Abstract
Prevention of stroke represents a goal of primary importance in health systems due to its associated morbidity and mortality. As several patient groups with increased stroke rates have been identified, multiple approaches have been developed and implemented: oral anticoagulation (OAC) for patients with atrial fibrillation, surgical and percutaneous revascularisation in patients with carotid disease, device closure for patients with patent foramen ovale, and now, left atrial appendage occlusion (LAAO) for selected patients with non-valvular atrial fibrillation (NVAF). The latter group of patients are the focus of this review which evaluates the pathophysiology, selection of patients, procedural performance, outcomes of treatment both during and post-procedure, adjunctive therapy, complications, and longer-term outcomes.
Introduction
An understanding of the pathophysiology and the development of preventive strategies are the cornerstones to reducing stroke-related morbidity and mortality. Among all strokes, 87% are ischaemic, 10% are intracerebral haemorrhage, and 3% are subarachnoid haemorrhage (SAH) (Supplementary Figure 1, Supplementary Figure 2).
There is a strong association between atrial fibrillation (AF) and stroke which has been found to be related to 15%-20% of all ischaemic strokes12345. Patients with AF have a 5-fold increase in stroke events with increased mortality, morbidity, and recurrence. The temporal relationship between AF and stroke is variable; some patients present with AF at the time of a stroke, while in others, AF is detected during outpatient monitoring6. In non-valvular AF (NVAF), the aetiology of cardioembolic stroke has been identified as thrombus in the left atrial appendage (LAA) in approximately 90% of cases7.
Stroke prevention relies on appropriate risk screening. Although several scores are available, the CHA2DS2-VASc is the most commonly used8. The specificity and precision of this score, however, reveals it to be of modest benefit, and several of its components are similar to those used for the prediction of bleeding events (HAS-BLED)9. Other important risk elements include LAA and left atrial (LA) function and size, the specific features of AF (paroxysmal or permanent) and the presence or absence of other cardiac disease.
Although oral anticoagulation (OAC) has been recommended as a standard of care, its implementation has been problematic510. In 6,195 acute stroke admissions in NVAF patients from 2018-2019 (Gurol EM, et al. Abstract 38: Hemorrhagic Strokes in Patients with Atrial Fibrillation: The Neuro-AFib Study. Stroke. 2021;52), ischaemic strokes predominated (5,153 patients, 83.2%); 77% of these patients had a history of prior AF, while in 23% the diagnosis was new. In patients with previously known AF, only 44.9% were on an OAC11. In addition, midterm compliance remains suboptimal; at 1.3 years, only approximately 60% had continued with the OAC12. In a systematic review and meta-analysis of 39 studies and 593,863 patients, 1-year compliance with a direct OAC (DOAC) was <80%. This data, plus the information that in NVAF approximately 90% of the thrombi were identified in the LAA, formed the rationale for the development, testing, and subsequent approval of LAA occlusion (LAAO) devices713.
The LAA (Figure 1) is exquisitely fragile with multiple interstices between the pectinate muscles. It is sometimes described by surgeons as being almost translucent and termed “the most lethal human attachment”, which is related to the ease with which it may be damaged during surgery (Figure 2A, Figure 2B)13. Multiple morphologies have been described, often with multiple lobes, each of which may serve as the nidus for thrombus (Figure 3A, Figure 3B). While differences in the rate of stroke, depending on the specific morphology, have been described, this information is typically not used as a clinical indication for closure. In addition, the orifice of the LAA is typically oval while LAAO devices are usually round, leading to the potential for residual leak.
The myocardium of the LAA is rich in both atrial and brain natriuretic peptides (ANP and BNP, respectively); and its shape may foster its utility as a regulator of left ventricular end-diastolic pressure (LVEDP). Accordingly, it may have an important haemostatic endocrine function. In some patients, particularly those with left ventricular hypertrophy, atrial systole may be haemodynamically important. Finally, in some patients, the LAA itself may be responsible for the electrical activation of AF14. These considerations in AF patients have not been found to be of general clinical significance but remain under investigation.
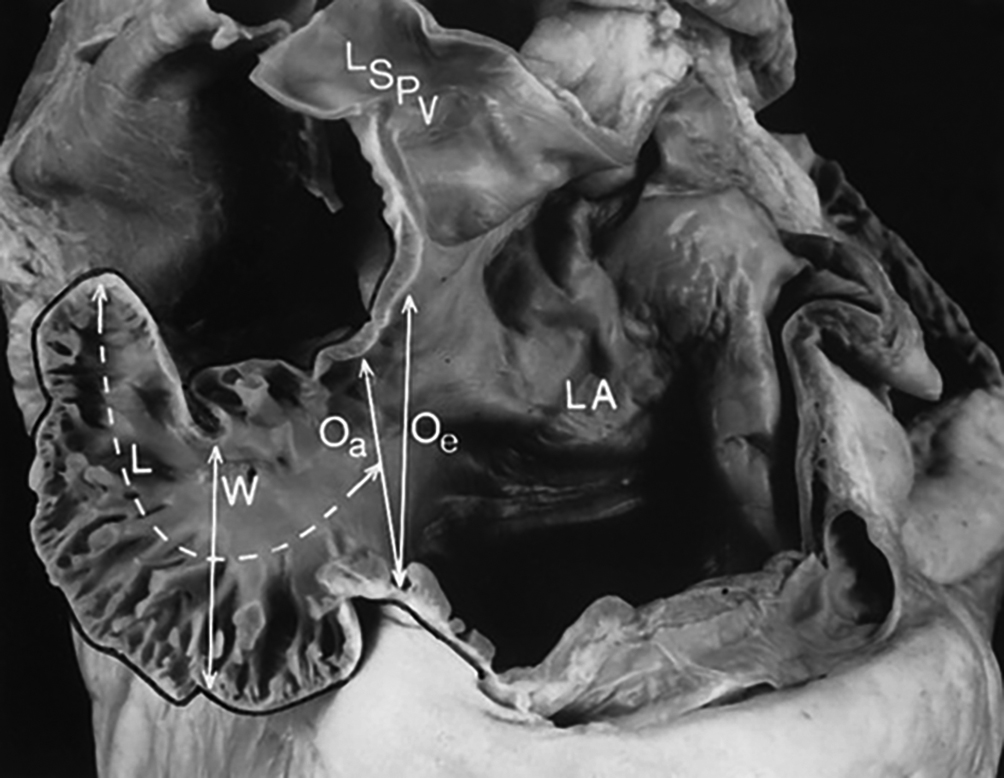
Figure 1. Measurement of the left atrial appendage. The left atrial appendage (LAA) is formed during the 4th week of embryogenic development. The relationship between the LAA, the left superior pulmonary vein (LSPV) and LA can be seen. A prominent feature is the presence of multiple pectinate muscles between which the atrial myocardium is very thin; thrombi may develop and become the source for an embolic stroke. Important measurements include length (L), width (W) and the ostial dimensions defined both echocardiographically (Oe) and anatomically (Oa). These dimensions and the angulation are important for device selection. (Reproduced with permission from31)
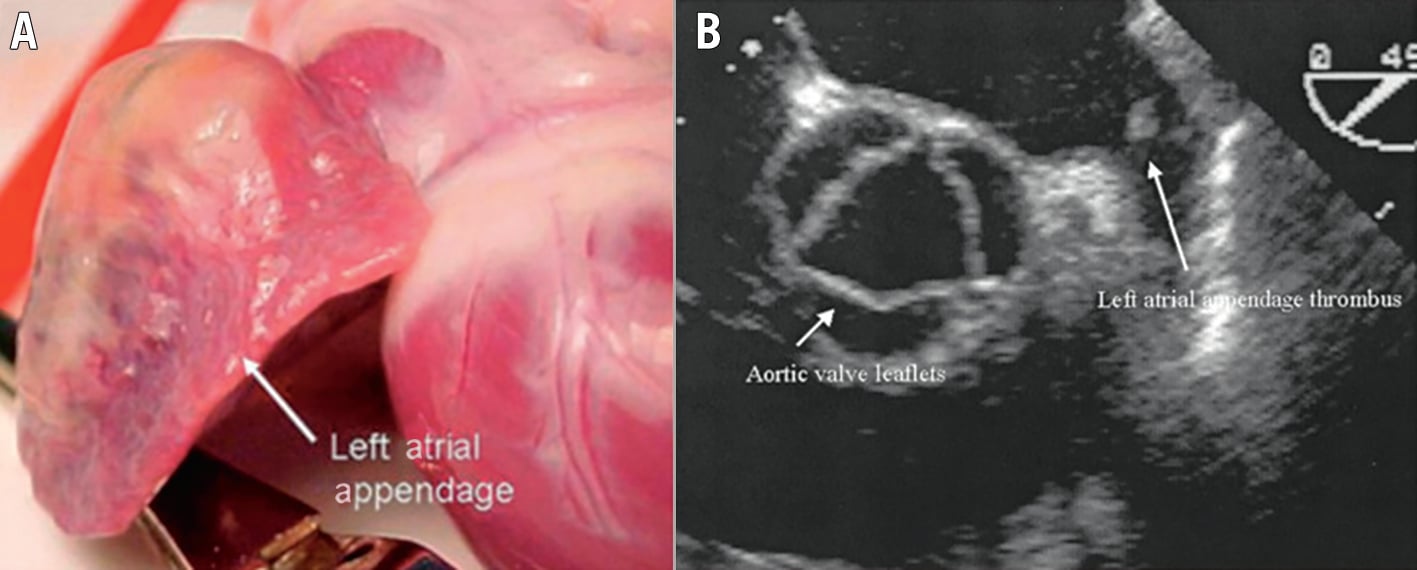
Figure 2. “The most lethal human attachment”. LAA external view and TOE with thrombus − document specific anatomic characteristics including a thin-walled LAA with the crevasses identified produced by the pectinate muscles (A) and the thrombus which may be present (B). (Reproduced with permission from105). LAA: left atrial appendage; TOE: transoesophageal echocardiogram
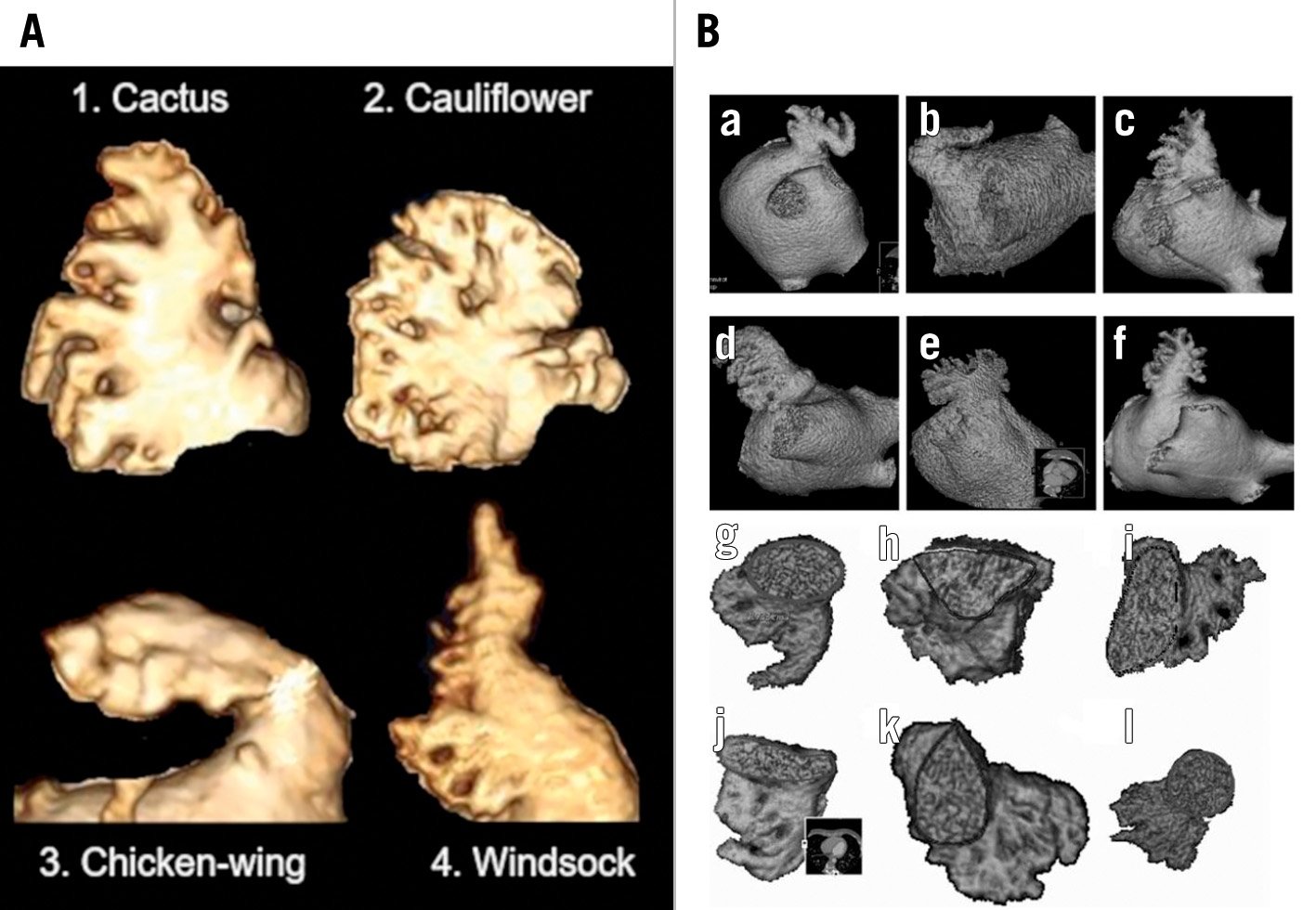
Figure 3. Morphology classifications. A) Modified caricatures of the 4 types: 1. Cactus, 2. Cauliflower, 3. Chicken-wing, and 4. Windsock. B) More detailed subtypes have been identified by CT. Four general shapes have been identified: chicken-wing (a, b), windsock (c,d), cauliflower (e) and cactus (f). In addition, there is a variability in the shape of the ostium ranging from oval (g) to triangular (h), foot-like (i,j), water drop (k), and round (l). The specific frequencies vary. Some specific features have also been associated with increased potential for ischaemic stroke/systemic embolic events. This morphologic information may be helpful in selecting access for LAAO or even for selection of a more optional device configuration. (A) reproduced from Adukauskaite A, et al. ECR 2019 Congress, C-2357, Innsbruck AT; (B) reproduced with permission from30. CT: computed tomography; LAAO: left atrial appendage occlusion
Current data
Over the past 20 years, LAAO has emerged as a safe and effective alternative to OAC for stroke prevention in the expanding number of patients with NVAF at increased stroke risk1315161718192021 (Central illustration). There are multiple randomised clinical trials (RCT) and numerous single, multicentre, and postapproval studies in aggregate including >250,000 patients treated with one of 2 American devices. Multiple other devices are undergoing clinical trials. Professional societal guidelines (including the American College of Cardiology [ACC], the American Heart Association [AHA], the Heart Rhythm Society [HRS], the Society for Cardiac Angiography [SCAI], the European Society of Cardiology [ESC], and the European Association of Percutaneous Cardiovascular Interventions [EAPCI]) have labelled LAAO as a Class IIB indication for stroke prevention relative to OAC in selected patients34. Despite this, many patients worldwide do not receive either LAAO or an OAC.
Long-term data analyses of the WATCHMAN 2.5 device (Boston Scientific) are now available from the randomised PROTECT AF and PREVAIL trials and accompanying registries. At 5-year follow-up of the RCTs which enrolled 1,114 patients for 4,343 patient-years, LAAO with the WATCHMAN provided stroke prevention that was comparable to vitamin K antagonists (VKA). There were significant reductions in haemorrhagic stroke (hazard ratio [HR] 0.20; p=0.002), disabling/fatal stroke (HR 0.45; p=0.03), cardiovascular/unexplained death (HR 0.59; p=0.027), and all-cause death (HR 0.73; p=0.035). There was also a marked reduction in post-procedural bleeding (HR 0.48; p=0.0003). In the 2 nested registries accompanying each RCT (Continued Access to PROTECT AF [CAP] and Continued Access to PREVAIL [CAP2]), with a total of 1,144 patients followed for an average of 50 months, there was a dramatic decrease in event rates compared to that predicted by the CHA2DS2-VASc score. At a mean of 5 years of follow-up, haemorrhagic stroke was 0.17 per 100 patient-years in CAP and 0.09 per 100 patient-years in CAP2. There was also a marked reduction in ischaemic stroke in each registry compared to what had been expected with the CHA2DS2-VASc score (1.30 and 2.20 respectively per 100 patient-years for CAP and CAP2, respectively) (Supplementary Figure 3).
A third trial, PRAGUE-17, randomised 402 patients with a CHA2DS2-VASc score of 4.7±1.5 and a HAS-BLED score of 3.1±0.9 to LAAO versus DOAC (frequently apixaban) and followed them up to 20.8±10.8 months. The primary endpoint was a composite of combined ischaemic, bleeding, and procedural events which was non-inferior when comparing LAAO with a DOAC (Table 1). The combined outcome data on PROTECT AF, PREVAIL, and PRAGUE-17 document excellent outcomes in the combined total of 1,516 patients22 (Figure 4).
