Abstract
Aims: In patients with atrial fibrillation, a relevant stroke risk (CHA2DS2-VASc score ≥2) and a relative or absolute contraindication for oral anticoagulation, catheter-based LAA occlusion is performed increasingly in Europe. The present article summarises the rationale, clinical data, devices, implantation techniques and follow-up drug regimens.
Methods and results: European survey data on patients with atrial fibrillation support the need for non-pharmacological approaches for stroke prevention in patients with atrial fibrillation. A relevant bleeding risk remains with novel oral anticoagulants (NOACs), which are also dependent on drug compliance. Recent long-term data from the PROTECT-AF trial and the CAP registry regarding the WATCHMAN LAA occluder device suggest safety and efficacy. First registry data support the safety of two other CE-marked devices, the AMPLATZER Cardiac Plug (ACP) and the Coherex WaveCrest device, which have become available in Europe. Other LAA occlusion devices are in clinical development.
Conclusions: Catheter-based LAA occlusion is now being developed further as an interventional approach for stroke prevention in patients with atrial fibrillation. Implantation techniques and devices are being improved, which will probably result in better procedural safety. Appropriate operator training is of major importance for this approach.
Introduction
Atrial fibrillation (AF), the most common arrhythmia, is a major cause of severe strokes, in particular in the elderly. Chronic anticoagulation can substantially reduce the risk of stroke by approximately 60-70%; however, it is associated with a significant annual risk of major bleedings, particularly in the elderly (Figure 1)1. To assess the risk of bleeding with anticoagulation the HAS-BLED score has been developed based on a real-world patient cohort treated with warfarin from the EuroHeart Survey on Atrial Fibrillation2. Of note, the persistent use of anticoagulation with warfarin has been observed to be below 50% even in patients after an ischaemic stroke. This is probably explained, at least in part, by the associated bleeding risk, but also by the general limitations of drug compliance3.
Rationale for left atrial appendage (LAA) closure in atrial fibrillation
In patients with “non-valvular atrial fibrillation” (no mitral stenosis or prosthetic heart valve) >90% of thrombi have been detected in the LAA4. This has stimulated development of catheter-based approaches to LAA occlusion for stroke prevention5, an approach that has a lower risk of major bleedings as compared to chronic anticoagulation and is not dependent on drug compliance. Moreover, recent data suggest that for patients with CHA2DS2-VASc score ≥2, pulmonary vein isolation may not eliminate the need for stroke prevention by anticoagulation or other approaches6.
Currently, one randomised, multicentre study has been completed comparing the safety and efficacy of LAA occlusion to warfarin in patients with non-valvular atrial fibrillation, i.e., the PROTECT AF trial7. In this study, patients were randomised to either LAA occlusion (n=463) employing the WATCHMAN™ device (Boston Scientific, Natick, MA, USA) or continued oral anticoagulation with warfarin (n=244). Patients in the intervention group continued to take warfarin for 45 days, then switched to dual platelet inhibition until six months and were changed to aspirin monotherapy thereafter. At 1,065 patient-years follow-up, the probability of non-inferiority of the WATCHMAN device compared to warfarin was 99.9% for the primary efficacy endpoint, defined as a primary composite endpoint of stroke, cardiovascular death, and systemic embolism7.
Safety events were more common in the device intervention group (7.4% vs. 4.4% per 100 patient-years) and were mainly periprocedural complications, including pericardial tamponade7. After trial completion, a continued access registry (CAP) was started. Data from 1,002 patients demonstrated a significant decline in the rate of procedure- or device-related safety events with increasing operator experience. No additional safety issues appeared8. Subgroup analysis of patients aged >75 years in this series confirmed the safety and efficacy of the procedure in this patient subgroup, in whom the bleeding risk as well as the thromboembolic risk are increased9.
