Introduction
Atrial fibrillation (AF) is the most common clinically relevant cardiac arrhythmia. The estimated prevalence in the general population is 1-2% and increases with age.1-8
Patients with AF are at increased risk of thromboembolism, in particular ischaemic stroke. The risk of stroke in patients with non-valvular (essentially non-rheumatic) AF is ~5% per year.9 Moreover, strokes related to AF are associated with a higher mortality and morbidity when compared with non-AF strokes, emphasizing the need for more effective stroke prevention in these patients.10
The CHADS2 score (cardiac failure, hypertension, age, diabetes, stroke counted double) was established to assess the risk of thromboembolic events in patients with AF of non-valvular origin.11 Although there is a clear relationship between the CHADS2 score and stroke rate, the CHA2DS2-VASc score has recently been introduced and adopted by the European Society of Cardiology (ESC) as well as by American Heart Association, American College of Cardiology, and Heart Rhythm Society and other national bodies’ guidelines for AF in an attempt to improve risk stratification in the low-risk group by considering additional stroke risk factors (gender, vascular disease) in addition to old factors including cardiac failure, hypertension, age (divided to two risk classes) diabetes, and stroke that may influence a decision for anticoagulation therapy.12
Prospective and randomized studies show that oral anticoagulation (OAC) significantly reduces the risk of thromboembolism.13 However, this treatment is underutilized in patients with AF due to poor patient compliance, contraindications, and potential bleeding complications.14-18
The pathogenesis of thrombogenesis in AF is multifactorial and includes the Virchow triad of events leading to thrombus formation, i.e. endothelial or endocardial damage or dysfunction, abnormal blood stasis, and altered haemostasis, platelet function, and fibrinolysis.19
There is evidence for endothelial damage, as well as intense fibrosis and inflammation in the left atrium (LA) in patients with AF.20 These changes are especially prominent in the left atrial appendage (LAA), a particularly low flow area, and may enhance to thrombus formation by their effect on the endocardial surface.21,22
There is also strong evidence for the presence of a prothrombotic and hypercoagulable state in AF, as manifested by increased blood levels of markers reflecting coagulation activity (prothrombin fragments 1 and 2, fibrinopeptide A, thrombin-antithrombin complexes, and D dimer).23,24
The LAA is the remnant of the embryonic LA. The LAA is a tubular blind-ended structure with different lobes and variable morphology. Its complex structure with areas of relative low flow predisposes to stasis, especially during AF when blood flow velocity decreases, as can be visualized on transoesophageal echocardiography (TOE) examination with spontaneous contrast (smoke) or on pulsed-wave Doppler during paroxysms of AF.25-28 It has been shown that in patients with non-valvular AF, 90% of thrombi are located in the LAA.29 Thrombi detected in the LAA as well as a reduced LAA peak flow velocity were identified as independent predictors of an increased thromboembolic risk,30,31 and also for recurrence of stroke among non-valvular AF patients recovering from ischaemic stroke.32
Moreover, patients with certain LAA morphologies have been shown to have different levels of thromboembolic risk further supporting the role of LAA in embolization.33
Left atrial appendage occlusion or exclusion in AF34-50 is based on the concept that only ~10% of clinically relevant emboli in non-valvular AF do not originate in the LAA.51-61 The rationale is that, after excluding the LAA as an embolic source, the remaining small risk does not longer warrant OAC with its inherent risk for major bleeds. The risk of embolism from the LAA or the LA increases with age, but so does the risk of bleeding under OAC. Various surgical and catheter-based methods have been developed to exclude the LAA and the success of catheter-based methods attests to the validity of this concept.62 This document reviews the catheter-based methods and their results.
History
SURGICAL TECHNIQUES FOR LEFT ATRIAL APPENDAGE EXCLUSION
Incidental surgical LAA exclusions during heart surgery have been performed for decades.63 A first report by Madden dates back to 1949.34 The popularity of this procedure remained low as it did prolong the surgical procedure and required some specific techniques. Moreover, follow-up TOE often detected residual flow in the LAA in case of a simple suture64 and in a large number of these patients the need for lifelong OAC with a vitamin K antagonist (VKA) remained due to indications unrelated to AF, most commonly mechanical valve prostheses in the mitral position. An electrocardiographically guided thoracoscopic technique for isolated surgical LAA occlusion65 and a percutaneous endocardial/epicardial approach (Lariat, SentreHeart)46,47,50 where an epicardial sling suture is guided by a magnet inside the LAA have been introduced more recently. In addition, a number of other minimally invasive surgical and percutaneous devices including the AtriClip, Cardioablate, and Aegis, are at various stages of advanced animal studies or first in man experiments.
