Abstract
Left atrial appendage closure (LAAC), a device-based therapy for stroke prevention in patients with atrial fibrillation, is considered an alternative to oral anticoagulation therapy, particularly for patients at high risk of bleeding. Proof of concept has been demonstrated by the PROTECT AF and PREVAIL trials which evaluated the WATCHMAN device (Boston Scientific, Marlborough, MA, USA) versus warfarin, showing favourable outcome for the device group. The most commonly used devices for LAAC are the WATCHMAN and its successor, the WATCHMAN FLX (Boston Scientific) and the AMPLATZER Cardiac Plug and more recently the AMPLATZER Amulet device (both St. Jude Medical, St. Paul, MN, USA). The procedure is typically performed via a transseptal puncture under fluoroscopic and echocardiographic guidance. Technically, it is considered quite demanding due to the anatomic variability and fragility of the appendage. Careful material manipulation, adequate operator training, and good cardiac imaging and device sizing allow a safe, uneventful procedure. Post-procedure antithrombotic drug selection is based on the patient’s history, indication and quality of LAAC.
Introduction
Percutaneous left atrial appendage closure (LAAC) or occlusion (LAAO) is a device-based therapy for stroke prevention in patients with non-valvular atrial fibrillation (AF). Atrial fibrillation is the most common non-sustained cardiac arrhythmia, affecting 1.5-2% of the population in developed countries. It is associated with a fivefold increased risk for stroke, and its occurrence increases with age. Moreover, the clinical consequences for patients with AF suffering a stroke become more dramatic with increasing age1. Stroke prevention remains the mainstay of treatment strategies in AF. The standard therapy in 2016, derived from several large, randomised clinical trials including >100,000 patients, favours the use of oral anticoagulation (OAC) with direct oral anticoagulants (DOAC) in all patients with AF and at least one additional stroke risk factor such as diabetes, coronary artery disease (CAD), previous stroke, etc. These drugs come with a reduced risk of intracranial bleeding compared to warfarin and do not require international normalised ratio (INR) monitoring. In certain situations, such as renal insufficiency, DOAC dosage may be reduced, but these dose adjustments may result in decreased efficacy for stroke prevention2.
The rationale for LAAC originates mainly from echocardiography studies showing that in non-valvular AF >90% of thrombi are found in the LAA3. Proof of concept has been demonstrated by PROTECT AF and PREVAIL, two randomised clinical trials which evaluated the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA) versus warfarin, showing favourable outcome for the device group4-6. In fact, the four-year results of the PROTECT AF trial showed a significant reduction in all-cause mortality for the intention-to-treat population, a remarkable achievement for such a hard clinical endpoint6. The most commonly used devices for LAAC are the WATCHMAN (FDA approved and Conformité Européenne [CE] marked) and its successor, the WATCHMAN FLX (Boston Scientific) device (CE marked), and the AMPLATZER™ Cardiac Plug (ACP) and more recently the AMPLATZER Amulet™ device (both St. Jude Medical, St. Paul, MN, USA, and both CE marked) (Figure 1). Several new devices are in development and have been covered in recent reviews. This review will focus on indications and patient selection for LAAC, device description and technical considerations, and post-procedure drug selection.
Indications for LAAC
According to the 2012 ESC Guidelines for the management of AF, “interventional percutaneous LAAC may be considered in patients with high stroke risk and contraindications to long-term OAC”1. In addition, according to the 2014 ESC/EACTS Guidelines for myocardial revascularisation, “percutaneous LAAC and antiplatelet therapy may be considered in patients with AF undergoing PCI if a high stroke risk and a contraindication for long-term combined antiplatelet and OAC is present”7. In both documents, LAAC received a IIb recommendation (level of evidence B), mainly based on the results of the PROTECT AF and PREVAIL studies4,5. Noteworthy, in those two studies LAAC was only evaluated in patients eligible for warfarin, whereas patients with contraindications to long-term OAC were excluded. Nevertheless, the majority of patients were at moderate to high risk of bleeding on the basis of their HAS-BLED score6. Currently, percutaneous LAAC has not yet been studied in a randomised setting for patients with contraindications to long-term OAC. Therefore, in March 2015 the Food and Drug Administration (FDA) made the following statement of device approval in the United States: the WATCHMAN device is indicated to reduce the risk of thromboembolism from the LAA in patients with non-valvular AF who:
– are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy,
– are deemed by their physicians to be suitable for warfarin, and
– have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.