Additional observational data from other multicentre registries and another RCT are available. Analysis is complicated for several reasons:
1. The specific device used (first- vs second-generation devices; plug-based vs disc- and lobe-based devices). The outcomes with first- versus second-generation WATCHMAN devices were considerably different. Although most of the existing literature pertains to the initial WATCHMAN 2.5 device, the current FLX device (Boston Scientific), which featured several design improvements, was recently studied in several registries. In a prospective non-randomised multicentre registry of 400 high-risk patients (mean CHA2DS2-VASc 4.2±1.5), the primary efficacy endpoint (composite of death, ischaemic stroke, systemic embolism) was met and the 12-month LAA closure rate, defined by ≤5 mm peri-device leak (PDL) as assessed by the echocardiographic core laboratory, was 100%23. Two patients experienced the primary safety endpoint: 1 patient had an ischaemic stroke 1 day after unsuccessful closure and the second a transient ischaemic attack (TIA) 2 days post-procedure. Post-procedural management included a DOAC and low-dose acetylsalicylic acid (ASA) for at least 45 days. At 2-year follow-up of the 395 successfully treated patients, all-cause mortality was 9.3%, cardiovascular death 5.5%, and all-cause stroke 3.4%. In addition, the rates of complete closure were maintained. Based on these data as well as changes in design (Figure 5), the FLX device is now the only WATCHMAN device clinically available.
Real-world outcomes of the WATCHMAN FLX device have been reported from the National Cardiovascular Data Registry (NCDR), with 16,446 patients treated (Kapadia SR, et al. Real-world Outcomes with WATCHMAN FLX: Early Results from SURPASS. CRT 2022. Washington, D.C., USA). Procedural success was achieved in 97.6%. Post-procedure, the majority (70.1%) were treated with a DOAC alone or in combination with ASA. The composite safety outcome of all-cause death, ischaemic stroke/systemic embolisation, or device/procedure complications requiring either open cardiac surgery or endovascular intervention during the hospital stay was seen in 60 patients (0.37%). Compared with the first WATCHMAN device, the FLX results improved for death (0.91%), stroke (0.38%), ischaemic stroke (0.28%), pericardial effusion requiring intervention (0.51%), and device-related thrombus (DRT) (0.23%); 95% of recipients had leak <3 mm and 82% had no leak.
The Amplatzer Amulet device (Abbott) consists of 2 elements – a lobe and a disc (Figure 6). The recent Amulet IDE Trial randomised 1,878 high-risk patients to LAAO with the Amplatzer Amulet versus the WATCHMAN 2.5 device. The residual leak at 45 days was markedly improved with the Amulet (Figure 7) with no residual leak in 63% versus 46% with the initial WATCHMAN 2.5 device. Peri-device leak >5 mm was infrequent with both devices (1% with Amulet and 3% with WATCHMAN 2.5). The 12-month device composite safety endpoint event rate was not different between the two limbs of the trial (Figure 8). The frequency of major bleeding, typically pericardial effusion up to 10 days, was increased with the Amulet. This may have important implications, as by that time the patients would have been dismissed home. Typically, pericardial effusions with WATCHMAN devices occur within 24 hours of the procedure when the patient may still be hospitalised. There were no differences in 12-month ischaemic events between the 2 groups despite the significant difference in leak size, raising the question of the clinical importance of residual leak. Finally, post-procedure, dual antiplatelet therapy (DAPT) was used with the Amulet in contrast to the WATCHMAN 2.5 group, in which most were treated with a combination of OAC and ASA as per recommendations in the US Food and Drug Administration (FDA) instructions for use (IFU).
2. The specific patient populations in whom the devices were used (i.e., randomised vs selected patients, in whom the indication was a relative or complete contraindication to OAC), or the specific agent used (e.g., VKA or DOAC). This is increasingly important, because there are now 4 alternative DOAC agents.
3. The variable protocol designs for either RCTs or registries, which in the case of the latter the presence and inclusion of a “control group” for comparison was used. The analysis of the WATCHMAN Registries included the now-accepted concept of expected versus observed adverse events. While this has disadvantages because of a lack of a randomised, carefully controlled group, it does offer a comparison of observed versus expected rates in a clinical population which, when adjusted, then serves as a control.
4. The post-procedure medications used; they were used by either protocol or clinical judgement, and with variable combinations of single antiplatelet therapy (SAPT), DAPT, VKA, and DOAC (either full strength or half strength). Any of these might affect early outcomes as well as late adverse events.
5. The follow-up imaging performed either by protocol or clinical design, and the specific modality and timing of assessment (e.g., computed tomography [CT] vs transoesophageal echocardiography [TOE]). If follow-up imaging is only performed because of a clinical event, it is not possible to ascertain accurate information about the prevalence of the specific endpoint being evaluated by DRT. In addition, the definition of events obtained using CT may be different than that of those obtained using follow-up TOE (e.g., hypoattenuated thickening [HAT]).
6. The variable endpoints for comparison: stroke (either ischaemic, haemorrhagic, or both), neurologic adjudication or not, death (cardiac or all-cause), peri-device leak and the assessment modality used for ascertainment, DRT, and device leak, either as a composite or as individual elements. This has important implications as there is typically a hierarchical order of clinical importance; in such a case, if significant bleeding from vascular access is included, it may be dominant in frequency and affect an overall endpoint that includes only stroke or death. In addition, local site-specific therapy will only be able to decrease ischaemic events related to the LAA but will not be able to decrease events related to other pathophysiologic substrates.
7. The statistical analysis used, either frequentist or Bayesian methodology, and strategies used for meta-analyses, irrespective of whether patient level data was used or not.
The FDA approval process for LAAO mandated a national registry for all commercial (non-investigational device) patents24. Data on 38,158 procedures from 495 hospitals performed between January 2016 and December 2018 documented a dramatic increase in the cumulative number of procedures and implanting physicians and hospitals24 (Supplementary Figure 4, Supplementary Figure 5). Compared with the pivotal trials, patients currently treated are at higher risk; the CHA2DS2-VASc score was 4.6±1.5 compared with 3.4±1.5 in PROTECT AF, and 60.8% are elderly, of whom many are 85 years or older (Table 2). The distribution of CHA2DS2-VASc and HAS-BLED scores documents a relatively wide variability (Supplementary Figure 6). The initial WATCHMAN 2.5 device was successfully deployed in 35,417 patients (92.8%) (Supplementary Table 1, Supplementary Table 2). Major adverse in-hospital events occurred in 2.16%, most commonly major bleeding (1.25%) or pericardial effusion requiring intervention (1.39%) (Figure 8). Subsequent planned analyses will include changing patient characteristics, device-related complications (DRT, leak, embolisation), longer-term outcomes, post-procedural medications, and new technology. This data will supplement the 2 large RCT (CHAMPION-AF [ClinicalTrials.gov: NCT04394546] and CATALYST [ClinicalTrials.gov: NCT04226547]), each of which is enrolling in aggregate approximately 5,000 patients.
The most recent RCT, SWISS-APERO, evaluated 3 groups of patients – Amulet (111 patients, 50.2%), WATCHMAN 2.5 (25 patients, 12%), current WATCHMAN FLX device (85 patients, 42%) using a superiority design. The primary endpoint was a composite of crossover to a non-randomised device during LAAO or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days. Secondary endpoints included procedural complications, device-related thrombus, peri-device leak assessed by CCTA at 45 days, and clinical outcomes.
The primary superiority endpoint of the device – crossover or residual LAAO patency – was not met. For secondary endpoints, there were important differences: 1) at 45 days, the endpoint of cardiovascular death, stroke, or systemic embolism occurred in 3 Amulet patients and 5 WATCHMAN patients (HR 0.59, 95% confidence interval [CI]: 0.15-2.43); 2) there were more major procedural complications with the Amulet including bleeding and pericardial effusion as compared to WATCHMAN (9.0% vs 2.7%; p=0.047); and 3) using the prespecified peri-device leak assessed by CCTA, leaks were common in each group, but the type of leak varied. With TOE, there were more WATCHMAN leaks. Irrespective of imaging technology, no leak was major (defined as >5 mm).
This is an important trial that demonstrated excellent results with both techniques in patients who are at high risk for stroke or systemic embolisation. Both can be utilised in these patient groups to decrease subsequent stroke. It must be remembered that not all embolic strokes come from the LAA and that leaks are common. Although the large NCDR documented that there was a gradient with even small leaks ≥1 mm being associated with major embolic or ischaemic events, the overall event rate was relatively low25.
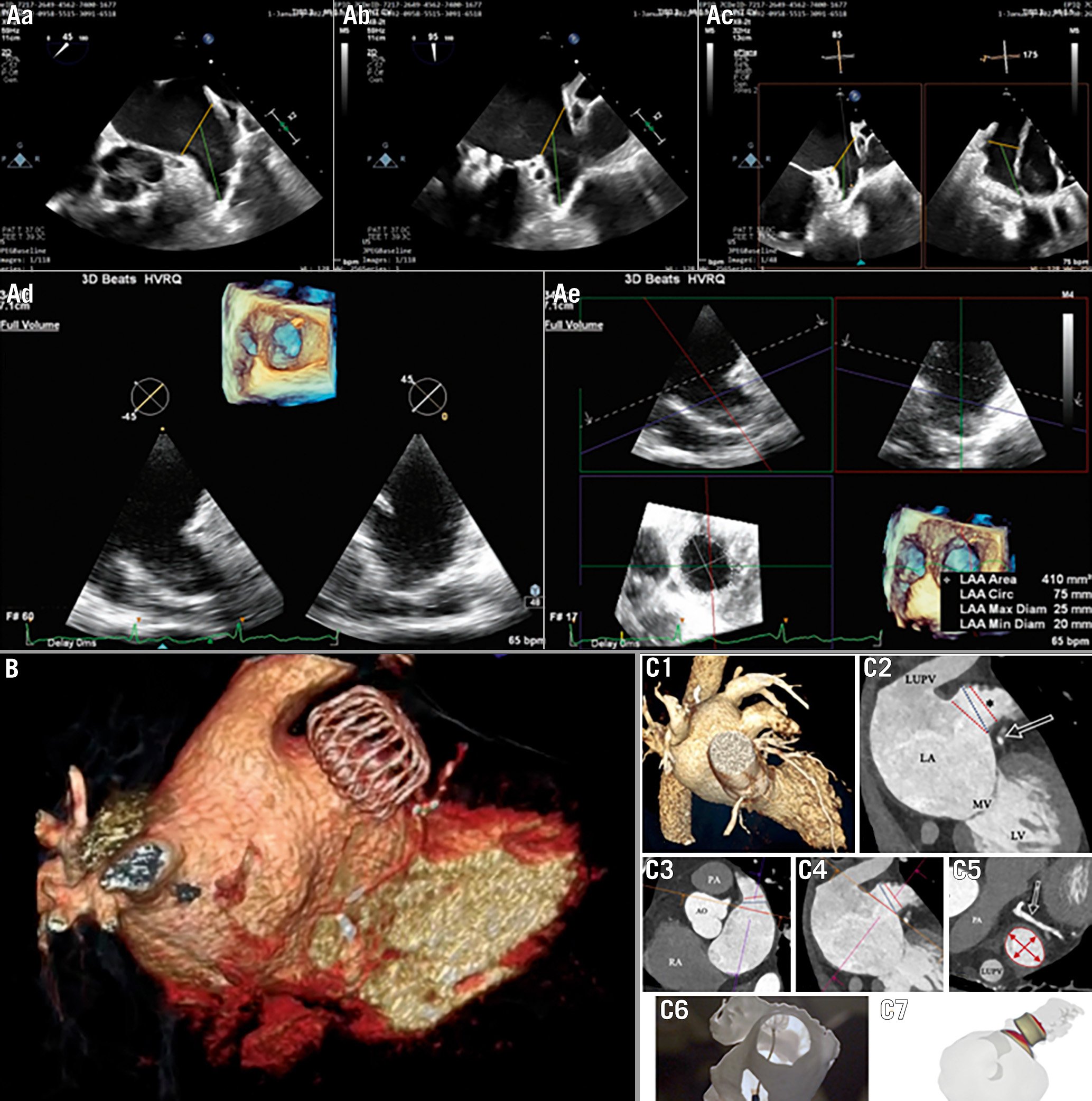
Central illustration. Imaging strategies for LAAO. Aa-Ae) Procedural planning based on TOE images: multiple views with 2D technique, 3D full volume of LAA with component 2D images. MultiVue of 3D full volume shows the blue plane's alignment with the landing zone and landing zone orifice measurements. B) 3D volume rendering reconstruction of CT scan imaging of successful LAA occlusion with WATCHMAN FLX. C) Procedural planning with CT scan: 3D image volume rendering 3D MPR analysis (1), identification and measurements of the LAA ostium and landing zone (2), (3), (4), (5), (6) and CT-based software simulation of Amulet implantation (7). CT: computed tomography; LAA: left atrial appendage; MPR: multiplanar reconstruction; TOE: transoesophageal echocardiogram
Table 1. Key findings of the LAAO randomised clinical trials.| Trial | Design | Patients | Key findings |
|---|---|---|---|
| PROTECT AF (PMID: 19683639) 66 |
WATCHMAN vs warfarin non-inferiority |
Control (n=244)Device (n=463) |
|
| PREVAIL (PMID: 24998121)67 |
WATCHMAN vs warfarinnon-inferiority | Control (n=138)Device (n=269) |
|
| PRAGUE-17 (PMID: 32586585)21 |
LAAO device vs DOACnon-inferiority | Control (n=201)Device (n=201) |
|
| CI: confidence interval; CV: cardiovascular; DOAC: direct oral anticoagulant; LAAO: left atrial appendage occlusion; SE: systemic embolism; HR: hazard ratio ; RR: risk ratio; TIA: transient ischaemic attack | |||
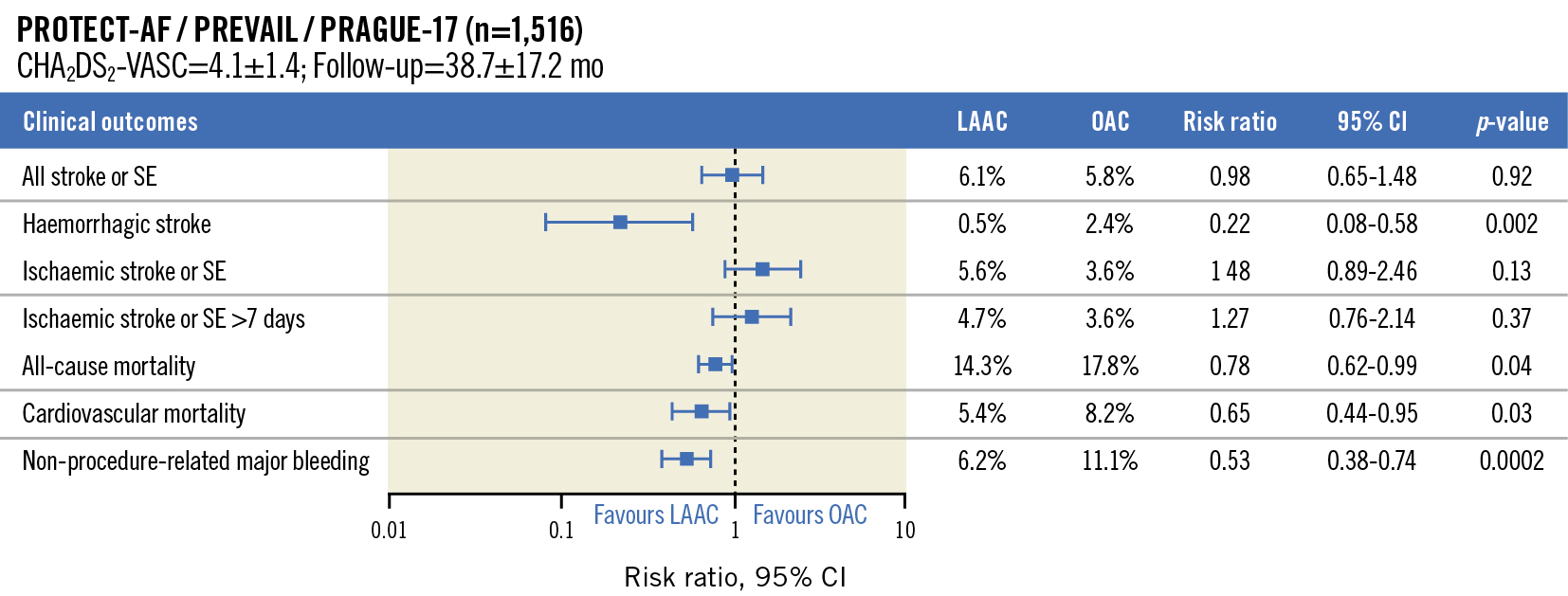
Figure 4. Summary of clinical outcomes. Data continue to accrue on the longer-term outcomes of the patients treated in the first 2 WATCHMAN 2.5 RCT of device versus warfarin and now the PRAGUE-17 with device versus DOAC. Several metrics have been used for comparison. As seen, there is no significant difference between LAAC and OAC in all stroke or systemic embolism. There is a marked reduction in haemorrhagic stroke (RR 0.22), and a significant reduction in CV and all-cause mortality. Non-procedure major bleeding was also significantly decreased. (Adapted with permission from22). CI: confidence interval; CV: cardiovascular; DOAC: direct OAC; LAAC: left atrial appendage closure; OAC: oral anticoagulants; RCT: randomised controlled trial; RR: risk ratio; SE: systemic embolism
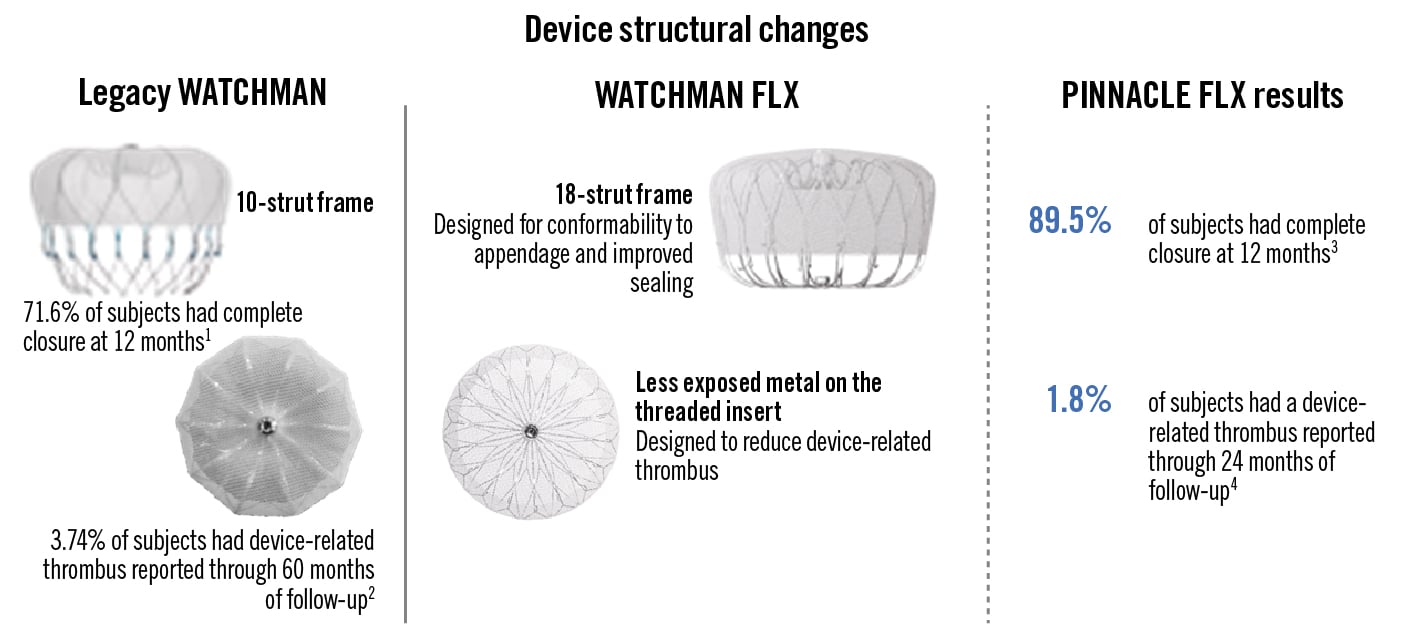
Figure 5. Addressing leak and device-related thrombus. Since the initial WATCHMAN 2.5 device (legacy device), a new device has been introduced and is now the only device available. As seen, there are structural differences in strut frames, with 18 frames in the new generation, and changes to the threaded metal insert. These changes have been associated with a marked improvement in closure rates at 12 months and a decrease in DRT at 12 months in the current FLX device. (Courtesy of Boston Scientific). DRT: device-related thrombus

Figure 6. Amulet device. The Amulet device has a dual-seal technology with a lobe that fills the left atrial appendage cavity and a disc to seal the ostium.