The four-year follow-up data of PROTECT AF were presented at EuroPCR 2013. A superiority was observed regarding all-cause mortality in patients assigned to WATCHMAN device treatment which was accompanied by reductions in haemorrhagic stroke (0.4% vs. 2.9%, p<0.01). In addition, patients receiving LAA occlusion with the WATCHMAN device showed favourable changes in quality of life (QoL) at 12 months as compared to warfarin treatment in PROTECT AF10. Early in 2014, these data led to a favourable vote of an FDA panel on the indication for a WATCHMAN LAA occluder in patients eligible for warfarin, as studied in PROTECT AF. Already in 2012 European Society of Cardiology guidelines gave a class IIb indication for LAA occlusion in patients with a relevant stroke risk who are not eligible for long-term anticoagulation.
RISK OF MAJOR BLEEDING WITH NOVEL ORAL ANTICOAGULANTS (NOACS)
The novel direct anticoagulants, i.e., dabigatran, rivaroxaban and apixaban, reduce the risk of intracranial bleeding. However, major bleedings still occur at a rate of approximately 2-3% per year even in low-risk patients (Figure 1)11-17.
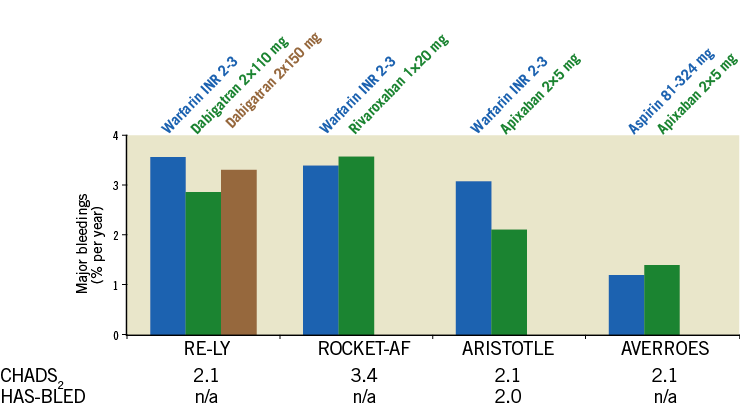
Figure 1. Risk of annual bleeding events under warfarin and the direct oral anticoagulants dabigatran, rivaroxaban and apixaban according to CHADS2 and HAS-BLED score (when available). Definition of bleeding event: intracranial bleeding, hospitalisation due to bleeding, haemoglobin decrease >2 mg/dl and/or transfusion of red blood cells.
A subanalysis of the RE-LY trial has suggested that the benefit of low-dose dabigatran (2×110 mg/day) regarding bleeding events compared to warfarin is lost in patients ≥75 years12. Rivaroxaban increased gastrointestinal bleedings as compared to warfarin13. Apixaban at the 2×5 mg dose has a reduced risk of bleeding as compared to warfarin in all subgroups, yet bleeding events still occurred at a rate of 3.46%/year in the patient group with a HAS-BLED score ≥314. The published data regarding bleeding risk associated with warfarin and NOACs are summarised in Figure 1. In summary, both warfarin as well as NOACs should be used with caution in elderly patients with increased bleeding risk. Alternatives for stroke prevention are needed.
PATIENT SELECTION FOR LAA CLOSURE
Figure 2 suggests a potential algorithm for patient selection for LAA closure based on the above-mentioned data regarding safety and efficacy of LAA occlusion (by the WATCHMAN device) and the risk of bleeding with chronic anticoagulation. In particular, LAA occlusion can be considered for those patients with a relevant risk of stroke (as defined by the CHA2DS2-VASc score) and a significantly increased risk of major bleedings (such as in patients with recurrent GI bleedings, intracerebral haemorrhages, etc.).
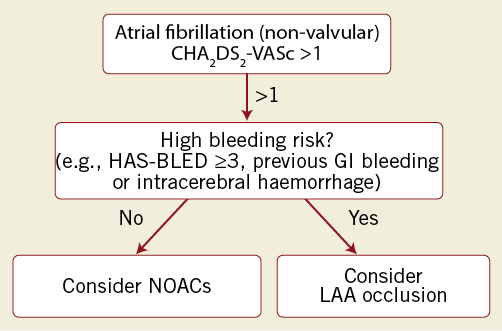
Figure 2. Potential algorithm for decision making regarding stroke prevention in atrial fibrillation patients (AFib). Oral anticoagulation or LAA occlusion depending on stroke risk (CHA2DS2-VASc score) and bleeding risk (HAS-BLED score). Calculation of scores refers to recent ESC publications on atrial fibrillation. The risk of bleeding with a HAS-BLED score of 3 is 3.7%/100 patient-years.