CATHETER-BASED LEFT ATRIAL APPENDAGE OCCLUSION
The electrophysiologist Michael Lesh conceived a device called PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for percutaneous plugging of the LAA, intrigued by the fact that during ablation of AF the LAA was easily accessible. He assisted Horst Sievert’s first such intervention on 30 August 2001.38 The PLAATO device (Medtronic) had a number of significant drawbacks and the implantation technique was fairly difficult and perilous. The device was pulled off the market although clinical results were favourable.43
On 15 June 2002, percutaneous LAA occlusion without general anaesthesia or echocardiographic guidance in awake patients was introduced by Bernhard Meier using the technically simpler Amplatzer approach41 and taking advantage of the double-disc devices routinely used for occlusion of an atrial septal defect (ASD) or a patent foramen ovale (PFO). The disc destined for the right side of the interatrial septum in ASD or PFO occlusion covered the entrance of the LAA not unlike the plate of a pacifier outside a toddler’s mouth (pacifier principle). Subsequently, the Amplatzer devices and introducer sheaths (St Jude) were adapted for LAA occlusion. The dedicated Amplatzer Cardiac Plug (ACP) and LAA sheath were introduced in 2008.
On 12 August 2002, the Watchman device (Boston Scientific) was introduced into clinical practice by Eugen Hauptmann and Eberhard Grube. It has since undergone several modifications and is approved in many countries worldwide. It remains the only device studied in randomized trials, such as PROTECT AF45 and the PREVAIL.66 In December 2013, an Food and Drug Administration (FDA) advisory committee voted favourably for approval of this device for use in the USA as an alternative to warfarin.
The WaveCrest device (Johnson and Johnson) has recently received CE mark, as well. It was developed with separately applicable fixation anchors and a different design intended to provide more superficial deployment at the entrance to LAA with little or no manipulation within the LAA body.
Since 2010, a percutaneously inserted intra-LAA patch has been used by a group around Eleftherios Sideris.49 Other devices, currently in early animal or human trials, have been developed by, Occlutech, Gore, and Lifetech.
The feasibility of the mentioned Lariat non-surgical combined endocardial/epicardial suture ligation of the LAA was first demonstrated in animals by Lee et al. in 2010,46 then in humans by Bartus et al.47 in 2011, and subsequently evaluated in clinical routine. The device has CE mark and is approved by the FDA.
Currently available devices and techniques including some surgical techniques
A variety of surgical approaches have been examined mainly in observational studies and with mixed results.37,40,42,64,67 Two alternative concepts to achieve LAA occlusion are obstruction of the LAA orifice with an occlusion device41,45,48,68 or percutaneous suture ligation using an endocardial/epicardial approach.47
Currently, three entirely catheter-based devices are commercially used for mechanical orifice obstruction, the Watchman and WaveCrest devices and the ACP. The Lariat device is used for percutaneous endocardial/epicardial suture ligation.46,47,50 They all have obtained CE mark.