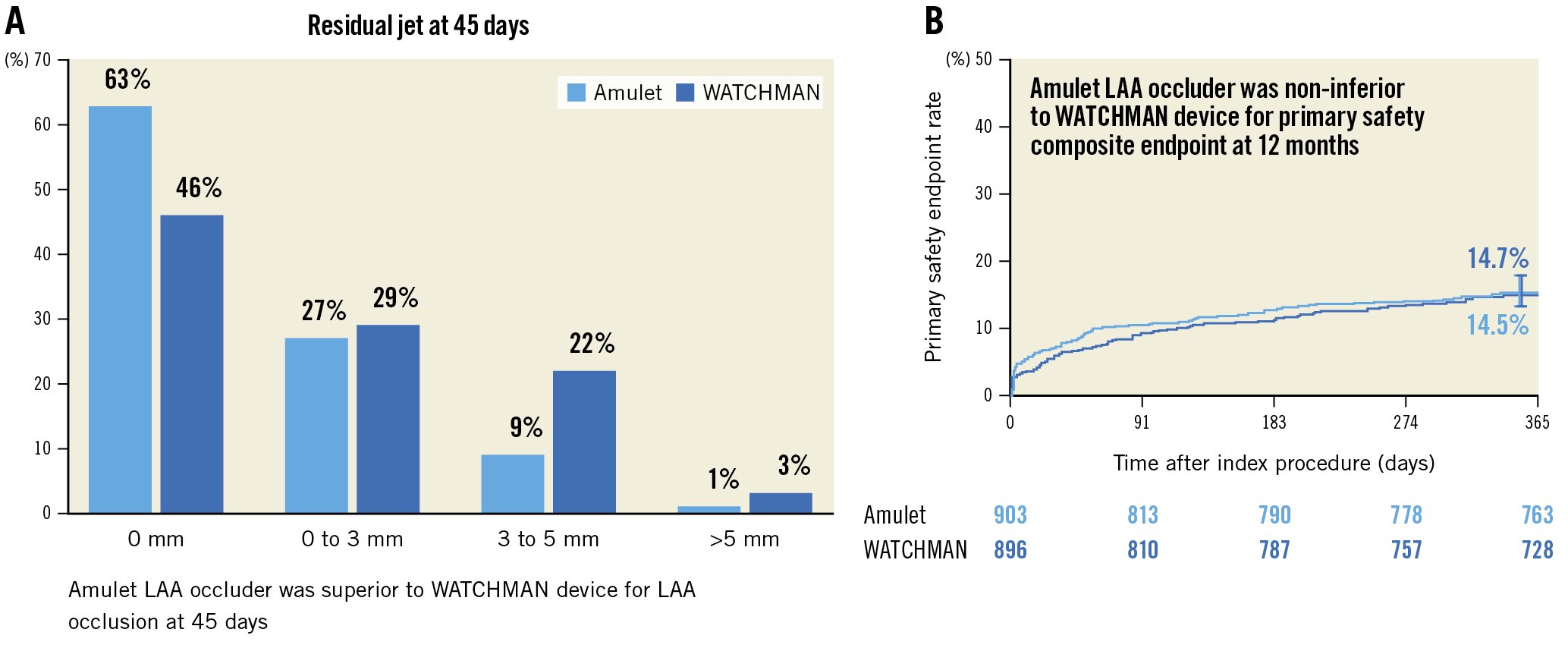
Figure 7. Mechanism of action (45 days); Primary safety endpoint (12 months). A) The mechanism of action was assessed as residual leak; differing significantly between the Amulet (63% –no residual leak) and the WATCHMAN 2.5 (46% – no residual leak). B) Safety was assessed using as procedural complications all-cause death or major bleeding at 12 months and was not significantly different. LAA: left atrial appendage
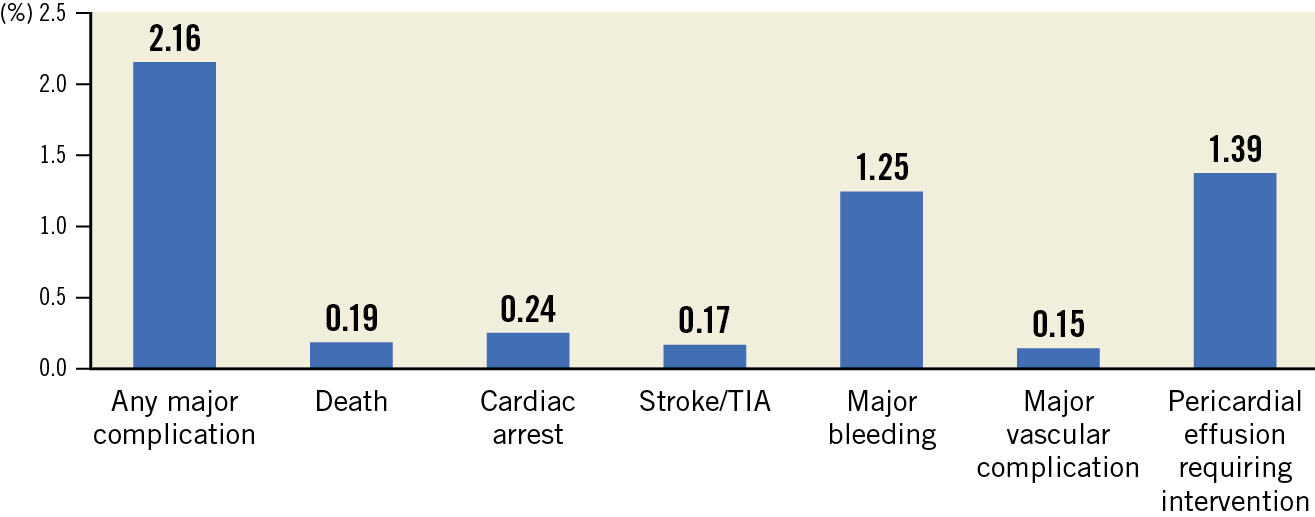
Figure 8. In-hospital adverse event rates in the NCDR-LAAO Registry. Compared with the pivotal trial results of LAAO, in-hospital adverse rates in this clinically indicated (non-research protocol) document improved outcome. In particular, pericardial effusion and procedure-related stroke were markedly decreased. (Reproduced with permission from24). LAAO: left atrial appendage occlusion; TIA: transient ischaemic attack
Table 2. Selected characteristics for WATCHMAN patients enrolled in the LAAO registry between 1 January 2016 and 30 June 2018 compared with patients from the PROTECT AF and PREVAIL Trials and the EWOLUTION Registry.| PROTECT AF 2005-2008 (n=463 Interv Arm) |
PREVAIL2011-2013(n=269 Interv Arm) | EWOLUTION Registry 2013-2015 (n=1,025) |
LAAO Registry 2016-2018 (n=38,158) |
|
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 71.7±8.8 | 74.0±7.4 | 73.4±8.9 | 76.1±8.1 |
| Women | 137 (29.6) | 87 (32.3) | 411 (40.1) | 15,672 (41.1) |
| Race | ||||
| White/European | 425 (91.8) | 253 (94.1) | NA | 35,345 (92.6) |
| Black/African American | 6 (1.3) | 6 (2.2) | NA | 1,768 (4.6) |
| Asian/Pacific Islander | 5 (1.1) | 1 (0.4) | NA | 670 (1.7) |
| Hispanic ethnicity | 25 (5.4) | 6 (2.2) | NA | 138 (0.4) |
| Medical history | ||||
| CHA2DS2-VASc score | 3.4±1.5 | 3.8±1.2 | 4.5±1.6 | 4.6±1.5 |
| Prior ischaemic stroke/TIA | 82 (17.7) | 74 (27.5) | 312 (30.5) | 11,389 (29.9) |
| Prior congestive heart failure | 124 (26.8) | 63 (23.4) | 350 (34.2) | 14,266 (37.4) |
| Prior diabetes mellitus | 113 (24.4) | 91 (33.8) | 304 (29.7) | 14,396 (37.7) |
| Prior hypertension | 413 (89.2) | 238 (88.5) | 885 (86.4) | 35,148 (92.1) |
| HAS-BLED score | NA | NA | 2.3±1.2 | 3.0±1.1 |
| Prior intracranial bleeding | NA | NA | 155 (15.1) | 4,550 (11.9) |
| Prior clinically relevant bleeding | NA | NA | 396 (38.7) | 26,466 (69.4) |
| Reproduced with permission from24. LAAO: left atrial appendage occlusion; TIA: transient ischaemic attack | ||||
Patient selection
While fundamental selection criteria have not changed since the inception and implementation of the technology, namely patients with NVAF at increased risk for stroke, estimates of patient risk have varied and will continue to evolve as data accrue. For example, in current trials, a CHA2DS2-VASc score of 1 may be used as an entry criterion, whereas in the past a score of >2 might have been required. With further information, indications may become modified and expanded. An important new area for growth will include combined procedures such as transcatheter aortic valve replacement (TAVR) and LAAO or pulmonary vein isolation (PVI) and LAAO2627. In these patients, a combined procedure will have advantages in terms of patient comfort as well as laboratory efficiencies. The primary endpoint for evaluation will be of increasing importance, either as a composite or individual parameter. There are important issues that will also affect the considerations of the risk/benefit ratio of LAAO versus the standard of care – VKA or DOAC. As seen (Table 3) there are both absolute as well as relative contraindications to long-term OAC. There are, however, patients in whom an LAAO device is not indicated (Table 4).
Another important issue regarding selection criteria relates to the results of PROTECT AF and PREVAIL. In the preclinical animal models, warfarin and ASA were used because of concern that the device may be thrombogenic. At that time, DOAC were either not available or approved. Accordingly, warfarin and ASA were used for 30 days. Based on the results of preclinical testing, which used warfarin and ASA for 30 days, the pivotal trials followed a similar strategy of warfarin and ASA. In those trials, warfarin and ASA were used for 45 days during which time TOE was performed. If the device appeared to be healed by TOE, warfarin was discontinued, and a combination of ASA and clopidogrel (Plavix, VIDAL) was used for a total of 6 months, then ASA alone.
In considering a trial design randomising patients to either device or control therapy, both groups had to be eligible for warfarin. The control group continued warfarin and ASA indefinitely. These protocols became embedded with IFU for patients in the US (Table 5).
In contrast, LAAO deployed in other countries was used in patients with an absolute or relative contraindication to OAC. Accordingly, several different post-dismissal regimens were clinically used28. In the EWOLUTION registry, the post-procedure medication regimens were VKA (16%), novel oral anticoagulants (NOAC; 11%), DAPT (60%), SAPT (7%), and no anticoagulation (6%). At 1 year, there were no differences in death, stroke, or bleeding rates, irrespective of whether or not there was a contraindication to OAC, and there was no relationship with the specific OAC. Since US approval, there have been multiple iterations of post-device implantation therapy.
Table 3. Contraindications to LAAO.| Absolute contraindication to long-term OAC |
|---|
| a. Severe uncorrectable anaemia (e.g., thalassaemia) |
| b. Prior life-threatening bleeding |
| c. Multiple significant falls |
| d. Prior ICH or OAC |
| e. Prior SAH – known cerebral aneurysm |
| f. Untreatable HHT |
| Relative contraindication to long-term OAC |
| a. Non-compliance with OAC |
| b. Malignancy |
| c. Chronic end-stage renal disease |
| d. Amyloid |
| e. Chronic bacterial endocarditis |
| f. High-risk occupation – active duty military, fireman |
| g. Failure of prior OAC |
| HHT: hereditary haemorrhagic telangiectasia; ICH: intracerebral haemorrhage; LAAO: left atrial appendage occlusion; OAC: oral anticoagulant; SAH: subarachnoid haemorrhage |
Table 4. Patient exclusion criteria.
| Patients for whom long-term anticoagulation is required e.g., structural medical heart valves |
|---|
| Mitral valve disease with a significant gradient – mitral annular calcification |
| Patients with increased potential for left atrial thrombus formation e.g., amyloid |
| Anatomy in which a device cannot be placed safely or effectively |
| Prior surgical extirpation of the left atrial appendage |
Table 5. WATCHMAN FLX intended use/indications for use by geographic region.
| United States | European Union | China |
|---|---|---|
|
Intended use/indications for use WATCHMAN FLX is indicated to reduce the risk of thromboembolism from the LAA in patients with NVAF who:
|
Intended use WATCHMAN FLX is intended for percutaneous, transcatheter closure of the LAA Indications for use WATCHMAN FLX is intended to prevent thrombus embolisation from the LAA and reduce the risk of life-threatening bleeding events in patients with NVAF who are eligible for anticoagulation therapy or who havea contraindication to anticoagulation therapy. |
Indications for use WATCHMAN FLX is used for patients with NVAF with a CHA2DS2-VASC score ≥2 (candidates for anticoagulant therapy or long-term oral anticoagulant contraindications) to prevent left atrial thromboembolism and reduce the risk of fatal bleeding events. |
| LAA: left atrial appendage; NVAF: non-valvular atrial fibrillation | ||
Procedural planning
Preprocedural imaging
The LAA is most often multilobed, with variable lobe orientations, bends, and tapering29. The most widely used classification consists of 4 shapes: chicken-wing, windsock, cactus, or cauliflower30 (Figure 3A). This classification has also been expanded with consideration of different shapes and sizes of the ostium of the LAA (Figure 3B). Anatomic complexity includes the take-off and spatial location of the LAA. This may affect the optimal positioning of the transseptal puncture to achieve an optimal trajectory of the device for stable device orientation without excessive torsion. Optimal criteria for device deployment by either TOE, CT, or both are shown in Table 6.
LAA imaging
An essential component is the assessment of pre-existing thrombus, as it has been considered an absolute contraindication. TOE has been the most commonly used approach. When identified prior to the procedure (Figure 9), alternative strategies still need to be considered, typically either initiation, resumption, or continuation of an OAC therapy for 3 months followed by repeat imaging to document resolution.
Recently a “no touch” approach has been described in 28 carefully selected patients with persistent LAA thrombus; 26 had distal LAA thrombus and the other 2 had thrombus in the mid- to distal portion. Using either an Amplatzer or a LAmbre (Lifetech) LAAO was successful in all cases. The “no-touch” technique included avoiding or minimising contrast or minimising catheter manipulation in the LAA. Distal embolic protection devices were used in 6 of the patients. While these findings are of great interest, they should not be incorporated into routine practice patterns which try to eliminate thrombus before LAAO31.
Preprocedural TOE imaging also allows evaluation of the size of the LAA with measurements of depth, annular dimension, and LAA morphology which should be based on multiplane views (45°, 95°, 85° and 175°) and 3D images (Figure 10).
While TOE was required for the pivotal initial trials, the limitation of its invasiveness and requirement for fasting are increasingly recognised as a constraint, particularly in elderly patients. In addition, when used as intraprocedural guidance, general anaesthesia has usually been required, which delays the recovery time after extubation. While uncommon, absolute and relative contraindications may also exist relating to oesophageal pathologies, coagulopathy, or severe thrombocytopaenia.
For these reasons, CT has been applied with increased frequency and, in some institutions, is preferred. CT is highly sensitive in screening for thrombus but with a substantive risk of false-positive results if appropriate acquisition adaptations are not applied. The addition of a delayed-phase imaging acquisition increases the positive predictive value and specificity of cardiac CT to >95%. The non-invasiveness of cardiac CT acquisition, along with the very high spatial resolution providing accurate multiplanar and 3D reconstructions of the LAA and surrounding structure, makes it ideal in both the pre- and post-procedural phases of LAAO323334. However, the contrast requirement reduces the applicability in patients with severely reduced renal function or contrast allergies32. Although radiation concerns are important, the effective radiation exposure has been reduced to 1-2 mSv32353637 with current scanners and dedicated acquisition protocols. A consensus paper including LAAO-specific acquisition technique considerations and protocols has been published32.