Devices in clinical development for LAA occlusion
There are several devices in clinical development for catheter-based LAA occlusion. The most commonly used devices in Europe at present are the WATCHMAN (Boston Scientific) and the AMPLATZER™ Cardiac Plug (ACP) device (St. Jude Medical, St. Paul, MN, USA). The Coherex WaveCrest device (Coherex Medical Inc., Salt Lake City, UT, USA) has recently received CE marking and is now available in Europe.
WATCHMAN LAA OCCLUSION DEVICE
The WATCHMAN device (Figure 3A) was used in the PROTECT AF study. Oversizing of the device by 15-30% rather than the initially recommended 10-20% is now a strategy in many experienced centres in order to optimise implantation results18. A further randomised study, the PREVAIL study, showed rates of pericardial effusion comparable to the CAP registry (1.9% vs. 2.2%). The next-generation device (named generation V) is expected to cover a wider range of LAA ostium sizes with a reduced number of device sizes in order to simplify the procedure further. The release in Europe is expected for 2014. A large, 1,000-patient European registry, called EWOLUTION, was recently started and is collecting procedural and outcome data in Europe.
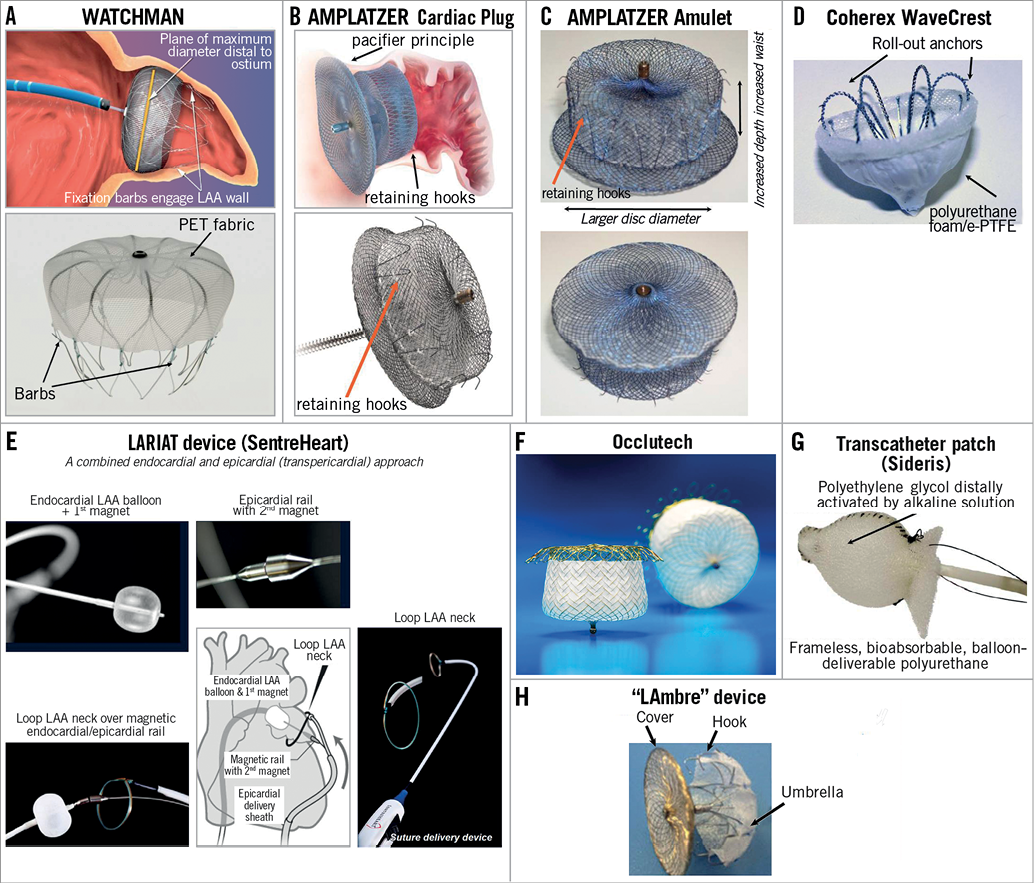
Figure 3. Catheter-based LAA occluders in clinical development. A) WATCHMAN occluder (in clinical use, endpoint studies available: PROTECT AF, CAP registry, PREVAIL, ASAP, ALSTER-LAA). B) AMPLATZER Cardiac Plug (in clinical use, registry data available, large clinical endpoint study initiated). C) AMPLATZER “Amulet” (CE mark available, currently in revision for delivery system). D) Coherex WaveCrest (CE mark in 2013). E) SentreHeart transcutaneous ligation (CE mark, in use in the USA). F) Occlutech LAA occluder. G) Transcatheter patch: bioresorbable, frameless LAA occlusion. H) “LAmbre” device consisting of a fabric-enriched cover and an umbrella connected by a short central waist.