The “real-world” indication and use of LAAC has been demonstrated in two large multicentre European registries. In the AMPLATZER Cardiac Plug (ACP) Registry, which included 1,047 patients between December 2008 and November 2013, the most common indication for LAAC was previous major bleeding (47%), followed by high bleeding risk (35%) and avoidance of triple therapy in CAD and stenting (22%)8. In the prospective EWOLUTION registry, the most common indication for LAAC with the WATCHMAN device was contraindication to warfarin (62%) and previous or high risk of bleeding (39%)9.
It should be noted that the patient population which may benefit from LAAC is not homogeneous. For example, a young patient who suffers a stroke while being on optimal OAC therapy is totally different from an 85-year-old patient with recurrent intracranial haemorrhage on OAC or aspirin therapy. In principle, when evaluating candidates for LAAC it is important to “know the patient”: acquiring a comprehensive history, reviewing in detail the patient’s medical records and obtaining adequate information from cardiac imaging may assist in identifying which particular patients would benefit more from LAAC. This strategy may counterbalance the lack of hard evidence regarding patient indications and can help to achieve a favourable introduction of a relatively new technology such as LAAC in everyday clinical practice.
Devices
WATCHMAN
The WATCHMAN device consists of 10 nitinol frames that carry small barbs around the device perimeter, which engage into the LAA tissue (Figure 1). The device is covered with a 160 µm membrane consisting of polyethylene terephthalate (PET) that entraps any thrombi behind the device, avoiding their entrance to the blood circulation. The device is self-expanding and its proximal part maintains position in the LAA; the open distal part has no radial force and anchors the device in its longitudinal axis. The device is available in five different sizes: 21, 24, 27, 30 and 33 mm (Figure 2). Correct sizing is fundamental for successful LAAC with the WATCHMAN; clinical experience finds the device to be very forgiving regarding oversizing, which is routinely carried out by around 20% up to 30%, resulting in less leakage than the initial recommendations to use 10%-20%. In other words, the device size is usually selected two sizes larger than the maximum measured diameter (i.e., a 20 mm LAA ostium receives a 24 mm device). Implantation technique has been standardised resulting in successful LAAC in >95% of patients without prior screening for specific LAA anatomy9. The WATCHMAN device may be implanted in a wide range of LAA orifice dimensions (16-30 mm) provided that the LAA depth is equal to or larger than the LAA orifice.

Figure 1. WATCHMAN, WATCHMAN FLX, and AMPLATZER Amulet devices. The WATCHMAN (A), the WATCHMAN FLX (B), and the AMPLATZER Amulet devices (C) are specially designed for LAAC. LAAC: left atrial appendage closure
The WATCHMAN sizing chart is shown in Figure 2, and a cardiac imaging work-up is depicted in Figure 3. An inferior and posterior transseptal puncture is considered essential for optimal sheath and device alignment. Most centres use an SL-1 sheath with a BRK-1™ needle (St. Jude Medical) that may be given extra curve if the right atrium is dilated and a stable position at the fossa ovalis is difficult to achieve. Following the transseptal puncture, a stiff wire (AMPLATZER Super Stiff™; St. Jude Medical, or other stiff wire) is positioned in the left superior or inferior pulmonary vein to allow safe exchange to the delivery sheath. The wire should not be placed directly into the LAA as that manoeuvre comes with a high risk of (sometimes delayed) pericardial effusion. Next, a pigtail catheter inside the delivery sheath is used to negotiate deep into the LAA, which allows a better understanding of the commonly variable anatomy and the number of lobes present (Figure 4A-Figure 4C, Moving image 1). Operators need to identify the location of the interlobar ridges as these are important to predict device unfolding. If the ridge is very proximal, 50% of the device needs to sit proximal to the interlobar ridge in order to cover the entrance to the other LAA lobes. After release, the “PASS” criteria are checked, namely position (“shoulders” protruding into the left atrium <50% of device size), anchoring (tug test without change of device position in transoesophageal echocardiography [TEE] and angiography), sealing (TEE views 0°, 45°, 90°, 135° without leaks >5 mm), and compression (aimed to be around 20%) (Figure 4D). If the device position is correct, it is released by unscrewing the connector wire. Clinical experience shows that the superior, anterior lobes give the best support for distal anchoring of the WATCHMAN device. Inferior lobes mostly result in large “shoulders” that protrude into the left atrium. These are acceptable up to 10-14 mm sizes, since 50% of the device is PET-covered. If the depth of the LAA is not sufficient, clinical experience shows that the WATCHMAN device can be pushed distally after 50% of the device has been released without an increased risk for pericardial effusion. A final tug test is mandatory to prove the device is anchored safely.