Detailed information is extremely important (Figure 11). The left circumflex coronary artery and the left upper pulmonary vein (LUPV) ridge constitute important landmarks. The LAA orifice is defined by a line connecting two landmarks and is intended to be covered by the 2-component devices (lobe-disc) (Figure 12). The anatomical orifice is defined by a line connecting the circumflex and a point 10-20 mm inside the LAA from the LUPV ridge (Figure 11), described as the beginning of the trabeculated LAA29, and is intended to be covered by plug-based devices. Studies have found an association between device implantation depth (>10 mm from the LUPV ridge) and risk of subsequent DRT38. With CT, multiplanar reconstructions aid in determining the maximal and minimal diameters and the circumference of the LAA landing zone (10-12 mm from the ostium and perpendicular to the LAA wall) seen in both WATCHMAN and Amulet planning (Figure 12, Moving image 1, Moving image 2).
Dedicated CT-LAAO software allows 3D printing, device implant simulation, access route planning, and overlay/fusion imaging during the intervention32333940 (Figure 13). The superiority of 3D-based sizing has been documented with cardiac CT demonstrating the largest LAA dimensions compared to both 2D and 3D TOE32354143. CT preprocedural planning predicts the device size more accurately than 2D TOE and has been documented to decrease procedure time and contrast use and potentially reduce complications33. Sizing algorithms vary across devices, but generally device sizing relies on the maximum diameter of the landing zone. The value of the mean, perimeter- or area-derived LAA diameter may be valuable in pronounced elliptical-shaped landing zones. Planning for the optimal transseptal puncture site may help coaxial alignment of delivery sheaths and devices into the LAA. An inferoposterior transseptal puncture is preferred in the majority of anatomies, but in selected anatomies, like reverse chicken-wing, a more central or anterior puncture might improve coaxial alignment43. Software application protocols can facilitate simulation of various puncture sites, sheath shapes, and orientations into the LAA and visualisation of actual device types and sizes at the predicted landing zone. This has been shown to affect the operator’s choice of sheath compared to traditional TOE planning39. Fluoroscopic simulation in addition to this allows determination of the optimal intraprocedural C-arm projection based on the CT dataset. Three-dimensional printing may be useful in selected anatomic settings40, although the logistics and cost remain limiting. A computational simulation of device implantations may improve implantation results, as illustrated by a recently presented smaller RCT44.
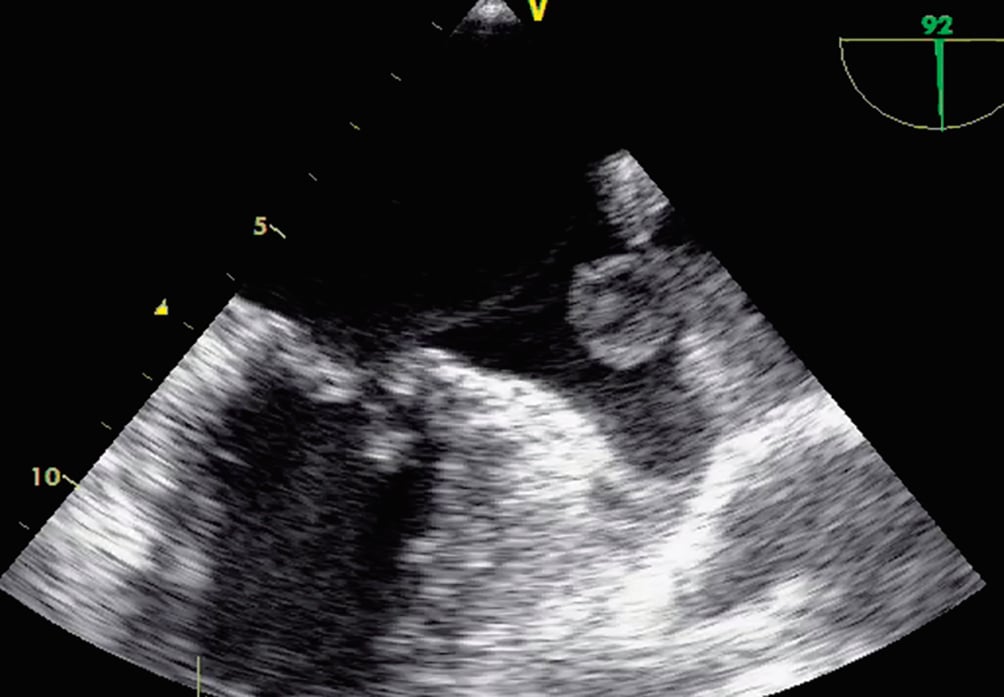
Figure 9. TOE imaging documenting a large LAA thrombus. At the time of preprocedural evaluation, the presence of LAA thrombus represents a contraindication because of the potential for embolisation during catheter placement. LAA: left atrial appendage; TOE: transoesophageal echocardiogram
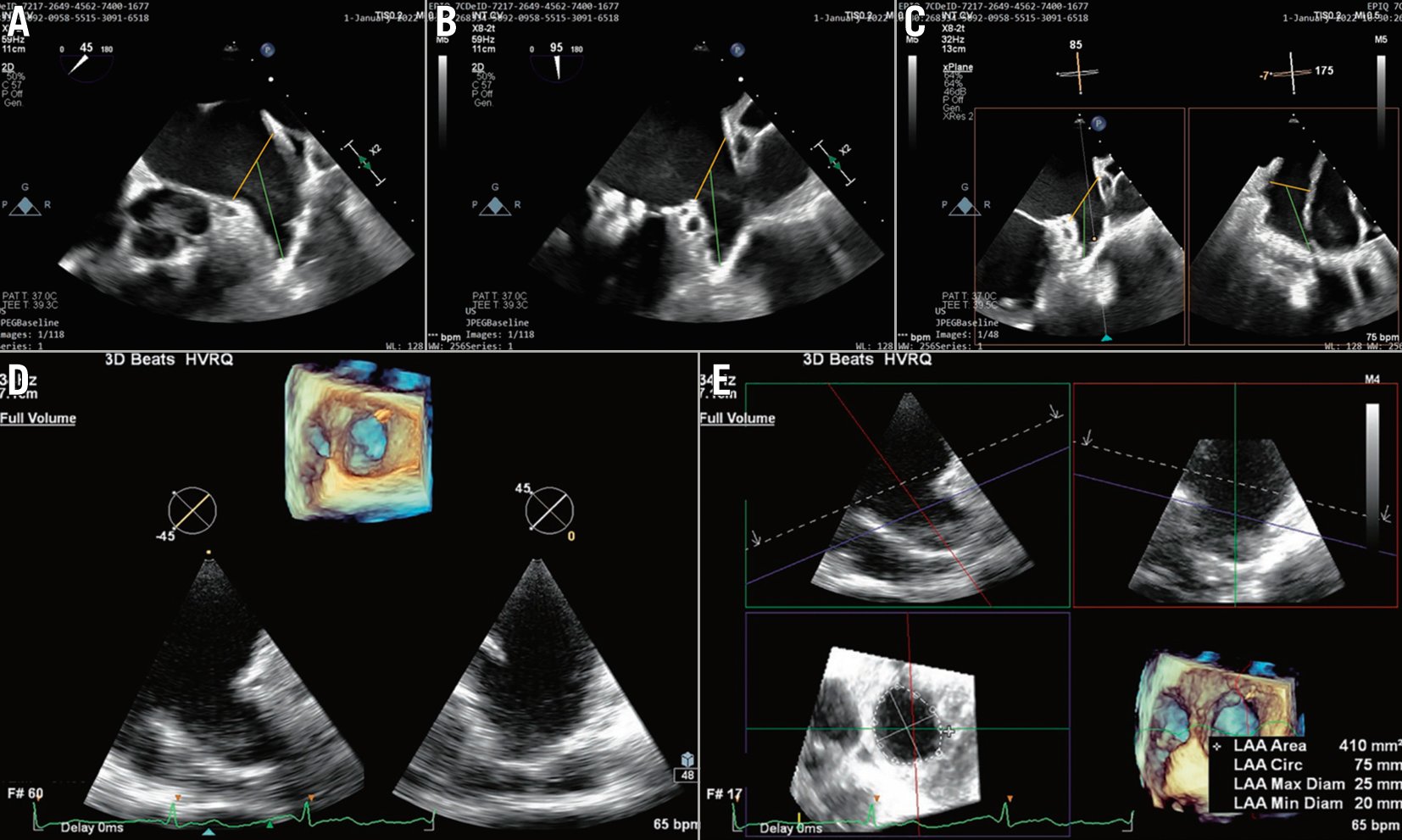
Figure 10. 2D TOE planning for LAAO. Four views are evaluated: A) 45°, B) 95°, C) Biplane view, 85°, 175°. In addition, 3D full volume of the LAA with the component 2D images are helpful. Finally, D) 3D full volume images showing alignment of the blue plane allows measurement of the landing zone orifice. E) The measurement box cannot be removed because it is the layout of the machine – the same image in 3D can be seen in the picture on the left – 3D MPR for accurate measurements of LAA diameters, an image without measurements would be incomplete. LAA: left atrial appendage; LAAO: left atrial appendage occlusion; MPR: multiplanar reconstruction; TOE: transoesophageal echocardiogram
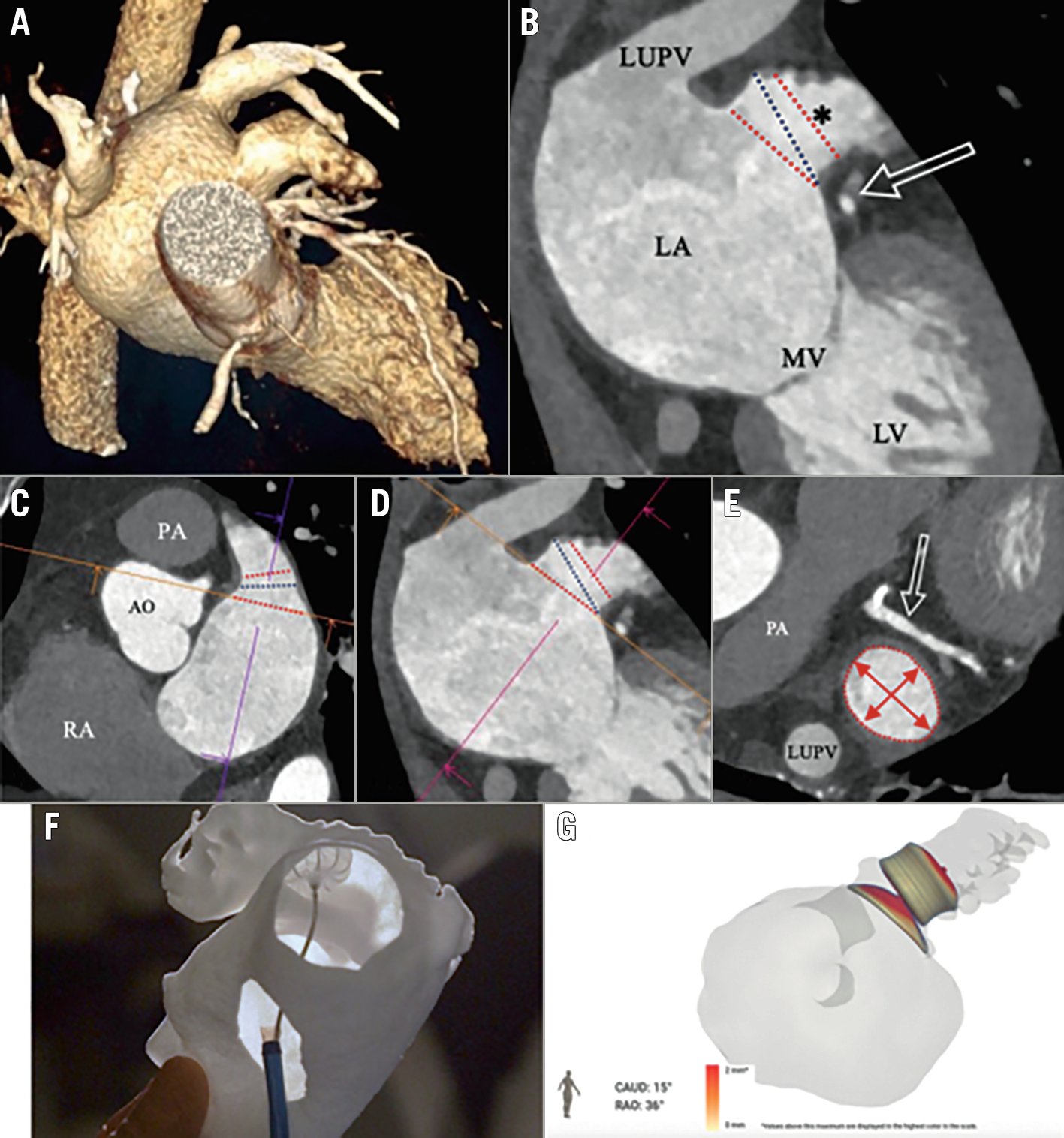
Figure 11. CT has become widely used for preprocedural evaluation. Different views as seen are useful to identify the relationships between the left upper pulmonary vein (LUPV), left atrium (LA), LAA, and mitral valve (MV). The circumflex coronary artery (arrow) is an important landmark. Lines drawn with that from the circumflex to the LUPV identify the anatomical orifice. A) Overall CT image documenting relationship between the pulmonary vein and LAA; B) Landmark can be used to identify the anatomical orifice of the LAA. It is also useful for measuring the ostium of the landing zone; C) Another view documents the relationship between the tip of the LAA and pulmonary artery. It also documents the position of the left atrium in relation to the aorta which is important for avoiding inadvertent puncturing of the aorta. The lines are useful for identifying the orifice of the LAA as well as planning optimal location of the implant; D) The route of the transseptal to the LAA can be identified. This is used to identify optimal position for transseptal puncture; E) A cross-section area of the LAA and its relationship with the circumflex coronary artery (arrow); F) Composite documenting the optimal transseptal location leading to the Watchman implant (see at the top of frame); G) The FEops can be used for preprocedural planning to identify optimal position of the Amulet device. AO: aorta; LAA: left atrial appendage; PA: pulmonary artery; RA: right atrium
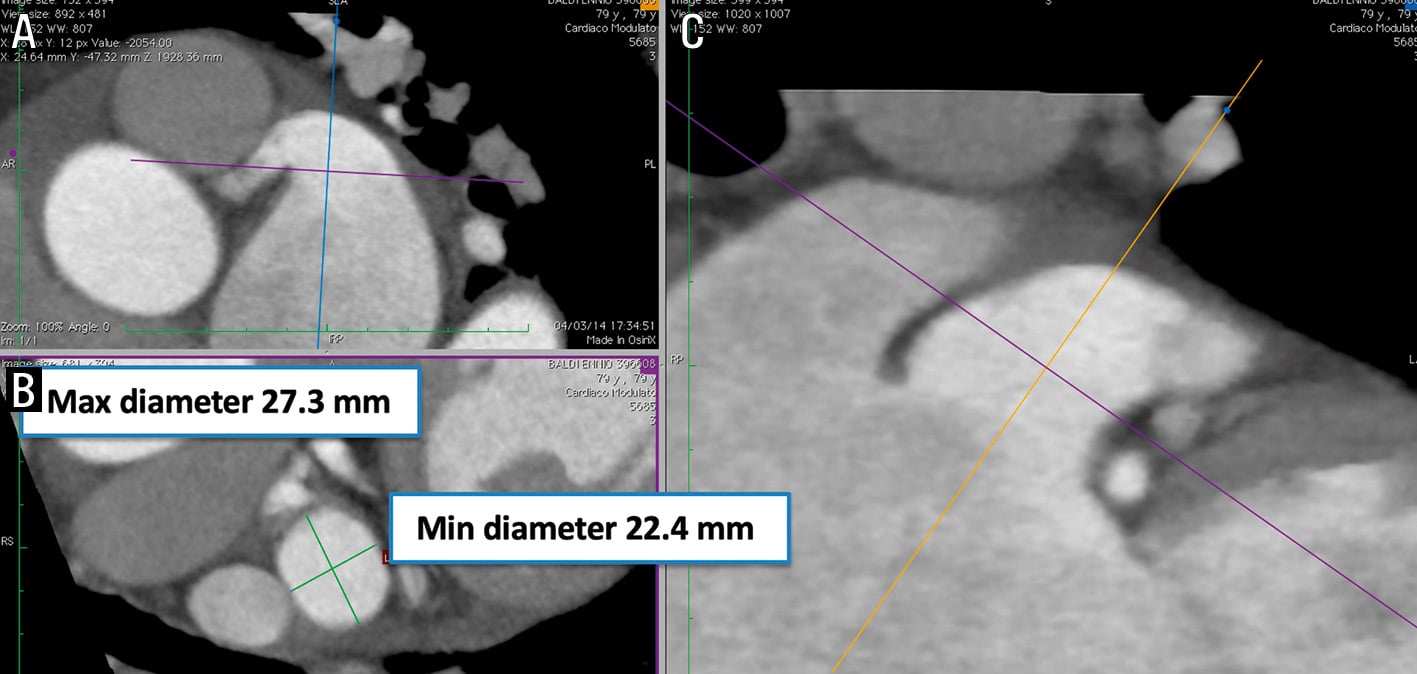
Figure 12. MPR analysis of preprocedural CT images for AMPLATZER Amulet implantation. Multiplanar reconstructions aid to determine the maximum diameter, minimum diameter, and circumference of the LAA landing zone (10-12 mm from the ostium and perpendicular to the LAA wall) as well as the length of the LAA. A) Length of LAA; B) and C) circumference of LAA. CT: computed tomography; LAA: left atrial appendage; MPR: multiplanar reconstruction
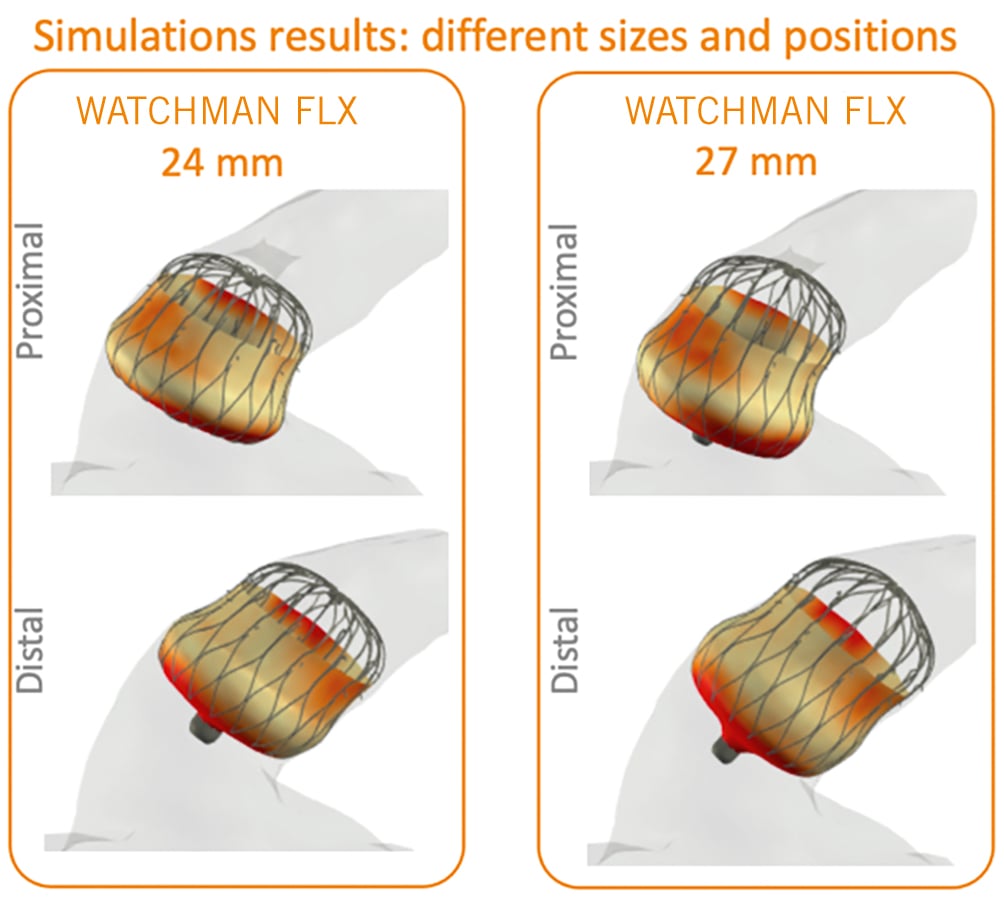
Figure 13. CT-based software simulation (FEops) of WATCHMAN FLX implantation. Computational simulation is based on patient-specific morphology. The geometry has been reconstructed from the CT dataset. The software demonstrates potential opportunities to attempt the prediction of device interaction with LAA anatomy. CT: computed tomography; LAA: left atrial appendage
Intraprocedural imaging
Optimising the site of safe transseptal puncture is of essential importance in facilitating coaxial access to and device deployment in the LAA. Typically, fixed curved sheaths of various shapes have been used, although deformable curved sheaths are now available. Several stainless-steel needles are also available depending on operator preference. The lumens should be carefully flushed to provide the ability to measure intracardiac pressures. In addition, speciality needles utilising radiofrequency energy may be helpful to facilitate more controlled septal punctures.