AMPLATZER CARDIAC PLUG (ACP) AND AMULET LAA OCCLUSION DEVICE
For another LAA occlusion device, the AMPLATZER Cardiac Plug (ACP) (Figure 3B), a large clinical trial was initiated in 2013 which was designed to compare LAA occlusion to anticoagulation with warfarin or dabigatran, i.e., the ACP trial. This trial is currently on hold and will be redesigned. The results of several registries regarding procedural results and short-term follow-up have been published or presented, including patients with a contraindication for anticoagulation19,20. A second generation of the device (ACP2 or Amulet™; St. Jude Medical) has recently been introduced in Canada and Europe for first clinical testing (Figure 3C)21. It has several modifications including a larger LAA orifice disc, a low-profile end screw and a longer lobe length to improve sealing performance and further reduce the risk of complications21. The low-profile end screw may facilitate endothelialisation of the device with the aim of reducing the risk of device-associated thrombi22. The company (St. Jude Medical) has decided to revise the flexible delivery cable system after feedback from the first clinical implantation in expert centres in Canada and Europe. The redesigned ACP2/Amulet device will be introduced into the market in the second half of 2014.
COHEREX WAVECREST LAA OCCLUSION DEVICE
The WaveCrest Left Atrial Appendage Occlusion System (Coherex Medical Inc.) (Figure 3D) completed initial preclinical testing and first-in-man studies in New Zealand in 2010. Enrolment in the WaveCrest I phase 2 clinical study began in 2011 and the acute results of 63 patients were presented at EuroPCR 2013. The current-generation device comes in three sizes (22, 27, and 32 mm) to cover LAA ostia between 18 and 30 mm. The anchors are rolled out after proximal positioning of the device, thereby allowing a very controlled release. CE marking was granted in 2013, and the device is now marketed in Europe.
LARIAT DEVICE (SENTREHEART) – A COMBINED ENDOCARDIAL AND EPICARDIAL (TRANSPERICARDIAL) APPROACH
The LARIAT snare device (SentreHeart, Redwood City, CA, USA) takes a different approach: after placement of a magnet-tipped guidewire endocardially and epicardially (connected by the magnet tips of both wires across the intact wall of the LAA), a “loop” is placed through the epicardial sheath via the rail established by the two guidewires and tied firmly around the neck of the LAA (Figure 3E). This procedure has been performed by a few centres in cohorts of >20 patients, and procedural safety was favourable23,24. In a recent report of 89 patients, two cases of severe pericarditis postoperatively were reported25.
OCCLUTECH LAA OCCLUSION DEVICE
The Occlutech® Figulla® Flex II ASD device (Occlutech, Helsingborg, Sweden) (Figure 3F) consists of a self-expanding flexible nitinol meshwork and a patch to close the LAA orifice. Instead of hooks to anchor the device in the LAA, expandable loops at the very distal end allow for larger radial force with less depth of the device compared to the WATCHMAN. The device comes in sizes between 17 and 39 mm. There are preclinical data available for this device, and human studies are ongoing to receive CE mark in 2014.