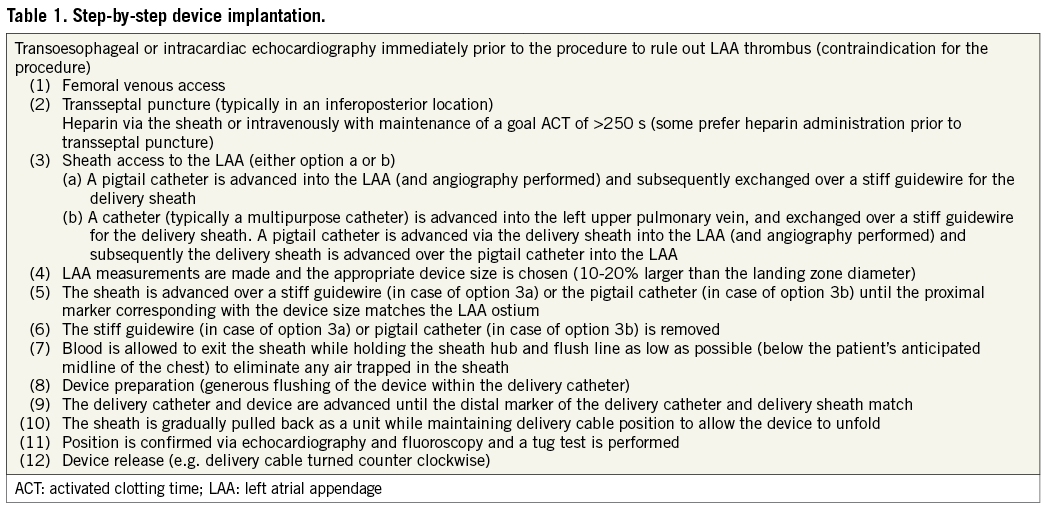
The Watchman device consists of a nitinol cage (Figure 1) with a 160 mm polyethylene terephthalate (PTFE) membrane covering the surface facing the LA. Fixation barbs are attached to the portion facing the circumference of the appendage minimizing the risk of dislodgement and embolization. It is attached to a delivery cable and delivered via a 14 French (Fr, outer diameter 4.7 mm) access sheath. A single curve or double curve configuration sheath can be used depending on the appendage orientation. After transseptal puncture (a low posterior puncture location is preferred to allow coaxial alignment with the appendage), intravenous heparin is administered maintaining an activated clotting time (ACT) >250 s and a pigtail catheter is positioned into the LAA over a soft J-tipped 0.035 inch wire. Angiography of the LA focusing on the LAA is performed in several views [right anterior oblique (RAO) caudal and cranial projections typically outline the LAA best], delineating shape and size. Sizing of the device, taking advantage of both cine angiography and TOE, is discussed under the ‘Imaging for left atrial appendage occlusion’ section. The device size is typically chosen 10-20% larger than the diameter of the landing zone (measured from the area of the left circumflex coronary artery across the LAA to ~1 cm inward from the tip of the ridge separating LAA and left upper pulmonary vein). Subsequently, an extra-stiff J tipped 0.035 inch wire is advanced into the distal LAA and the pigtail catheter and transseptal sheath are exchanged for the access sheath while maintaining wire position. Some operators introduce a catheter into the left upper pulmonary vein first instead of aiming at the LAA. In this case, after transseptal puncture, an extra-stiff 0.035 inch wire is positioned into the left upper pulmonary vein and the transseptal sheath is exchanged over the wire for the access catheter. Subsequently, a pigtail catheter is advanced through the access sheath to the LAA. The access sheath has three markers corresponding to device size and is advanced into the LAA until the marker aligns with the ostial plane of the appendage. After purging, the device is advanced via a delivery catheter to the distal end of the access sheath. Finally, the access sheath and delivery catheter are slowly withdrawn while maintaining device position, allowing it to unfold. Once deployed, appropriate position is confirmed by both angiography and TOE. A tug test is performed under fluoroscopy or TOE demonstrating simultaneous movement of the device and appendage. Optimally, the device should not protrude >4-7 mm beyond the LAA ostium (depending on device size outlined in the manufacturer’s instructions for use manual) and should cover the entire ostium with no or minimal (<5 mm by colour Doppler) residual flow and a compression grade of 8-20% (some recommend a higher compression grade of 15-30%). The compression grade is expressed in per cent comparing the diameter of the implanted device with the unconstricted diameter indicated by the manufacturer in the size label. When optimal positioning is confirmed, the device is released. If position or size appears suboptimal, the device can be retrieved and exchanged or repositioned. Table 1 lists the basic steps of LAA device implantation.

Figure 1. Watchman LAA occlusion device.