Typically, a trans-right femoral venous approach is used to position the guiding catheter in the left atrium. The venous sheath can be used for multiple catheters if needed. Preprocedural CT is increasingly utilised; there are now commercial products that facilitate the selection of an optimal site prior to the procedure. Alternatively, TOE or intracardiac echocardiography (ICE) catheters can be used. Thoracoscopy alone does not allow for optimal selective positioning.
The LAA is an anterior structure. In general, an inferoposterior septal position in a thin part of the intra-atrial septum is selected. However, in some patients, depending upon their specific anatomy, other positions might be selected. Increasingly, deeper device placement has been used and is associated with less DRT. Placement of the device through a patent foramen ovale should be avoided because of problems obtaining coaxiality of the device.
Following the puncture of the intra-atrial septum, which can be documented by echocardiography, pressure monitoring, or small amounts of contrast, the sheath can be advanced. The characteristics of the intra-atrial septum vary depending on the location of the puncture itself or fibrosis and/or thickening related to prior procedures such as ablation. The use of ancillary adjunctive devices, such as SafeSept (Pressure Products) with its floppy stable wire position, may be important. In some patients with a very fibrotic intra-atrial septum, dilators may be required to access the left atrium.
Once the needle position has been documented in the left atrium, a variety of procedures can be performed and used as support to deliver the sheath which varies in size by the device manufacturer. These techniques are important to avoid damage to either the wall of the left atrium (typically the dome) or the LAA. These include among others a .035 inch soft wire placed in the left superior pulmonary vein, a pigtail catheter, or other curved guidewires that are less traumatic to the left atrium itself.
Both TOE and ICE guidance are used – the latter not requiring general anaesthesia. With TOE, a bicaval view (90°) allows assessment of the superior anterior location of the puncture and a short access view of 0-30° allows assessment for the AP location. ICE guidance, as mentioned, is now increasingly used.
Although various approaches have been used for guidance (TOE, ICE, fluoroscopy alone), the combination of fluoroscopy and TOE remains the standard at most laboratories. Device manufacturers have proposed specific imaging criteria to ensure the optimal position and stability of the occluder device and adequate occlusion (Table 7). Suitability for device release is typically ascertained in several TOE echocardiographic views (0°, 45°, 90°, 135°) and in at least 1 angiographic view (right anterior oblique caudal projection). Recently, the use of paediatric-size TOE probes has been shown to provide adequate imaging to guide LAAO without the need for general anaesthesia.
Intracardiac echocardiography has emerged as a feasible alternative for guidance. The rationale for this change includes (a) avoidance of general anaesthesia in elderly patients; (b) mitigation of TOE-related complications; and (c) logistical advantages such as facilitation of same-day discharge and improving lab efficiency4546. However, the adoption of ICE in current practice has been limited for several reasons: 1) there is a learning curve associated with ICE imaging, especially for interventional cardiologists who uncommonly utilise ICE for left atrial procedures; 2) ICE-associated costs are a limiting factor in some laboratories; and 3) there are concerns about the limited data on the validation of device release criteria using ICE alone. Nonetheless, the growing data on the safety, efficacy, and cost-effectiveness of ICE-guidance along with the recent advances in ICE technology have mitigated some of these concerns and ICE-guided LAAO will probably continue to increase474849505152 (Figure 14, Figure 15).
A survey of the published literature on ICE-guided LAAO yields several practical recommendations for the incorporation of ICE guidance into practice:
1. Understanding the nuances of the ICE equipment. The majority of ICE-guided LAAO procedures can be performed safely and efficiently with 2D probes. The AcuNav (Siemens) and ViewFlex (Abbott) probes are the most common and provide excellent 2D imaging but lack biplane or 3D imaging. 3D ICE probes have been introduced in recent years (e.g., VeriSight; Philips; NUVISION; Biosense Webster). Optimal use requires more advanced operator knowledge of 3D reconstruction and console management.
2. Utilisation of cardiac CTA for preprocedural planning. This will greatly facilitate accurate sizing, shorten procedure time, and minimise the potential complications associated with excessive manoeuvres and prolonged indwelling of the ICE probe. Modern software (e.g., TruPlan [Boston Scientific/Circle CVI]) also allows imaging simulations where an ICE probe can be placed in different locations in the heart and the corresponding expected images created.
3. Use a simplified imaging protocol. Reproducing the 4 TOE angles (0°, 45°, 90°, 135°) with ICE is challenging. Most experts have suggested that obtaining 2 orthogonal views with ICE to assess the PASS and CLOSE criteria might be adequate47. Achievement of these criteria are essential to minimise the potential for device embolisation. Increasingly optimising the initial position is felt to be important in minimising leaks and DRT. Validation studies for this simplified ICE protocol are currently underway (ClinicalTrials.gov: NCT04569734).
4. Accept a learning curve. The migration from TOE- to ICE-guided LAAO is associated with a learning curve. Positioning the ICE probe in the LA is unfamiliar to many interventional cardiologists but can be mastered after 10 cases47.
5. Use ICE routinely. Limiting the use of ICE to only a small number of highly selected cases will hinder an operator’s ability to gain and maintain proficiency. Several studies have documented the excellent performance of ICE when adopted routinely for the majority of procedures474952.
Other intraprocedural imaging approaches include fluoroscopy-only guided LAAO, zero-fluoroscopy echo-only guided LAAO, contrast-free echo-guided LAAO, and ECHO fusion. Some have specific advantages, such as limiting contrast administration in patients with chronic kidney disease. However, data supporting these approaches are limited5354. Echocardiography will remain, at least for the foreseen future, an integral part of the LAAO procedure and essential for the ongoing safety and longer-term outcomes55.
Table 7. Release criteria for the WATCHMAN FLX and the Amulet devices.| WATCHMAN FLX | AMULET |
|---|---|
| PASS Position: adequate shape and positioning of the device with exclusion of all LAA pectinate muscle Anchor: satisfactory anchoring assessed with a tug test to ensure device stability Size compression: size deformation with compression of 10-30% at the level of the shoulders Seal: adequate seal without peri-device leak by colour Doppler and/or angiographic assessment |
CLOSE Closure: 2/3 of the device lobe is distal to the left circumflex artery Lobe compression: there is adequate device lobe compression Orientation: the orientation of the device lobe is in line with the axis of the LAA neck Separation: there is adequate separation between the lobe and disc and there is an elliptical shape to the disc |
| LAA: left atrial appendage | |
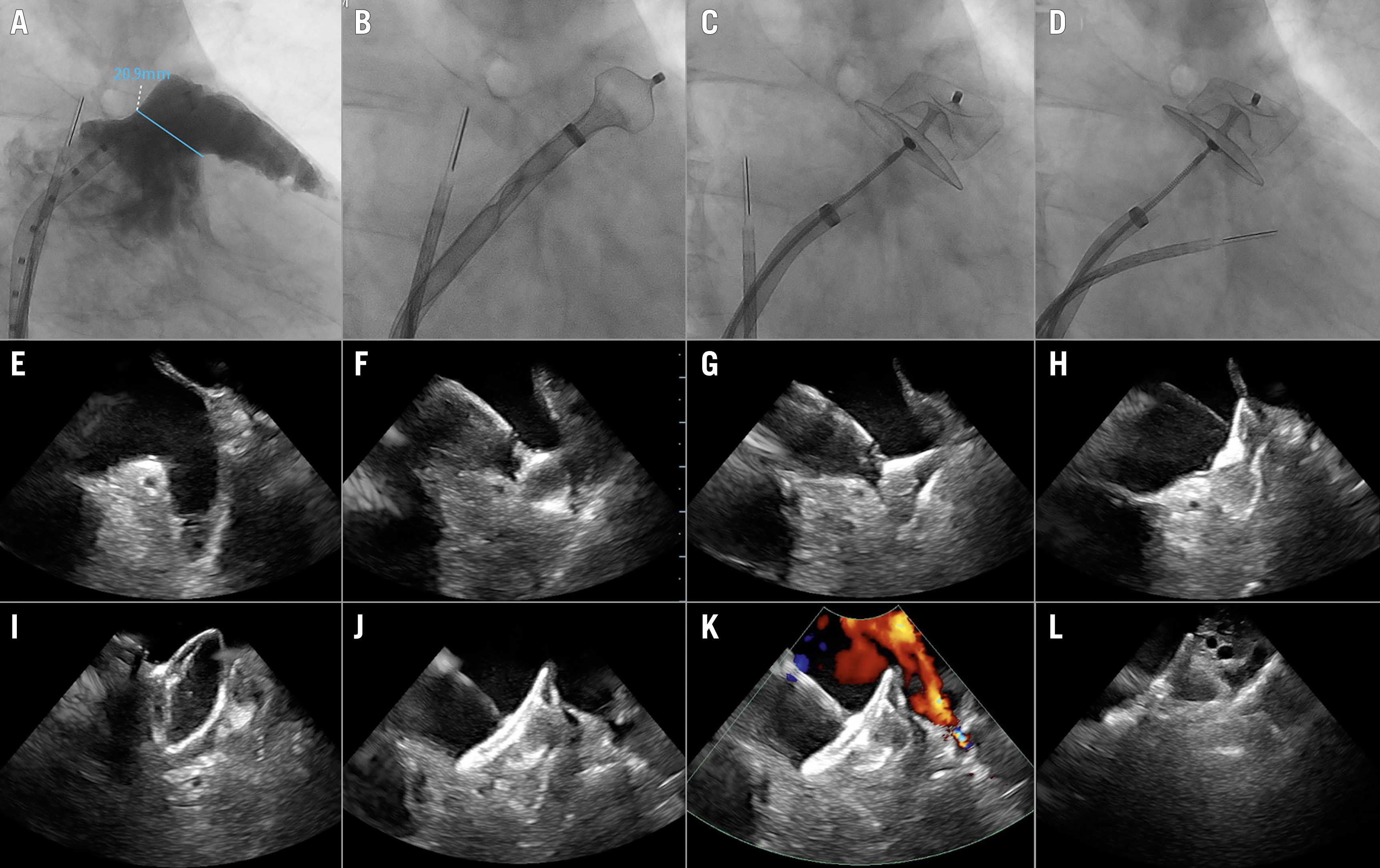
Figure 14. Step by step ICE-guided LAAC with an Amulet device. Top panel: A) Angiography showing a chicken-wing LAA anatomy. B–D) Illustration of the ICE positions in the 3 standard views: B) left upper pulmonary vein (LUPV) view, C) mid-LA view, and D) supra-mitral view. Middle panel: E–H) LAA from the LUPV; Amulet lobe in the ball configuration; lobe in the triangular configuration and lobe deployed in the neck of the LAA. Lower panel: I–L) Amulet disc being deployed (American football configuration); disc fully deployed; colour Doppler without signs of peri-device leakage; the Amulet device seen in the supra-mitral view. (Reproduced with permission from45). ICE: intracardiac echocardiography; LA: left atrium; LAA: left atrial appendage; LAAC: left atrial appendage closure
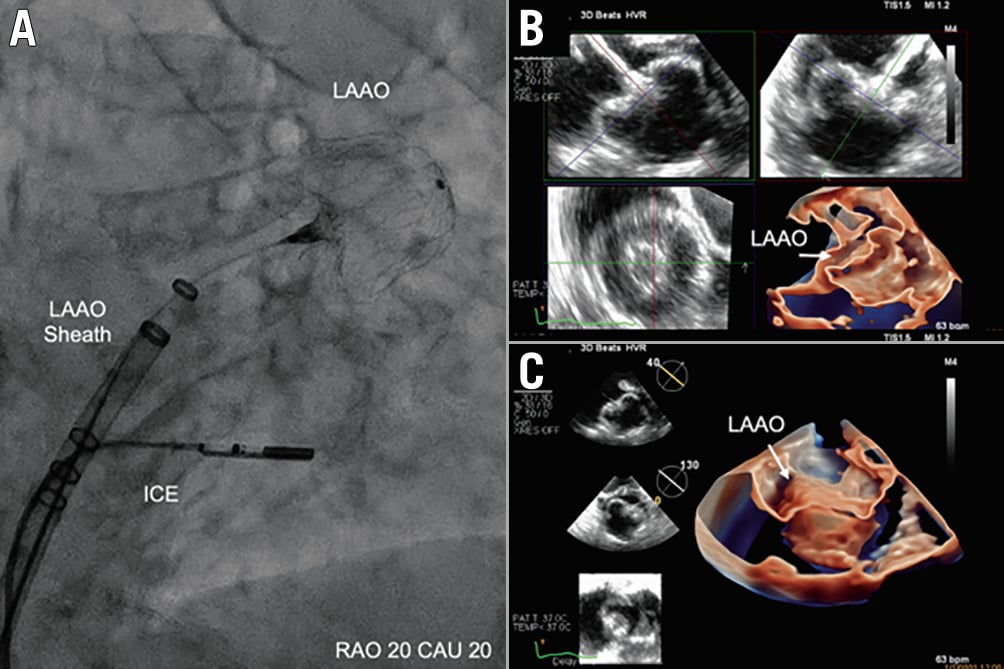
Figure 15. LAA closure guided by 3D intracardiac echo. A) The location of the ICE probe on fluoroscopy during deployment. B, C) Multiplanar reconstruction and 3D images of the LAA after closure with the WATCHMAN FLX device. (Reproduced with permission from53). ICE: intracardiac echocardiography; LAA: left atrial appendage; LAAO: left atrial appendage occlusion
Complex anatomies
The anatomy of the LAA is highly variable and attaining adequate closure in some challenging anatomies (e.g., acute shallow chicken-wing, double lobe, rigid trabeculations, etc.) can be challenging. Several important factors need to be considered when treating such anatomies to increase procedural success rate and mitigate the risk of peri-device leak. First, utilising computational simulation software provides a wealth of information about the likelihood of successful closure for each specific anatomy. It can also predict the optimal location of the transseptal puncture needed to achieve coaxiality with the appendage. Although most LAA anatomies are amenable to closure with any transseptal access location, closing certain anatomies (e.g., anterior chicken-wing) is particularly difficult when the transseptal access is suboptimal. Second, if access to multiple devices is available, the operators may be able to choose the device that provides the best closure of specific anatomies. A number of types of LAAO are in development for testing including plug, disc and lobe, and ligation/obliteration (Supplementary Table 3)56. For example, a disc and lobe device might be better suited to close a shallow chicken-wing anatomy than a plug-type device. Even when landing the lobe in the LAA neck is not possible, a sandwich technique may be used. With this, the lobe may be lodged in the wing with the disc becoming the primary source of LAA seal. Third, steerable sheaths are now available. Although speculative, the use of these sheaths would improve coaxial engagement with the LAA, facilitating better device deployment and improved sealing. Finally, it should be emphasised that certain anatomies are not suitable for percutaneous closure and may be better managed medically or by surgical closure.