TRANSCATHETER PATCH OCCLUSION (SIDERIS DEVICE)
This is a bioabsorbable approach to LAA occlusion (Figure 3G). The transcatheter patch is positioned in the LAA and “released” by distally activating the surgical adhesive by direct injection of alkaline solution (distal of the balloon sealing the LAA)26. This frameless, balloon-deliverable device has been used before for occlusion of heart defects. The patches are tailored from polyurethane foam and delivered using a supportive balloon26. The supportive balloon catheter is removed 45 min after surgical adhesive activation. A clinical registry of 20 patients has been reported in 2011, in which 17 patients underwent successful LAA closure. In three patients, the device was retrieved because the patch did not attach26.
LIFETECH LAMBRE DEVICE
The name “LAmbre” is derived from “an umbrella in the left atrial appendage”. LAmbre™ (Lifetech Scientific Corp., Shenzen, China) is a nitinol-based, self-expanding device consisting of two parts, i.e., a fabric-enriched cover and an umbrella connected by a short central waist (Figure 3H)27. The device is delivered by an 8-10 Fr sheath27. The preclinical experience has been reported in 2012; no clinical data have been reported so far for this LAA occlusion device.
Insights into procedural optimisation of catheter-based LAA occlusion
Independent of the particular LAA occlusion device used, many procedural steps are similar. Here we focus in particular on aspects to optimise procedural success.
TRANSSEPTAL PUNCTURE SITE
The location of the transseptal puncture is key for an optimal access to the LAA and delivery of the LAA closure device. As the LAA is mostly located in an anterior and superior position, an inferior (or mid-inferior), and mid-to-posterior transseptal puncture site frequently facilitates the access to the LAA and allows for an optimal perpendicular positioning, i.e., of the WATCHMAN or ACP device in the neck and body of the LAA (Figure 4A). A high and/or anterior transseptal puncture will often result in a suboptimal positioning of the delivery catheter in the LAA (Figure 4A). Most centres choose a high transseptal puncture for LAA occlusion only in the rare cases in which a 90° TEE picture suggests a more inferior LAA localisation.
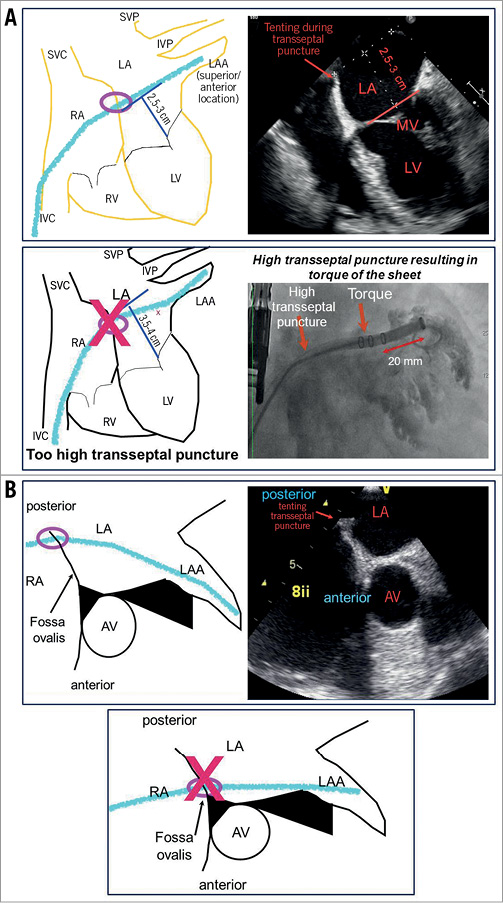
Figure 4. Optimal transseptal puncture sites for LAA closure. Medial/posterior and low transseptal puncture allows for straight intubation of the LAA with delivery catheter. Straight alignment of the delivery sheath to LAA orientation allows for easy release of LAA occluder devices. A) Too high transseptal puncture (3.5-4 cm above mitral valve plane, “clip position” for transseptal puncture) results in a torqued delivery sheath position as LAA localisation is mostly superior. Low transseptal puncture (2.5-3.5 cm above mitral valve plane) is mostly ideal for LAA occlusion. B) Anterior puncture results in torqued position of delivery sheath. Mid to posterior puncture is mostly ideal for releasing an LAA occluder.