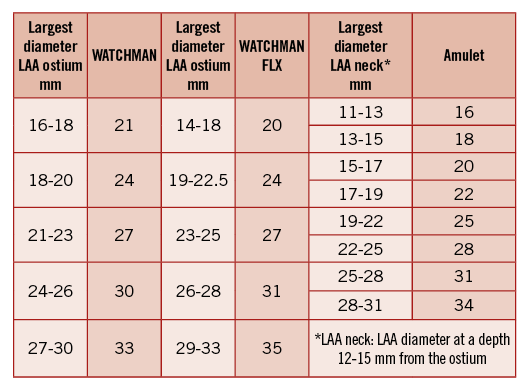
Figure 2. Current sizing charts based on clinical experience with the relevant devices. WATCHMAN oversizing by around 20%, WATCHMAN FLX by around 10%, Amulet by around 15% is recommended. LAA: left atrial appendage
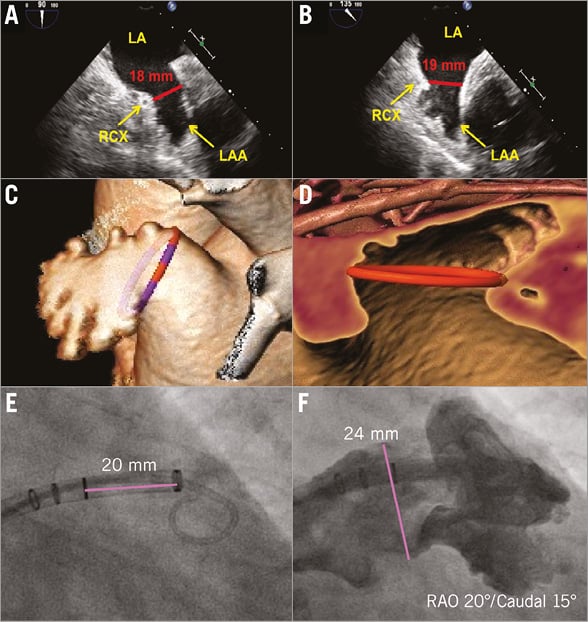
Figure 3. Imaging of the LAA and proposed measurements for sizing the WATCHMAN devices. A) & B) The diameter of the LAA orifice is taken approximately 20 mm from the ridge to the pulmonary vein on the superior margin and at the crossing of the circumflex coronary artery on the inferior side. On TEE, measurements are usually taken at 0°, 45°, 90° and 135°. The largest diameter is most often found at 135° (B). Cardiac CT (C & D) that can be used to measure all relevant dimensions more accurately provides three-dimensional reconstruction of the LAA and is considered a valuable tool for planning the procedure; the OsiriX and 3mensio (3mensio Medical Imaging BV, Bilthoven, The Netherlands) are useful software tools (this measurement done with 3mensio LAA module). Contrast angiography (E & F) offers real-time information about the LAA anatomy and is the main guiding tool during the procedure. CT: computed tomography; LAA: left atrial appendage; TEE: transoesophageal echocardiography
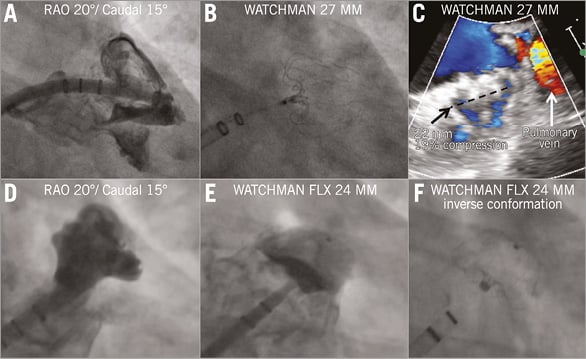
Figure 4. Step-by-step implantation of the WATCHMAN (A-C) and WATCHMAN FLX (D-F) devices. A) The WATCHMAN delivery sheath is positioned at the distal LAA edge by employing a pigtail catheter as a rail. The device is released from the delivery system by pulling back the system. The typical “strawberry” form of the WATCHMAN (B &C) indicates correct sizing. Prior to release, the PASS criteria are checked, namely position (device protruding into the left atrium), anchoring (tug test), size adequate (compression) and sealing. No peri-device leakage >5 mm should be accepted. Panels D-F show an exemplary picture of WATCHMAN FLX implantation. The device takes a classic “inverted” position.