LAAO procedure-related complications
In the early experience with LAAO, there were relatively high complication rates of pericardial tamponade, air embolism, and thromboembolism. The LA and LAA are thin-walled; manipulation of large-bore sheaths and devices (e.g., deployment, recapture, repositioning) within the LA can traumatise the wall. Transseptal puncture itself may also result in perforation. The low-flow low pressure of the left atrium can predispose to entrainment of an air embolus, in addition to stasis causing thrombus formation. Over the last decade, improved operator experience and meticulous technique, together with improved device iterations, have led to dramatic reductions in the incidence of major procedural complications (death, stroke, cardiac tamponade, device embolisation) (Figure 16).
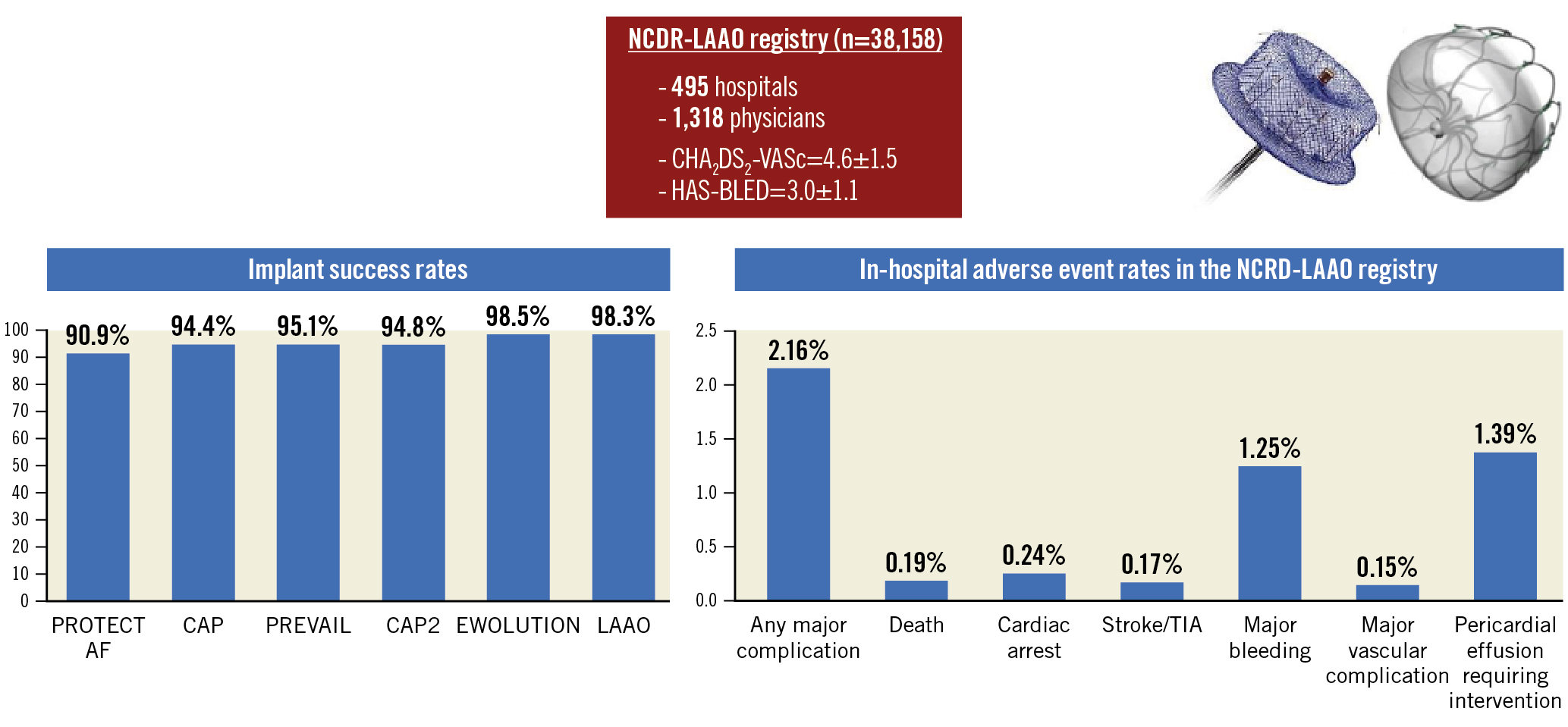
Figure 16. How are we doing with LAAC? As seen, the initial LAAO Registry documented that major complications are seen in 2.16% of patients. These rates have decreased since the early trials and registries. Implantation success has continued to improve. (Reproduced with permission from24). LAAC: left atrial appendage closure; LAAO: left atrial appendage occlusion; NCDR: National Cardiovascular Data Registry
Pericardial tamponade
Pericardial effusion, the most common significant complication, has declined. In PROTECT AF, pericardial effusion requiring intervention was reported in 4.3% of patients; PREVAIL and CAP2 documented 1.9%. The overall incidence of cardiac tamponade is ~1.3% in a pooled analysis of the WATCHMAN clinical trials and registries57. With the Amplatzer Cardiac Plug (ACP; Abbott) device the frequency ranged between 1.9% and 3.7%. In the Amulet IDE randomised trial including 1,878 patients, the incidence of effusion was 2.4% with the Amulet and 1.2% with the WATCHMAN58. Although there was a higher rate of pericardial effusion with the Amulet, the effusion resolved without sequelae in all cases; none required emergency surgery or caused death. With the most recent WATCHMAN FLX device, the incidence of pericardial tamponade was 1.0%23. In the SURPASS registry of 16,446 patients implanted with that device in the NCDR-LAAO Registry, the incidence of pericardial effusion was 0.51% (Kapadia SR, et al. Real-world Outcomes with WATCHMAN FLX: Early Results from SURPASS. CRT 2022. Washington, D.C., USA).
Most effusions occur within 24 hours of LAAO59. Manipulation of the guidewires, catheters, delivery sheaths, and transseptal system can be associated with trauma to the thin-walled left atrium, pulmonary veins, and LAA. The stabilising hooks on the LAAO occluders can also penetrate the thin LAA wall. Repeated device recapturing/repositioning can also traumatise the LAA wall. Repositioning at the initial implant may become more frequent in the attempts to optimise device placement to minimise residual leak and could potentially increase any damage. In the past, in ~30% of cases the specific aetiology could not be identified59. To minimise the occurrence of effusion, imaging guidance with TOE/ICE is recommended for all transseptal punctures. The use of pigtail-shaped wires (e.g., ProTrack wire, VersaCross [both Baylis]) to aid sheath exchanges and the use of a pigtail catheter to position large sheaths in the LAA are also recommended.
A pericardiocentesis kit should be readily available. Early recognition, prompt haemodynamic resuscitation, and percutaneous pericardiocentesis are critical. In the case of overt perforation from a large sheath or device, urgent surgical consultation should be obtained and discussion about the need for urgent surgery strongly considered. The protruding device/sheath should not be withdrawn until pericardial access is obtained. For active pericardial bleeding, autotransfusion should be considered to minimise blood loss and the need for transfusion. For smaller effusions, emergent pericardiocentesis may be sufficient. Typically, a pigtail catheter is inserted for continued drainage and may be required over the course of 1-2 days to ensure complete mitigation. During this time, colchicine should be considered to reduce inflammation. An initial consideration relates to management of heparin. If the perforation is very large, with heavy flow, a reversal of anticoagulation is indicated. If there is less flow and no severe haemodynamic embarrassment, reversal of heparin may not be needed because it would result in coagulation of the blood in the pericardium and subsequent inability to fully excavate it with the pigtail drainage catheter.
Device embolisation
Device embolisation is rare. With the legacy WATCHMAN 2.5 device, the reported incidence in trials was 0.25%57; the incidence in real-world practice in the NCDR-LAAO Registry was lower, at 0.07%24, and is even lower with the WATCHMAN FLX device: 0% in the PINNACLE FLX Trial23, and 0.1% in the SURPASS registry. In the Amulet IDE Trial, device embolisation with the Amulet was 0.7% versus 0.2% with the legacy WATCHMAN 2.5 device58.
Embolisation may be related to undersizing, implantation that is too proximal, or off-axis device orientation with torsion. Most embolisations are clinically silent; however, palpitations, heart failure, hypotension, and cardiac arrest have been reported. In a systematic review of 13 WATCHMAN 2.5 devices and 18 ACP device embolisations, 69% occurred acutely but 31% were detected late at 1-7 months post-LAAO60. The devices were identified in the left ventricle (LV; 43%), aorta (43%), and LA (14%). At the time of initial observation, urgent assessment of haemodynamics is required with prompt treatment. The location of the device must be identified. If the device is still in the LA, attempts to stabilise it by placing a pigtail at the mitral inflow is important to prevent embolisation into the LV. If the device is already in the LV, placement of a pigtail to move it towards and through the aortic valve should be considered. However, if the device becomes entangled in the mitral apparatus, urgent surgery is typically required as severe haemodynamic embarrassment is more frequent.
The optimal first-line strategy for management is percutaneous retrieval. A large sheath (≥2–4 Fr size larger than the embolised device delivery sheath size) should be used (e.g., 1-18 Fr, 80 cm length). In general, the gooseneck snares (length 120 cm) with a single large loop work better than other types of snares. The size of the gooseneck snare should be at least a 10 mm loop for ease of grabbing the device's end screw or the feet of the device. Bioptome or vascular retrieval forceps (e.g., Raptor; STERIS) have also been used successfully61. As mentioned, devices in the left ventricle typically require urgent surgical retrieval if they are entangled in the mitral valve apparatus.
Procedural strokes
LAAO procedure-related stroke is rare and largely avoidable, reported in <0.5% of cases, and usually manifests within 24 hours of the procedure. In the NCDR-LAAO Registry, an ischaemic stroke was documented in 0.12% and haemorrhagic stroke in 0.01% of the patient population24. Most are related to air embolism from inadequate device preparation but can be due to thromboembolism from pre-existing LAA thrombus, or de novo thrombus formation on equipment. Air embolism to the cerebral circulation can cause a transient ischaemic attack or stroke that may not be recognised if the patient is under general anaesthesia. Transient ST-segment elevation may be a clue if simultaneous coronary air embolism occurred. In the early PROTECT AF study experience, air embolism resulted in strokes in 1% (5/463) of cases59. However, in contemporary series with refined procedural techniques and device preparation, clinical air embolism is very rare. Preprocedural imaging to rule out pre-existing LAA thrombus is essential before embarking on LAAO. General anaesthesia and endotracheal intubation with positive thoracic pressure and maintenance of left atrial pressure >10 mmHg can be protective if air embolism occurs. Inadequate procedural anticoagulation and a prolonged procedure can result in thrombus formation on the device equipment. Administration of heparin is increasingly used prior to transseptal puncture. Maintaining an activated clotting time (ACT) >250 sec during the procedure is important. It should be periodically checked (every 15-20 min) and optimised, as necessary. TOE/ICE surveillance of the equipment is important for the early detection of strands of tissue that may be thrombus. Once a thrombus is detected, additional heparin should be administered, and thrombus aspiration should be considered with a large sheath. At the time of thrombus identification, the procedure should be completed expeditiously with either device deployment (if a clot is on the sheath and the device is already in the LAA), or removal of the sheath/device from the LA. A full neurological assessment should be performed when the patient awakes from general anaesthesia, and consideration given to thrombolysis for acute stroke management if deficits are identified clinically and documented angiographically.
Antithrombotic treatment following LAAO
Device healing is felt to be the result of the growth of a normal endothelium on the device surface to decrease thrombus formation and potentially decrease residual leaks. The time frame for endothelialisation varies – up to several weeks or even longer during which antithrombotic therapy is routinely prescribed and aimed at reducing the risk of thromboembolic events. The type and duration of antithrombotic therapy following LAAO has evolved with significant variability across different studies and devices.
Experimental and biomarker studies
Experimental studies document that WATCHMAN and Amulet devices are partially or completely endothelialised at 30 to 90 days post-LAAO62. Thus, LAAO devices are exposed to circulating blood and are potentially more thrombogenic during the initial weeks following implantation. Evaluation of the biomarkers of coagulation (prothrombin fragment 1+2, thrombin-antithrombin III) and platelet activation (soluble P-selectin, CD40 ligand) following procedures showed that LAAO was associated with a significant activation of the coagulation system, reaching peak levels at 7 days post-procedure, and partially returned to baseline levels by day 30. In contrast there was no significant increase in platelet activation63. These findings suggested that enhanced thrombin generation would drive the main haemostatic effect associated with LAAO. This is most likely related to fibrin deposition at the blood-device interface in accordance with preclinical studies showing surface fibrin deposition of devices within the days following LAAO62.
Two studies documented that OAC (either with VKA or DOAC) versus antiplatelet therapy was associated with a significant reduction in the biomarkers of coagulation activation within the initial weeks following implantation, further supporting the use of anticoagulation within the weeks following LAAO for preventing DRT65.
Vitamin K antagonist versus dual antiplatelet therapy
Short-term (6 weeks) anticoagulation therapy with VKA after LAAO (with or without aspirin), or DAPT within 3 to 6 months following the procedure, have been the 2 most common antithrombotic therapies. After this initial period, the use of lifelong single antiplatelet therapy (usually aspirin) has been the most frequent strategy.
VKA (+ aspirin) following LAAO was the antithrombotic regimen recommended in the 2 initial WATCHMAN randomised trials and their subsequent continued access registries666768. Overall, in a pooled analysis of 1,743 patients, this strategy was associated with a rate of DRT of 3.7%, with only 15 events (0.9%) occurring within the 6 weeks following the procedure (anticoagulation period)69.
Most patients undergoing LAAO currently have relative or absolute contraindications for chronic OAC prompting the use of DAPT in the majority of LAAO observational studies and registries, particularly outside the US70. The European expert consensus recommends the use of 3-month DAPT as a potential alternative to VKA after LAAO with the WATCHMAN and ACP/Amulet devices16 (Table 8). The reported DRT rate with this therapy has been variable, ranging from 0-17% (between 1% and 4% in the majority of studies)70. However, no randomised data exist comparing VKA and DAPT in this population.
Sondergaard et al 71 assessed the safety and effectiveness of short-term OAC (45 days) versus antiplatelet therapy after LA with the WATCHMAN device using a propensity score matching analysis. A total of 1,527 patients (oral anticoagulation: 1,018 patients [95% on VKA] and antiplatelet therapy: 509 patients [91% on DAPT]) were included. At 6 months, there were no differences between groups in thromboembolic or major bleeding events. However, DRT was higher in the antiplatelet group (3.1% vs 1.4% in the anticoagulation group; p=0.014). When patients with single antiplatelet therapy were excluded, there was still a significant difference (APT 3.3%, OAC 1.1%, p=0.0048). In addition to data from studies showing coagulation but not platelet activation as the main haemostatic response during the device endothelialisation process6263, the high rate of clopidogrel non-responders among LAAO recipients may have also contributed to these results72. In the global prospective Amulet registry, the rates of major bleeding in patients on DAPT was twice as high in comparison to patients on OAC without antiplatelet therapy (8.4% vs 4.1%, respectively), whereas the rate of DRT was similar between groups (1.6% vs 2.0%, respectively)17.
Table 8. Antithrombotic therapy before and after LAAO.| Clinical situation and therapeutic concept | Consensus statement |
|---|---|
| Acetylsalicylic acid 75-325 mg/day for the procedure and then continued long term (load 300-500 mg prior to procedure if not previously on acetylsalicylic acid) | “Should do this” |
| Anticoagulation, using unfractionated heparin, is recommended during the implantation procedure prior to or immediately after TSP, aiming for an activated clotting time of >250 s | “Should do this” |
| After WATCHMAN 2.5 implantation, warfarin (INR 2-3) should be given for 45 days, followed by clopidogrel for 6 months after the procedure in low bleeding risk group of patients, while in high bleeding risk group OAC should not be applied | “Should do this” |
| NOAC is a possible alternative to warfarin after WATCHMAN implantation | “May do this” |
| After WATCHMAN implantation in patients not suitable for oral anticoagulation, DAPT including clopidogrel 75 mg/day for 1 to 6 months after the procedure (load 300-600 mg prior to procedure if not previously on clopidogrel) | “May do this” |
| After AMPLATZER Cardiac Plug or Amulet implantation, DAPT including clopidogrel 75 mg/day for 1 to 6 months after the procedure (load 300-600 mg prior to procedure if not previously on clopidogrel) | “May do this” |
| Other options that may be considered on a case-by-case basis include a single antiplatelet therapy (acetylsalicylic acid or clopidogrel) for short periods of time, as long as approved by team consensus | “May do this” |
| Reproduced with permission from [16]. DAPT: dual antiplatelet therapy; INR: international normalised ratio; LAAO: left atrial appendage occlusion; NOAC: novel oral anticoagulants; OAC: oral anticoagulant; TSP: transseptal puncture | |
Direct oral anticoagulants
In NVAF, DOAC are superior to VKA for the prevention of both ischaemic and bleeding events5. This therapy has recently been considered as a potential alternative following LAAO in patients eligible for short-term OAC. Data from several observational studies have shown extremely low rates of DRT (0 to 1.3%, 0% in the majority of studies), and thromboembolic events in patients treated with DOAC following LAAO236465737475767778. Furthermore, some studies have also suggested a lower rate of bleeding events among DOAC patients compared to their DAPT counterparts7375. Data from a small RCT also reported a numerically lower rate of DRT with DOAC (0%) compared to DAPT (6%)65. Of note, preliminary data suggested that low- (versus full-) dose DOAC could result in the same beneficial effects regarding DRT and thromboembolic protection after LAAO7578. Multiple randomised trials are comparing DOAC versus DAPT following LAAO and will likely provide more definite data on the safety and efficacy of this therapy (Table 9).