DELIVERY SHEATH POSITIONING VIA STIFF WIRE
Following transseptal puncture, a pigtail catheter is usually positioned in the LA using a soft wire. After LAA angiography, the delivery sheath is delivered to the left atrium via a stiff wire (e.g., Amplatz Super Stiff or Extra-Stiff or Supra Core wire) that can be positioned in the superior left pulmonary vein or the left atrium. When exchange is performed with the wire in the LAA, very particular care is needed to avoid periprocedural cardiac effusion, given the thin wall of the LAA8. The use of a pigtail catheter in the delivery sheath will help to avoid cardiac tamponade during deep intubation into the LAA, as needed for example for the WATCHMAN implantation. Manipulating a pigtail catheter in the LAA was found to be safe by many operators (Figure 4B).
MEASURES TO AVOID AIR EMBOLISM
Air embolisation was a relevant problem in the beginning of catheter-based LAA closure; however, this has been markedly reduced with increasing operator experience. During several procedural steps a gentle blood aspiration is performed (“back-bleed”) and care must be taken to avoid air bubbles while loading the device into the delivery sheath. Importantly, the RA and LA pressure should be raised to above 5-10 mmHg by sufficient hydration of the patient, if possible.
IMAGING AND MEASUREMENT OF LAA SIZE
An appropriate measurement of the LAA landing zone is critical to avoid undersizing or oversizing of the device. This is frequently performed by TEE and angiographic views. Employing an RAO caudal projection corresponding to a TEE angle of 135° frequently visualises the largest ostial LAA diameter and distal LAA part; it is the standard angulation for releasing the WATCHMAN device. An RAO cranial projection (e.g., RAO 30, cranial 10-20) visualises the proximal “neck” of the LAA, and is particularly helpful for evaluation of the landing zone for AMPLATZER Cardiac Plug or Coherex WaveCrest device implantation (Figure 5A).
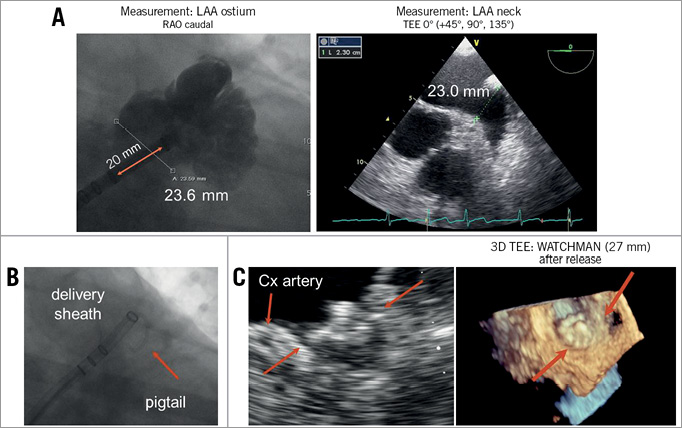
Figure 5. Step-by-step implantation of endocardial LAA closure device (e.g., WATCHMAN). After transseptal puncture the LAA is visualised by pigtail catheter. Ostium size is measured angiographically and by TEE in various projections and/or 3D TEE (A). The largest diameter is used to decide on the specific device size. Next, the pigtail catheter can be used as a rail to position the delivery sheath atraumatically into the distal part of an anterior LAA lobe (B). Now the device can be positioned and released. In the case of the WATCHMAN design, an optimal position is achieved if the device firmly covers the LAA ostium with minimal protrusion into the LA (C).
The decision on device size should be based on measurements of the LAA ostium/landing zone which is marked inferiorly by the circumflex artery; width measurement should be performed orthogonal to the delivery catheter. Generally 15-30% compression is recommended to achieve optimal results with the WATCHMAN device. To prevent acute or subacute pericardial effusion, the sheath and device are only moved backwards once the pigtail catheter serving as rail for the sheath has been removed. The positioning of the delivery catheter to the upper anterior lobe results in a good WATCHMAN position after release (Figure 5B). Release from a lower lobe often results in an inferior device protrusion. After positioning of the WATCHMAN device, four parameters are checked: 1) device location (should not be too far distal or proximal in LAA), 2) device anchoring (there should not be a significant device movement after tug test), 3) device compression (compression of 15-30% is aimed for), and 4) LAA sealing (there should not be a colour jet >5 mm next to the device). If these criteria are met, the device can be released (Figure 5C). After positioning of the ACP device, the following parameters are checked: 1) device location (at least 2/3 of lobe behind circumflex artery), 2) disc-lobe separation, 3) device compression, and 4) a gentle tug test to confirm stability of the device.
