 0.07% (1/1,420). This prevalence is significantly lower than the previous 10% frequency reported in the literature and may reinforce the indications for percutaneous appendage occlusion21. Left atrial cavity thrombosis is a very rare condition in non-valvular AF and has seldom been described in case reports, mostly in patients affected by a thrombophilic condition or acute heart failure.
|
Impact on daily practice – The prevalence of left atrial appendage thrombosis in atrial fibrillation is reported to be 90% but this refers to old studies, mostly in mixed populations of valvular and non-valvular atrial fibrillation. A new definition of what is “non-valvular atrial fibrillation” has recently been proposed. – In our study the localisation of thrombosis was inside the left appendage thrombi in 100% of the cases. In one case a thrombus was present in the atrial cavity too. – Left atrial cavity thrombosis is a very rare condition in non-valvular AF, mostly in patients affected by a thrombophilic condition or acute heart failure. In this case series, only 1.15% of atrial thrombi were located in the left atrial cavity. These data may increase the interest in percutaneous left appendage occlusion techniques. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Mid-oesophageal echo view showing a large left appendage thrombus protruding in the left atrium.
Moving image 2. Transthoracic two-chamber view showing a highly mobile left atrial cavity thrombus.
Moving image 3. Mid-oesophageal echo view showing a right appendage thrombus.<