WATCHMAN FLX
The WATCHMAN FLX (CE marked since October 2015) has several new design features compared to the WATCHMAN (Figure 1). A proposal for sizing is shown in Figure 2. The new device has closed distal nitinol loops to allow safe navigation of the partially deployed device in the LAA using the “ball” technique (Figure 4E). It has two rows of anchors and an increased number of struts to increase radial strength and safe anchoring. When fully deployed, the device has only half the depth as compared to the WATCHMAN and it is almost fully covered with the PET fabric. This feature, together with the increased radial strength, is intended to minimise peri-device leakage. It also allows sizing the device with less compression than before (10% of the LAA ostium width). The WATCHMAN FLX has two possible device configurations after release: the “classic WATCHMAN conformation” and the “inverse conformation” (Figure 4B, Figure 4F, respectively, Moving image 2). In order to check for device stability regarding the two conformations, a so-called “reverse tug test” may be performed by pushing the control wire distally after full deployment of the device.
AMPLATZER AMULET
The AMPLATZER Amulet is a self-expanding device made of nitinol. It has a different configuration from the WATCHMAN, with a distal lobe (that has six to ten pairs of stabilising wires around it) and a proximal disc, connected by an articulating waist (Figure 1)10. The device lobe is positioned in the proximal 10-15 mm of the LAA (landing zone or “neck”) serving as a first layer of closure and securing the device position, whereas the disc covers the LAA ostium from the left atrial side as a second layer of closure. The Amulet comes in eight different sizes ranging from 16 to 34 mm (16, 18, 20, 22, 25, 28, 31, and 34 mm) and can be implanted in a wide range of LAA sizes (11-31 mm landing zone). The Amulet sizing chart is shown in Figure 2. The four larger sizes (25 to 34 mm) have a lobe that is 2.5 mm longer than the four smaller sizes (16 to 22 mm); therefore, they need slightly more space for deployment (12 mm minimum LAA depth instead of 10 mm) and usually less oversizing. The Amulet is suitable for any LAA anatomy, including variations with lobes arising too close to the LAA ostium, very proximal interlobar ridges, very short “neck” and acute bending of the LAA body (the so-called extreme “chicken wing” morphology)11. Implantation success and complete closure rates are very high (98-99%), mainly due to the flexibility of the device, the dual-layer closure design, and the ability to anchor in the very proximal part of the LAA8,10,11.
A step-by-step approach for Amulet implantation is shown in Figure 5A-Figure 5I, Moving image 3. The first steps (transseptal puncture and stiff wire exchange in the pulmonary vein) are similar to WATCHMAN implantation. Originally, having a pigtail catheter inside the delivery sheath, deep negotiation in the LAA was recommended (as with the WATCHMAN device). However, a modified technique with partial deployment in the left atrium (ball position) (Figure 5D, Figure 5E) and advancement towards the landing zone is gradually being adopted. The reason for this is to avoid material and device manipulation in the distal part of the LAA, where a potential small thrombus (invisible on TEE) may get dislocated and cause a stroke. This technique is also applied in case an LAA thrombus is present and no other option is available. The device is released after verifying five signs of stability, and a gentle tug test is commonly performed before final release (Figure 5H, Figure 5I, respectively). The Amulet has replaced the ACP in the vast majority of countries. Implantation technique is similar for both devices. A randomised clinical trial with head-to-head comparison against the WATCHMAN device is expected to start in Q3, 2016, aiming for FDA approval in the USA.