PREVENTION AND HANDLING OF COMPLICATIONS
As the delivery sheath is soft, heavy compression of the vein and/or iliac kinking may pose a problem for device delivery. A larger, stiffer sheath, as used for TAVI (i.e., 16 Fr Cook sheath), may be helpful to allow the 14 Fr outer diameter sheath (e.g., WATCHMAN) to be delivered to the right atrium. Thrombus formation at the device or sheath during the procedure may occur if the ACT falls below the recommended 200 sec. ACT should therefore be checked carefully.
Pericardial effusion may occur following LAA occlusion either immediately or delayed, within 24 hrs after the procedure. Extensive manipulation within the LAA, device recapture and repositioning, stiff wire exchange in the LAA and extensive oversizing of the device, i.e., by accidental release of the device in a small distal lobe increase the risk of pericardial effusion. Frequently, pericardiocentesis with or without reversal of heparin is sufficient to control this complication. Reversal of heparin by protamine may lead to thrombus formation in the pericardial space limiting the possibility of drainage; therefore protamine needs to be used with caution.
Follow-up and medical therapy after catheter-based LAA occlusion
The available devices were found to be at some risk (although low) for thrombus formation on the device during the 3-6 months of device endothelialisation. A follow-up imaging (e.g., TEE) is usually performed between 45 days and three months after the procedure. The PROTECT AF algorithm (45 days warfarin, dual platelet inhibition until six months post implantation, then aspirin monotherapy) was associated with a 3-5% risk of thrombus formation at the device7. The multicentre ASAP study as well as the single-centre ALSTER LAA registry analysed the event rate when patients were started on dual antiplatelet therapy (DAPT) rather than warfarin as in the PROTECT AF study. Three months of platelet inhibition was found to be equally effective in preventing thrombus formation as compared to warfarin9,18.
Dual antiplatelet therapy is usually stopped in patients after 3-6 months, depending on the follow-up findings at three months in most centres. Thereafter, patients are currently mainly treated with low-dose aspirin. Whether patients without coronary heart disease or other manifestations of arteriosclerosis should completely stop antithrombotic therapy needs to be studied in future trials.
Summary and conclusion
Accumulating data suggest that catheter-based occlusion of the LAA is an effective and safe measure for stroke prevention in patients with AF. Data obtained with the WATCHMAN device suggest that this procedure is non-inferior to oral anticoagulation with warfarin. After four years of follow-up, the device group in the PROTECT AF trial had an improved overall mortality and quality of life. In patients with an increased bleeding risk identified by a HAS-BLED score of ≥3, previous gastrointestinal or intracerebral bleeding, caution with anticoagulation has been recommended, and LAA occlusion may represent a viable alternative for these patients28-30. Periprocedural events during LAA occlusion have been reduced when performed in experienced centres. Experienced device handling is necessary to maximise the benefits from this procedure. The most experience from clinical studies is available for the WATCHMAN device. For the ACP device, first registry data are available. In addition, the Coherex WaveCrest device has now become available in Europe. There are several novel devices for LAA occlusion in early clinical development (as described above) which may expand the device “armamentarium” for this procedure in the near future.
| Impact on daily practice Atrial fibrillation is a highly frequent cause of stroke and the persistent use of anticoagulation for stroke prevention is observed only in approximately 50% of these patients. Catheter-based left atrial appendage closure (LAA) is therefore currently being intensely developed as a novel approach for stroke prevention in patients with non-valvular atrial fibrillation, in part based on the observation that >90% of thrombi are detected in the left atrial appendage in patients with atrial fibrillation. It is thought that LAA closure can be particularly considered in patients with a relevant ischaemic risk and an elevated bleeding risk. |
Conflict of interest statement
M.W. Bergmann has received speaker honoraria and grant support from Boston Scientific, St. Jude and Coherex/J&J. U. Landmesser has received consultant honoraria from St. Jude.