The Amplatzer ACP consists of a cylindrical nitinol cage (lobe) securing the device in the LAA body connected by a short flexible waist to a nitinol plate (disc) covering the appendage ostium. Both are laid in with polyester fabric (Figure 2). Similar to the Watchman device, the cage is surrounded by fixation hooks. The flexible waist facilitates positioning and conformation to variable and complex appendage shapes. Of note, contrary to the Watchman device, the length of the ACP is shorter than its diameter. Therefore, whereas the Watchman device cannot be implanted in appendages shorter than wide, the ACP may be an option under those circumstances. In fact, ACP implantation can be attempted in virtually all appendages. The more recently introduced Amulet generation of that device may improve ease and safety of use and further expands the size range of appendages that can fit the device.69 It also has dimensional changes that are intended to improve stability and occlusion of the LAA os by the disc. The device fits landing zones from 11 to 31 mm. Femoral venous access (sheath sizes 9-14 F inner diameter depending on the device size and type), transseptal puncture, LAA angiography, and TOE imaging as well as delivery sheath positioning are as previously outlined (Table 1). The sheath may alternatively be directly advanced into the LAA. Like the Watchman device, the ACP is retrievable prior to disconnection from the pusher cable. With the delivery sheath at least 15 mm inside the LAA, the first half of the device (lobe) is delivered by sheath retraction and the second half by pushing it out. Then, the disc is produced by further retracting the sheath while still gently pushing on the device. With optimal positioning, the lobe should be visibly compressed (tire shape) with an appreciable distance to the disc, connected by a stretchable waist. The disc should assume a slightly concave shape and cover the entire LAA ostium or at least most of it (pacifier principle). After a sustained tug test and confirmation of an optimal position, the ACP is released.

Figure 2. Amplatzer Cardiac Plug.
The WaveCrest device consists of a nitinol structure without exposed metal hub and with a foam layer facing the LAA to promote rapid organization and a PTFE layer facing the LA to reduce thrombus formation (Figure 3). It is conformable to LAA anatomy and fixation anchors are separately actionable and radially positioned to provide effective fixation at the appendage once the desired position in attained. The WaveCrest delivery sheath is designed to optionally position the occluder in the LAA ostium during deployment and anchoring. Of note, the delivery sheath is not intended for deep access and manipulation inside the appendage as the device is designed and intended for proximal placement. Should the Watchman or the ACP devices be deemed too large for very short appendages, the WaveCrest may provide an alternative. As previously described for the Watchman and ACP devices (Table 1), LA access is provided by a 12 Fr sheath in the femoral vein and a preferably posterior transseptal puncture. The measurements of the projected landing zone on TOE include the distance from the left circumflex coronary artery to 10 mm distal to the apex of the lateral ridge (coumadin ridge). Most importantly, measurements should include the widest part of the ostium, since the positioning has to be in the proximal end of the LAA mouth, allowing headroom for anchors. Measurement at 0°, 45°, 90°, and 135° are recommended to capture the long axis and short axis of the ostium and the 135° view usually shows the widest diameter. The more proximal positioning of the WaveCrest device is related to the concept that distal deployment may compress the device itself and the anchors. Over-compressed anchors may become entangled. Therefore, a position proximal to all lobes guarantees best occlusion and the risk of pericardial effusion is minimized. Before detaching the device, the sheath needs to be pulled back ~2 cm from the occluder and contrast medium is injected through the delivery system port to visualize the distal LAA. A tug on the delivery catheter is performed until movement is seen (device and tissue move as a unit). In case repositioning appears necessary, the hooks are withdrawn before moving the device. After these steps, the device is set free.
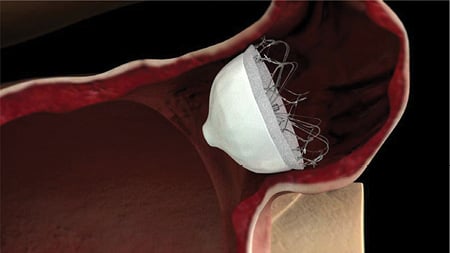
Figure 3. WaveCrest device.