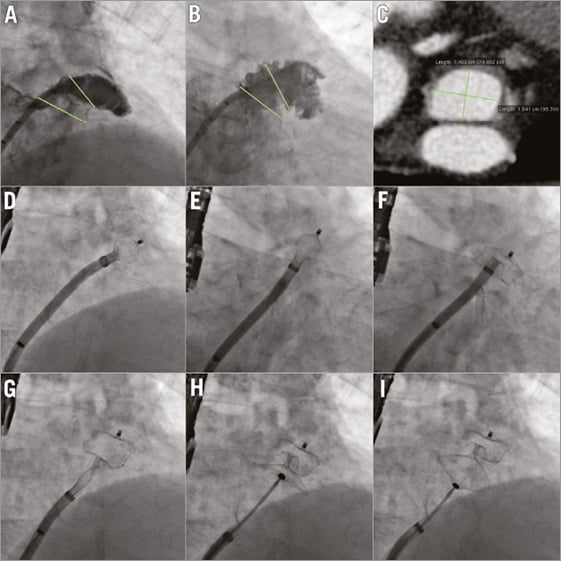
Figure 5. AMPLATZER Amulet: step-by-step. Through the transseptal sheath, by using a marked pigtail catheter, an LAA angiogram is performed in RAO-cranial 20 (A) and in RAO-caudal 20 (B) views. The dimensions of the LAA orifice and neck are measured (green lines). Panel C demonstrates the CT angiogram of the same patient at the level of the LAA neck (short axis). Panels D-I show the device deployment steps. D) Ball position. E) Counterclockwise rotation. F) Deployment of device lobe. G) Deployment of device disc. H) Device stability evaluation before release. I) Gentle tug test. CT: computed tomography; LAA: left atrial appendage; RAO: right anterior oblique
Imaging requirements, general anaesthesia, and surgical back-up
The usual workflow for LAAC starts with exclusion of LAA thrombi, which is considered a contraindication for device closure. This can be carried out in a separate TEE analysis some days prior to the procedure or just at the beginning of the procedure. In some centres, cardiac CT has replaced TEE for the exclusion of thrombus and is also used for procedure planning12. LAAC can be carried out under general anaesthesia (probably preferable for beginners) or with conscious sedation by propofol and midazolam. Production of saliva with the risk of aspiration when the patient is in the supine position and the TEE probe is in place can be minimised with 0.5 mg of atropine at the beginning of the procedure or an atropine analogue not passing the blood-brain barrier, glycopyrronium bromide (0.2 mg). Cardiac surgical back-up requirement varies among countries and centres. In many European facilities LAAC is performed without surgery on-site since life-threatening complications are considered rare (<1%) and can usually be treated by interventional means. However, according to SCAI/ACC/HRS Institutional and Operator Requirements for LAAO, cardiac surgical back-up is considered mandatory in the USA13.
The procedure is typically performed under fluoroscopic and TEE guidance, which allows accurate inferoposterior transseptal puncture (bicaval and aortic valve short-axis TEE views). If present, a patent foramen ovale (PFO) may be used, but the vast majority of operators prefer to perform a puncture because usually the wire and sheath alignment is suboptimal through a PFO, resulting in increased procedural time and more sheath manipulation that can also increase the risk of complications. Practically speaking, a PFO can be used if a wire that crosses it from the inferior vena cava goes directly into the LAA (high LAA position in the left atrium) and the LAA has “regular” anatomy (i.e., no chicken wing shape or very shallow LAA). Avoidance of transseptal puncture comes with a decreased risk of puncture-related complications, which, however, are very rare in TEE-guided procedures14. The dimensions of the LAA are measured in angiography and TEE or in CT, if available (Figure 3). It is advisable to perform at least two LAA angiograms in different C-arm projections (i.e., RAO-cranial 20° and RAO-caudal 20°) in order to understand the three-dimensional LAA configuration. Three-dimensional TEE (or CT 3D volume rendering) may also be helpful as the anatomy of the LAA is very variable. Some centres have reported workflows employing micro-TEE probes and also intracardiac echocardiograms (ICE) to avoid the need for a separate TEE operator. In addition, workflows employing just angiography-guided LAAC have been described, but this strategy is considered less safe, particularly for new operators.