Table 9. Ongoing studies on antithrombotic therapy following LAAO.| Study acronym | NCT/EuroCT number | Study design | Intervention | n | Target population | Main outcomes |
|---|---|---|---|---|---|---|
| ADALA (Antithrombotic therapy after left atrial appendage occlusion: double antiplatelet therapy versus apixaban) |
2018-001013-32 | Randomised trial | DAPT vs apixaban (5/2.5 mg bid) | 160 | LAAO closure (no specific device) | Combined of efficacy (thromboembolic events and device thrombosis) and safety (major bleeding incidence) at 3 months |
| ANDES (Short-term anticoagulation versus antiplatelet therapy for preventing device thrombosis following left atrial appendage closure) |
NCT03568890 | Randomised trial | DAPT vs any approved NOAC | 350 | LAAO with the WATCHMAN or ACP/Amulet devices | Device thrombosis as evaluated by TOE at 2 months |
| APPROACH (A multicentre study of apixaban) |
NCT04550637 | Prospective, observational | Apixaban (5 mg bid) for 3 months following LAAC | 200 | LAAO (no specified device) | All-cause death, stroke, transient ischaemic attack, systemic embolism at 6 months |
| ASPIRIN-LAAO (Aspirin discontinuation after left atrial appendage occlusion in atrial fibrillation) |
NCT03821883 | Randomised trial | Aspirin discontinuation vs continuation at the sixth month after LAAO | 1,120 | LAAO with the WATCHMAN device | Stroke, systemic embolism, acute coronary syndrome, cardiovascular/unexplained death, major bleeding, coronary/peripheral revascularisation at 2 years |
| DEA-LAA (Efficacy of short-term dabigatran etexilate followed by aspirin monotherapy after LAA (left atrial appendage) device closure |
NCT03539055 | Prospective, observational | Dabigatran 75 or 150 mg BID x 90 days plus ASA 81 mg daily | 100 | LAAO with the WATCHMAN device | Device-related thrombosis at 90 days (as evaluated by TOE or CT) |
| FADE-DRT (Efficacy of different anti-thrombotic strategies on device-related thrombosis prevention after percutaneous left atrial appendage occlusion) |
NCT04502017 | Randomised trial | DAPT, half-dose DOAC or clopidogrel in combination with ASA on the basis of CYP2C19 genotype or half-dose DOAC | 360 | LAAO (no specified device) | Composite of stroke, systemic embolism, and device-related thrombosis; incidence of major bleeding events at 1 year |
| ASA: acetylsalicylic acid; bid: twice a day; CT: computed tomography; DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; LAAC: left atrial appendage closure; LAAO: left atrial appendage occlusion; NOAC: novel oral anticoagulants; OAC: oral anticoagulant; TOE: transoesophageal echocardiogram | ||||||
Minimalist approach: single antiplatelet therapy or no antithrombotic therapy
Most patients undergoing LAAO present with an increased bleeding risk, and a significant proportion also have a history of major bleeding events on OAC. Furthermore, some patients are at extreme risk of life-threatening or disabling bleeding due to untreatable sources including intracranial (amyloid angiopathy, untreatable vascular malformations) or gastrointestinal diseases e.g., diffuse angiodysplasia. In these extreme cases, single antiplatelet therapy (SAPT) or even no antithrombotic treatment after LAAO have been used to reduce bleeding risk.
Small studies have evaluated the safety of SAPT following LAAO, with mixed results regarding the incidence of DRT (from 0% to 7%)79808182. Also, about one-quarter (between 14% and 44%) of patients included in real-world registries received either SAPT or no antithrombotic treatment after LAAO287383. Whereas some studies showed a similar rate of DRT and embolic events, others reported a much higher rate of DRT (>10%), particularly among patients not receiving any antithrombotic treatment84.
The optimal duration of antithrombotic treatments after LAA and lifelong single antiplatelet therapy remain the empirical standard. However, up to 15% of patients in real-world observational studies discontinued antithrombotic therapy within the first months following LAAO1785, mainly due to extreme bleeding risk or recurrent bleeding episodes. Mesnier et al85 showed that early antithrombotic treatment discontinuation (within the 6 months following LAA) was associated with a reduction in bleeding events without any increase in the risk of death or thromboembolic events after a median follow-up of 2 years. The ongoing ASPIRIN LAAO trial will provide more definite data on the safety of early (at 6 months) aspirin discontinuation (Table 8).
Post-procedural imaging
Post-procedural imaging is important for evaluation of device position, sealing, exclusion of interference with neighbouring structures and complications such as DRT (Figure 17A, Figure 17B). The timing of the follow-up and specific modality used may affect the incidence of certain findings. In consecutive patients examined by protocol, the incidence of specific findings may be more accurately determined than if imaging is only based on a single adverse clinical event, then frequencies and clinical associations or causation cannot be accurately determined. The recent European Heart Rhythm Association (EHRA)/EAPCI expert LAAO statement recommends imaging within 6-24 weeks post-procedure16 (Table 6). The timing of follow-up stems from experimental animal studies indicating complete device endothelialisation within 3 months6286. The pivotal PROTECT AF and PREVAIL follow-up included 45-day, 6- and 12-month imaging which has been extrapolated to clinical practice89. Changes in post-procedural anticoagulation regimen71 88 and studies demonstrating persistent PDL or signs of incomplete device endothelialisation at 1 year raise questions about the optimal timing of follow-up8990. Early imaging follow-up is, however, generally recommended, while longer-term imaging follow-up of patients with DRT or HAT and those with large PDL may warrant sequential follow-up imaging.
The 2 most frequent and problematic issues post-procedure are peri-device leak and device-related thrombus. For each, post-procedural imaging with either CT or TOE or both are essential.
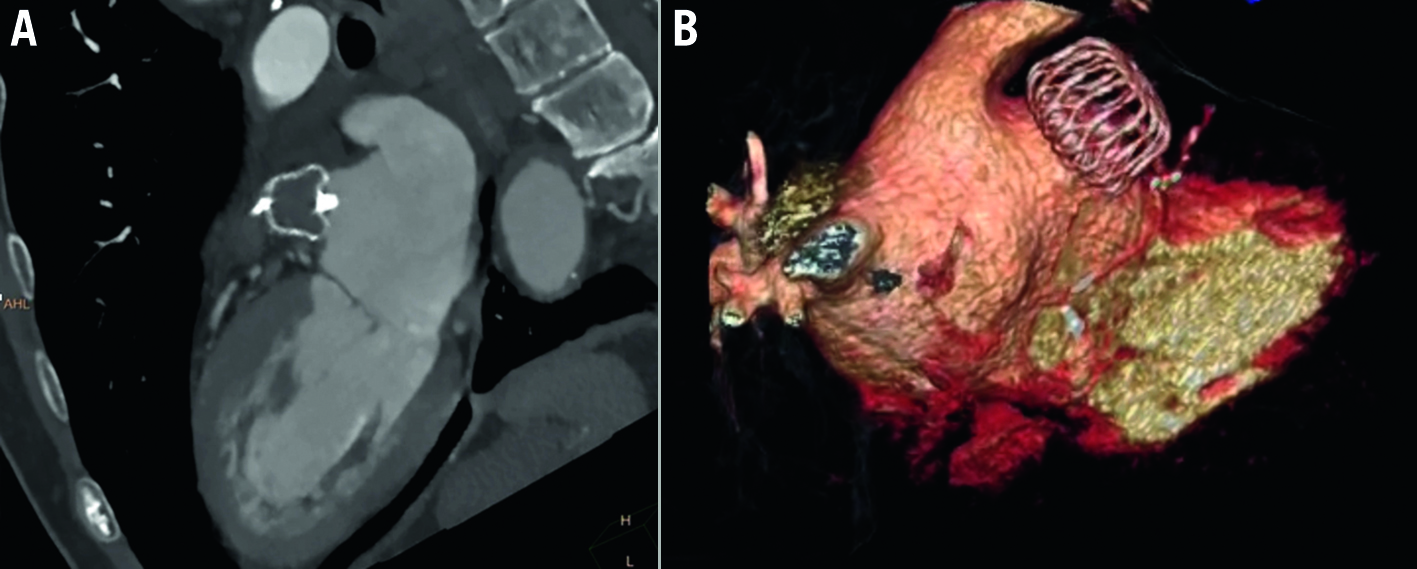
Figure 17. Successful LAA occlusion by WATCHMAN FLX seen on a 3-month follow-up CT scan. A) CT image shows the completely sealed LAA with a correct compression and complete exclusion. B) 3D volume rendering CT imaging of successful LAA occlusion. CT: computed tomography; LAA: left atrial appendage
Peri-device leaks after LAAO
Peri-device leaks after LAAO (Figure 18) are common, but their reported incidence varies considerably depending on definitions used, study design, core lab adjudication, imaging modality used (TOE vs CTA), and timing of the surveillance study. Until recently, data on the clinical significance and the optimal management of PDL after LAAO have been limited.
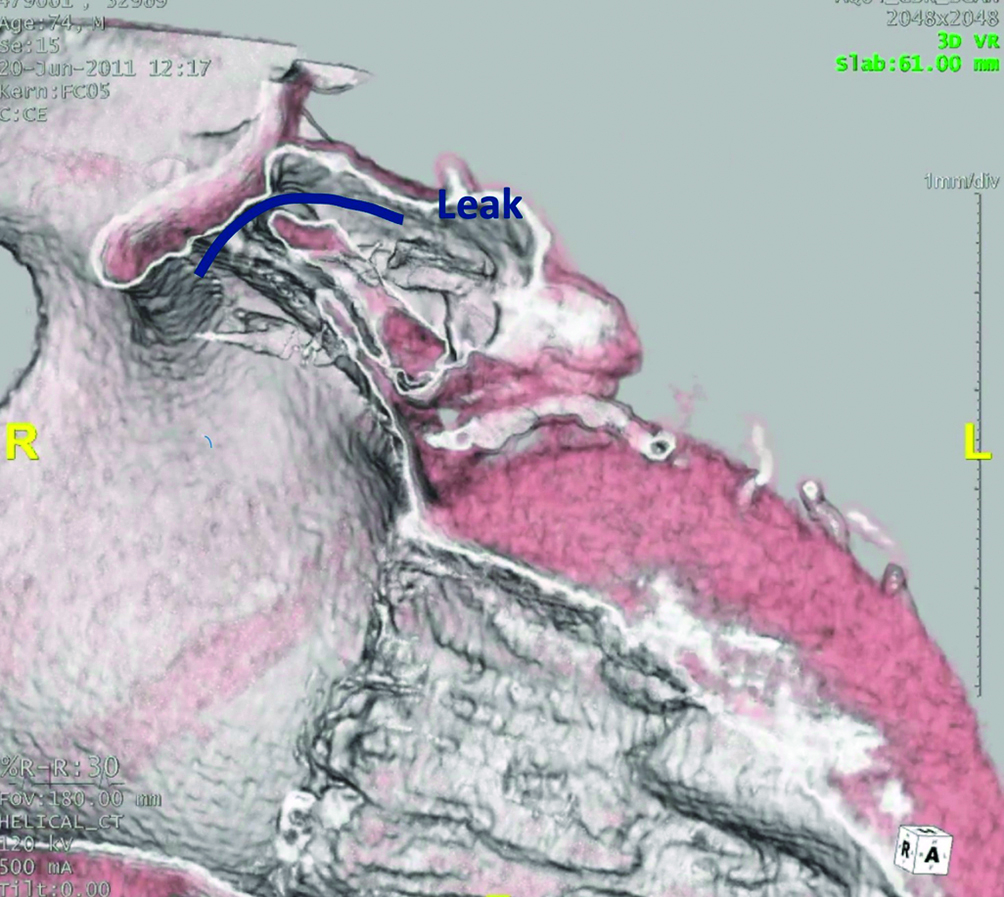
Figure 18. Peri-device leaks on 3D volume rendering CT images. LAA patency related to a superior leak (blue curved line) in a patient implanted with an AMPLATZER device. CT: computed tomography; LAA: left atrial appendage
Incidence
The rate of PDL after LAAO with the WATCHMAN family of devices was investigated prospectively in several studies. In the PROTECT AF trial, any PDL was detected in 40.9% of patients during follow-up TOE imaging at 45 days but decreased to 32.1% at 1 year. Large PDL (>3 mm in diameter) were present in 13.3% patients at 45 days and in 11.8% at 1 year90. In a large nationwide cohort of >50,000 patients enrolled in the NCDR-LAAO Registry, small leaks (1-5 mm) were present in ~25% of patients, while large (>5 mm) leaks were rare (<1%) 92 (Figure 19). As previously documented, the LAAO Registry study did not include the latest iteration of WATCHMAN FLX in which the rate of PDL was significantly lower. In the PINNACLE FLX registry, small (<5 mm) PDL at 45 days were present in 17.2% of patients, while no patient (0%) had a large (>5 mm) PDL23. In the Amulet observational registry (which included echo core lab adjudication), large (>3 mm) PDL were detected in 1.8% of patients only during the 45-day echo imaging83. However, in the Amulet IDE Trial, any PDL at 45 days was present in 37% and 54% of patients randomised to the Amulet versus the WATCHMAN 2.5 device, respectively58. Large (>3 mm) leaks were detected in 10% and 25% of patients in the Amulet versus WATCHMAN arms, respectively. Understanding the mechanism of PDL is also essential to assessing its risk and determining its most appropriate management strategy. For example, current instructions for use for the commercially available device utilise an arbitrary cut-off of a 3 or 5 mm leak diameter to define clinically significant leaks. In contrast, a definition that incorporates the leak mechanism might be more clinically relevant. A leak measuring 3 mm may be perceived as non-significant if it represents a small edge PDL in a proximal portion of a chicken-wing appendage, but not if it constitutes a narrow pathway into a large uncovered secondary lobe93. With CT assessment, the incidence is higher94.
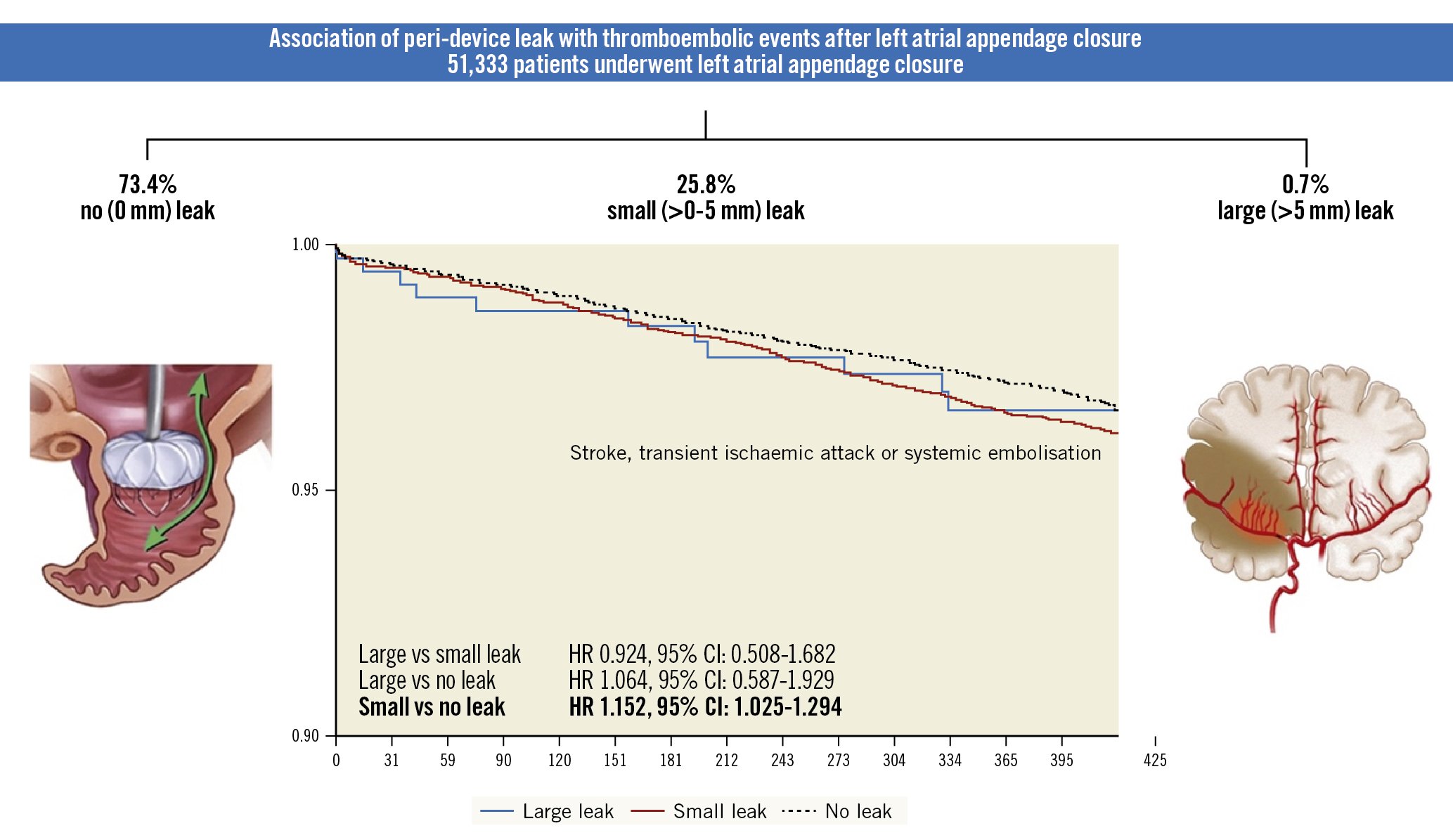
Figure 19. Association of peri-device leak with thromboembolic events after LAAO. Among >50,000 patients who underwent left atrial appendage occlusion in the United States (2016-2019), 1 in 4 patients had peri-device leak detected on follow-up imaging at 45 days. The majority of leaks were small (<5 mm in diameter). Small leaks, however, were associated with a modest increase in the composite endpoint of stroke, transient ischaemic attack, or systemic embolisation at 1-year follow-up. (Reprinted with permission from92). CI: confidence interval; HR: hazard ratio; LAAO: left atrial appendage occlusion
Clinical impact
Data on the clinical impact of PDL have been limited with conflicting conclusions9195. In practice, up to this time, the consensus remained that PDL >5 mm represented incomplete LAAO and required either continuation of anticoagulation or interventional leak closure. The large NCDR-LAAO Registry found that even small leaks (<5 mm) were associated with a modest but statistically significant increase in the risk of stroke/transient ischaemic attack/systemic embolisation at 1 year (adjusted hazard ratio [aHR] 1.15, 95% CI: 1.02-1.29) 92 (Figure 19). There were no significant differences in adverse clinical events between patients with large leaks and those with small or no leaks, although this is likely due to the small number of patients with large leaks and the fact that many of these patients were maintained on OAC. Long-term follow-up data from PROTECT AF, PREVAIL, and CAP-2 also documented that small leaks (<5 mm) that persisted beyond 1 year were associated with higher odds of stroke/systemic embolisation at 5 years (9.9% vs 5.1%, aHR 2.0, 95% CI: 1.2-3.4; p=0.008).
PDL management
No study has specifically assessed the net clinical benefit of continuing OAC in patients with PDL. Several single and multicentre studies have reported the outcomes of percutaneous closure using coils, plugs, and occluders93969798 and suggested that interventional closure of PDL is safe and effective in achieving complete closure of the LAA. Although patients who underwent PDL closure had low thromboembolic and bleeding event rates after leak closure, the limited follow-up and retrospective nature of these studies precludes solid conclusions. At present, the best approach for PDL is its mitigation which can now be increasingly achieved with newer devices with enhanced sealing abilities, steerable sheaths that facilitate coaxial alignment of the occluder device with the LAA, and preprocedural CT planning software that allows better LAA sizing and LAAO device selection23.
DRT
The second post-procedural complication is DRT38(Moving image 3) which, while uncommon, is associated with increased thromboembolic risk3684 (Figure 20). Most DRT occur in the early period after implantation, typically two-thirds within 180 days40. However, up to 20% are detected later than 1 year. The diagnosis can be challenging. Using TOE, DRT is defined as a homogeneous, echo-dense mass adherent to the atrial surface of the LAAO device visible in multiple projections36. Cardiac CT relies on the detection of subtle changes of HAT on the atrial surface which may not be readily detected by TOE3699 (Figure 21). Categorisation of HAT into low or high grade is based on imaging characteristics of the HAT localisation, extent and morphology which may help guide clinical management based on the assumption that subtle changes likely represent device healing and carry a low embolic risk36. DRT on TOE or high-grade HAT on cardiac CT requires intensification of anticoagulation therapy and repeated imaging follow-up to assess resolution. Repeated imaging follow-up may be necessary in patients with low-grade changes to follow potential progression as DRT appears to be a dynamic phenomenon. Future studies are needed to help understand the predictors of late occurrence, clinical risk, and optimal treatment92.
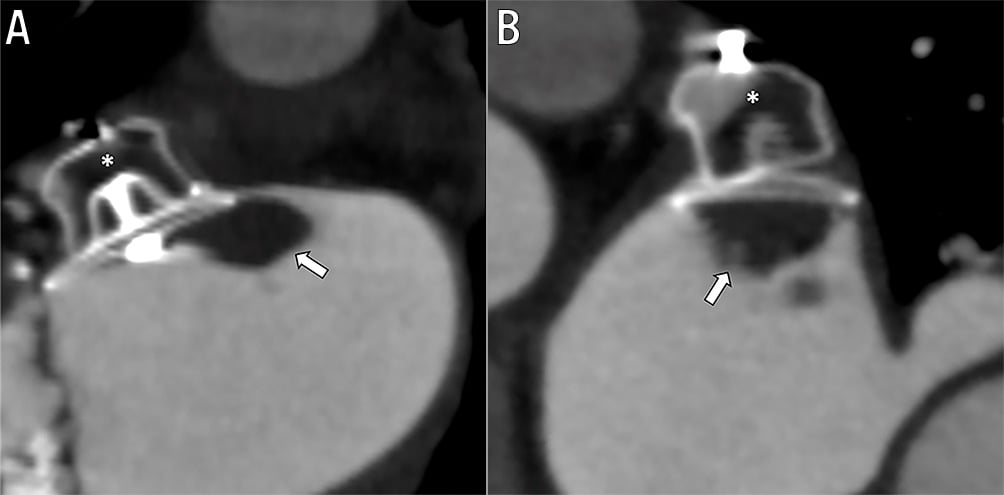
Figure 20. Device-related thrombus on left atrial apendage device. Amplatzer amulet left atrial appendage occulsion (LAAO) device (asterisk) secured within the left atrial appendage. Prominent device-related thrombus (DRT) attached to the proximal disc with protrusion into the left atrium. Visualised in two views (A) and (B).
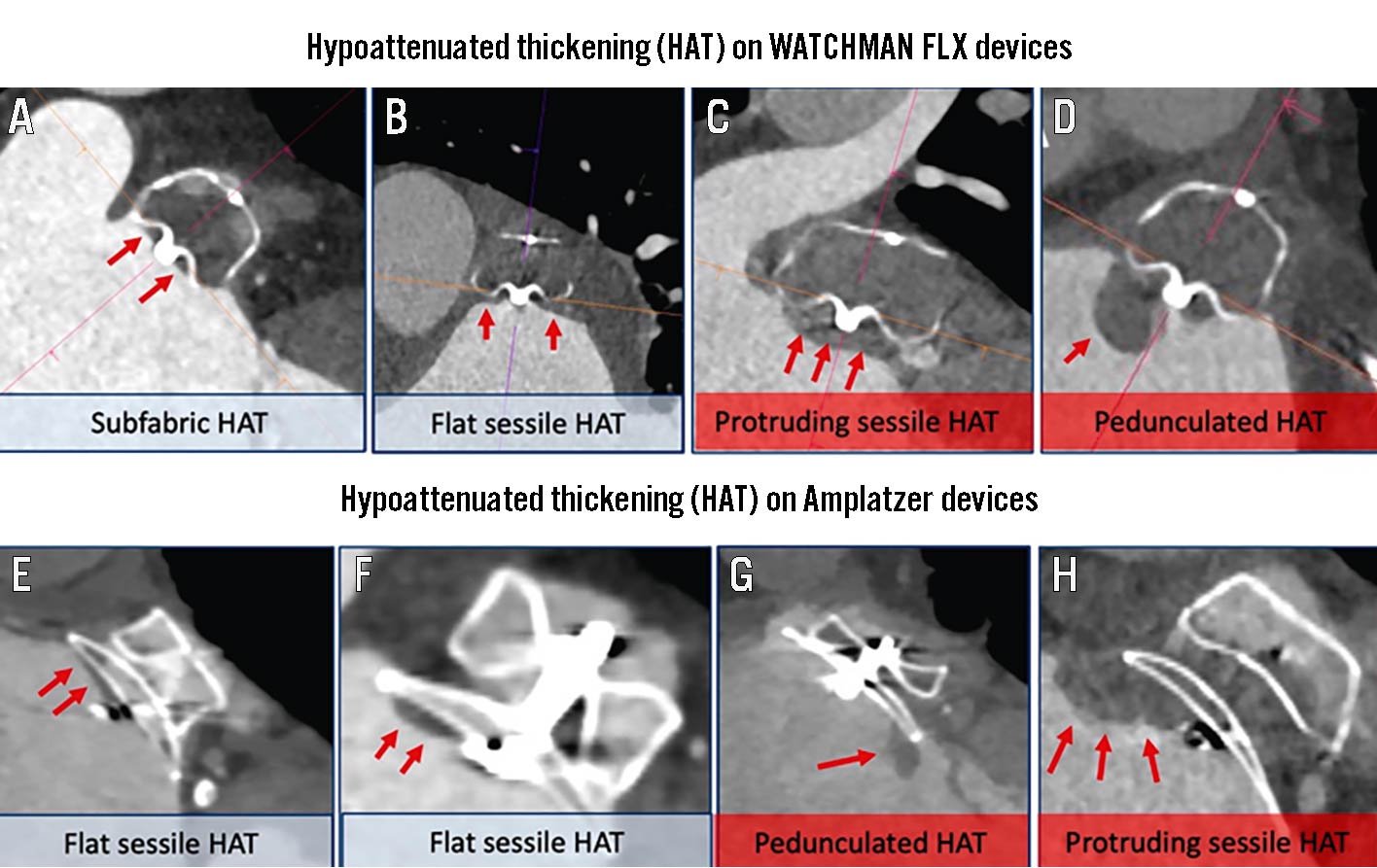
Figure 21. Hypoattenuated thickening in the WATCHMAN FLX and Amplatzer devices. Analysis of hypoattenuated thickening (HAT) includes several considerations: Localisation: isolated to screw-hub cove (A) or on atrial surface (B,C,D). Extent: smooth continuation onto the left atrium (LA) wall (B), thickness of HAT with <3 mm described as flat. Morphology: homogeneity of the HAT surface (E). Sessile (F) or pedunculated (G). High-grade features: protruding sessile or (H) pedunculated HAT without LA wall continuity or inhomogeneous surface (highlighted with red). (Reproduced with permission from99).
Frequency and timing
The incidence of DRT in PROTECT AF, PREVAIL and their nested continuous access registries was 3.74%69. A major limitation relates to the fact that approximately one-third of the cases were identified at the time of unplanned TOE studies. This may relate to the fact that there had been clinical concerns in these patients such as an ischaemic event, or bleeding that was problematic and discontinuation of the OAC was considered. Related to the selectivity of studies, the specific incidence of DRT will remain uncertain until follow-up protocol imaging is performed in consecutive patients. In a meta-analysis including >10,000 patients, the pooled incidence was 3.8%100. In this meta-analysis, the diagnosis was made at <90, 90 to 365, and >365 days in 42%, 57%, and 1% of patients, respectively. In the Amulet IDE Trial, DRT at 18 months was slightly higher in the WATCHMAN 2.5 arm of the study (4.5% vs 3.3% with Amulet)58. In the most recent evaluation of the second-generation WATCHMAN FLX device, the DRT rate was 1.7% at 1 year.
Clinical significance
In the initial WATCHMAN trials, 26.2% of patients with DRT experienced a stroke or systemic embolism event within 6 months of DRT detection69. In the largest global multicentre trial data to date of 37 international centres, 237 patients with DRT were compared with 474 patients without DRT. Identification of DRT was associated with a significantly heightened risk of the composite of death, ischaemic stroke, or systemic embolisation (HR 2.37, 95% CI: 1.58-3.56; p<0.001) and particularly an increase in stroke38. In the large meta-analysis of 66 studies and >10,000 patients, the odds ratio (OR) of ischaemic stroke in patients with DRT was 5.27, 95% CI: 3.66-7.5966. In the EUROC-DRT registry, DRT at 2 years was associated with stroke rates of 13.8% and death (20%)101.
Risk factors
In the large multicentre DRT, 5 factors were associated with DRT using multivariable analysis19. Three were clinical including a hypercoagulable disorder (OR 17.50, 95% CI: 3.39-90.45), renal insufficiency (OR 4.02, 95% CI: 1.22-13.25), and non-paroxysmal AF (OR 1.90, 95% CI: 1.22-2.97). One was related to a procedural complication, pericardial effusion (OR 13.45, 95% CI:1.46-123.52), and the final was related to procedural performance with a deeper implantation depth >10 mm from the pulmonary vein limbus (OR 2.41, 95% CI: 1.57-3.69). Using these factors a risk score was developed which may be useful in designing and studying post-deployment strategies. Although the type of post-LAAO antithrombotic therapy in this global registry did not impact the risk of DRT in this study, others yielded different conclusions1224. In the NCDR-LAAO Registry, a short course of anticoagulation with warfarin or a DOAC after the procedure was associated with a lower incidence of major adverse events at 6-month follow-up102. Finally, whether the risk of DRT is device specific remains uncertain. The Amulet device had a slightly lower DRT rate compared with the WATCHMAN 2.5 device in the Amulet IDE Trial. This is perhaps related to the geometry of closure and its effect on healing10102103.
Treatment
Although some studies suggested that oral or parenteral anticoagulants are effective in resolving DRT in a majority of patients, several issues remain. First, most patients referred for LAAO are not suitable candidates for intensified or prolonged OAC and may therefore be left with 2 high-risk scenarios: risk of embolic events with DRT and risk of major bleeding with resumption or initiation of OAC. Second, even among patients treated with OAC, DRT persists in 20-25% and those patients experience higher morbidity and mortality38. Third, even when DRT is resolved with OAC, recurrence rates are high (35% while still on anticoagulation, and 50% when anticoagulation is stopped)104. Finally, not all DRT are the same and the management of large and/or highly mobile thrombi remain uncertain. Iterative LAAO device design has also decreased the risk of DRT. For example, the metal screw on the WATCHMAN FLX is significantly less exposed on the surface of the device to reduce the risk of DRT23. Device manufactures are also exploring novel preventative approaches such as an antithrombotic coating to minimise the risk of DRT.
Conclusions
The last 2 decades have seen continued increases in patients with NVAF. While OAC remains a mainstay of AF-related stroke prevention, LAAO devices have been found to be safe and effective with the most recent data documenting procedural success rates of 97-98%. These extraordinary success rates have been reached by focusing on accurate preprocedural imaging, increased operator experience, and technical device evolution. In addition, the development of a larger amount of data has led to a better understanding of the clinical significance of DRT and peri-device leak, their predictors and management together with the understanding of different options for post-procedural antithrombotic management. As the field has matured, new groups of patients and disease have been added to the initial established indications. Current LAAO trials focus on expanding patient populations such as those with combined procedures (e.g. TAVR and MitraClip [Abbott]), those with specific indications (e.g., very low or very high risk for bleeding or stroke or cerebral amyloid angiopathy), those that optimise procedural strategies (e.g., ICE or composite angio/CT-fused imaging), and new technologies such as coated surfaces.
The current goal is to develop scientific data documenting at least equipoise with DOAC and perhaps superiority in some patient groups. That information could be used as a consideration for a class 1, level of evidence A indication for device placement. Achieving that goal will be enhanced by data obtained with the results of 2 large (approximately 5,000) patient groups randomised to device or DOAC (CHAMPION-AF and CATALYST) (Table 10). Each study will focus on 1 device (Amulet or WATCHMAN FLX), both of which are approved and will include broader diverse groups of patients with multiple secondary analyses. In addition, OPTION will address LAAO and PVI. It must be kept in mind that during the 5 years of follow-up of these studies, new iterations of the devices will become available which may dramatically improve outcomes and render current issues less important. The goal of this technology will be to broaden the options available to the increasingly large number of patients with NVAF at risk for stroke.
Table 10. Current pivotal LAAO randomised clinical trials.| OPTION | CHAMPION-AF | CATALYST |
|---|---|---|
| NCT03795298 | NCT04394546 | NCT04226547 |
| OAC eligibleCHA2DS2-VASc: ≥2 men; ≥3 women | OAC eligibleCHA2DS2-VASc: ≥2 men; ≥3 women | OAC eligibleCHA2DS2-VASc: ≥3 ( Gender distribution not specified) |
| PVI ablation within 10 days between 90 and 180 days | ||
| Randomisation: Watchman FLX vs DOAC | Randomisation: Watchman FLX vs DOAC1:1 | Randomisation:Amulet vs DOAC1:1 |
| # of patients: 1,600 | # of patients: 3,000 | # of patients: 2,650 |
| Primary outcomeComposite: stroke, all-cause death, systemic embolism 3 years, non-inferiorNon-procedural major or clinically relevant bleeding (3 yrs)Superiority Composite: stroke reduction in mortality, reduction in cardiovascular events |
Primary outcomeComposite: stroke, CV death, systemic embolism 3 years; non-inferiorityNon-procedural major or clinically relevant bleeding (3 yrs)SuperiorityComposite: ischaemic stroke/systemic embolism (5 yrs), non-inferiority | Primary outcomeComposite: ischaemic stroke, systemic embolism, CV death (2 yrs); non-inferiorityMajor or clinically relevant bleeding (2 yrs)SuperiorityComposite: ischaemic stroke/systemic embolism (3 yrs), non-inferiority |
| Enrolment complete | Enrolling | Enrolling |
| DOAC: direct oral anticoagulant; LAAO: left atrial appendage occlusion; OAC: oral anticoagulant; PVI: pulmonary vein isolation | ||
Conflict of interest statement
D.R. Holmes is a member of the advisory board of Boston Scientific; and reports an institutional research grant from Boston Scientific. K. Korsholm has received speaker’s honoraria from Abbott and Boston Scientific; and has received unrestricted institutional education grants from Abbott and Boston Scientific. J. Rodés-Cabau has an institutional research grant from Boston Scientific. J. Saw received consultation fees from Abbott, Boston Scientific, Baylis, and Gore; and proctored for Abbott and Boston Scientific. S. Berti is a proctor for Abbott, Edwards Lifesciences, and Boston Scientific. M. Alkhouli received consultation fees from Boston Scientific, Abbott, Philips, and Johnson & Johnson.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Tutorial on CT imaging for LAAO.
Moving image 2. Tutorial procedural planning for Amulet LAAO.
Moving image 3. TOE image documenting DRT which forms a cap on the device.