Combined procedures
Simultaneous LAAC procedures plus pulmonary vein isolation (PVI) as well as LAAC+MitraClip® (Abbott Vascular, Santa Clara, CA, USA) or transcatheter aortic valve implantation (TAVI) have been performed. While the combination of PVI and LAAC is appealing, the combination of MitraClip and LAAC might run the risk that any transmitral gradient will increase the risk of thrombi in the free left atrial cavity rather than the LAA. That is why LAAC is not recommended in valvular AF, more specifically mitral stenosis, patients. Combining MitraClip and LAAC should be performed with caution until more data are available on this specific subset. On the contrary, LAAC and TAVI might be an interesting option, since the risk of paroxysmal AF following TAVI appears to be increased15. More data are needed on these specific subsets of patients.
Post-procedure drug selection, device thrombosis, TEE follow-up
Some kind of antithrombotic and/or antiplatelet therapy is recommended after LAAC, for two main reasons: to prevent device thrombosis and to prevent stroke in case of a significant (>5 mm) peri-device leak. In the US PROTECT AF study, aspirin 80-100 mg plus warfarin was prescribed for 45 days after WATCHMAN implantation. A TEE study was then performed and, if there was no significant leak, warfarin was stopped and the patients were switched to dual antiplatelet therapy (DAPT) for six months post procedure. Then clopidogrel was stopped and aspirin was continued for life4.
The prospective ASAP registry which included patients with contraindication to OAC who were implanted with the WATCHMAN device tested DAPT for one to three months and then aspirin monotherapy16. The strategy was found to be safe: no increase in stroke risk was observed. For the AMPLATZER Amulet device, the current recommendation is one to three months of DAPT and a total of six months of single antiplatelet therapy with aspirin. Nevertheless, the choice and the duration of antithrombotic medication post LAAC are rather empirical, particularly for patients non-eligible to OAC8. Device endothelialisation is typically completed within one to three months. However, despite prescribing antithrombotic drugs for a short period after LAAC, the rate of device thrombosis is not negligible, averaging 3-4% for both devices17.
Fortunately, so far device thrombosis has not been associated with adverse events, but the phenomenon has not yet been thoroughly evaluated and device thrombosis is most probably under-reported. When discovered, device thrombosis is usually treated with a four-week course of OAC or LMWH therapy, followed by a TEE assessment. In the majority of reported cases, thrombus resolves permanently with this strategy18.
Again, similarly to LAAC indications and due to the lack of data, the choice and duration of antithrombotic therapy should be individualised. Potential parameters to be considered are the indication for LAAC (i.e., major bleeding on OAC or aspirin, stroke on OAC without high bleeding risk), patient comorbidities (i.e., CAD, diabetes, previous non-embolic ischaemic stroke, heart failure with significant systolic left ventricular dysfunction), left atrial appearance on echocardiography (i.e., very large atria, spontaneous echo contrast), and quality of LAAC (i.e., presence of significant or mild leaks, uncovered LAA lobes, device protrusion in the left atrium). The TEE follow-up depends on the preference of the particular centre but is typically performed within one to three months post LAAC and repeated at one year. Again, there is no standard approach and follow-up is usually decided on an individual basis.
Conclusions
In conclusion, percutaneous LAAC is a relatively new and quite challenging procedure for stroke prevention in patients with non-valvular AF. Good knowledge of individual patient characteristics and acquaintance with the special features of dedicated LAAC devices and tools may allow a safe implementation of this procedure in clinical practice. Further technology development and careful evaluation in clinical studies should be encouraged as LAAC is gradually being established worldwide.
Conflict of interest statement
A. Tzikas is a proctor for St. Jude Medical. M. Bergmann is a proctor for Boston Scientific and Coherex Inc.
Supplementary data
Moving image 1. WATCHMAN step-by-step.
Moving image 2. WATCHMAN FLX step-by-step.
Moving image 3. AMPLATZER Amulet step-by-step.
Supplementary data
To read the full content of this article, please download the PDF.
WATCHMAN step-by-step.
WATCHMAN FLX step-by-step.
AMPLATZER Amulet step-by-step.