The endocardial/epicardial Lariat approach to LAA occlusion leaving no foreign material in the heart is more complicated. A lasso-like suture, (snare), is positioned by a percutaneous technique epicardially at the base of the LAA and tightened followed by suture ligation. First, epicardial access is obtained similar to epicardial access for electrophysiological ablations70 and an epicardial soft tipped 14 Fr access cannula inserted into the pericardial space. Secondly, femoral venous access is established and transseptal puncture performed. Via an 8.5 Fr delivery sheath (e.g. SL 1 transseptal catheter by St Jude), a specially designed magnet tipped 0.025 inch endocardial guidewire is advanced into the LAA apex followed by a balloon mounted (compliant 20 mm balloon) catheter. The position of the endocardial guidewire is confirmed via contrast medium injection through the balloon catheter lumen. Via the percutaneous epicardial access sheath, a second 0.035 inch magnet tipped epicardial wire is advanced towards the LAA and aligned with a magnet located at the distal end of the endocardial wire already located in the LAA apex. The balloon in the LAA is inflated to help identify the appendage ostium and allow a lasso delivered via the epicardial sheath over the epicardial wire to grab the LAA ostium. Finally, the lasso is tightened. Appendage occlusion is confirmed by TOE and fluoroscopic imaging and a suture is deployed. The epicardial and endocardial delivery systems are removed. Subsequent necrosis of the strangulated LAA is likely but apparently not a problem.
Table 2 provides tips and tricks for use during implantation of intravascular LAA occluders.
Imaging for left atrial appendage occlusion
Adequate implementation of various imaging modalities is essential for developing a successful LAA occlusion programme. Imaging is important for pre-procedural and periprocedural assessment of the LAA and for follow-up. The LAA can occasionally be visualized with transthoracic echocardiography (TTE) but usually requires TOE, intracardiac echocardiography (ICE), cardiac magnetic resonance imaging (MRI), or computerized tomography (CT). Transoesophageal echocardiography is an integral part for guidance in most but not all41 LAA occlusion publications. Imaging modalities continue to evolve. The value of newer modalities should be compared against TOE, the gold standard for imaging the LAA and guiding LAA occlusion procedures.
PRE-PROCEDURAL ASSESSMENT
It is important to confirm the absence of LAA thrombi prior to LAA occlusion. The presence of mobile thrombi is a contraindication for percutaneous LAA occlusion, since dislodgement of thrombus may occur with manipulation of sheaths or devices in the LAA. Currently, TOE is considered the reference technique for the detection of thrombi in the LAA.71 In most patients, the LAA can be adequately visualized using TOE. Yet, in some patients there may be difficulties in obtaining unequivocal images, as for example, in patients with prominent pectinate muscles which may be falsely interpreted as LAA thrombus. The incidence of LAA thrombus on TOE among patients undergoing AF ablation who have been adequately anticoagulated was found to be very low and in those patients an elevated CHADS2 score was the strongest predictor of LAA thrombus.72 The prevalence of LA or LAA thrombus or sludge (dynamic gelatinous, precipitous echodensity without a discrete mass) in patients undergoing TOE examination for pulmonary vein isolation increased from 0% in patients with CHADS2 score of 0-11% in patients with CHADS2 score of 4-6.72 Of note, the prevalence of LAA thrombus may be higher in patients scheduled for LAA occlusion, since patients may not be anticoagulated because of previous bleeding complications. The diagnostic performance of a dual-enhanced cardiac CT protocol for detection of LAA thrombi was studied in patients with stroke.73-76 The overall sensitivity and specificity of CT for the detection of thrombi in the LAA were 96 and 100%, respectively.74 The role of cardiac MRI in management pathways for diagnosing LAA thrombus77 is not well enough defined and further studies are required. Ad hoc LAA occlusion using LA angiography for thrombus exclusion has been described in a small series.78
Pre-procedural TOE already hints to the device size or should reveal if the LAA appears difficult or impossible to occlude. The LAA is best imaged from the mid-oesophageal view. Using the multiplane function, the LAA is interrogated in multiple views (0°, 45°, 90°, and 135°). The morphology and presence of multiple lobes of the LAA are usually only appreciated at an angle beyond 100°. Characterization of the LAA shape and the presence of multiple lobes can be facilitated by three-dimensional (3D) TOE or pre-procedural MRI or CT.
Watchman and ACP devices require specific TOE measurements necessary for choosing the appropriate device sizes. The maximal width of the LAA ostium and depth of the LAA are first measured (Figure 4). The maximum LAA ostium width is measured from the level of the left circumflex coronary artery to a point 1-2 cm from the tip of the left superior pulmonary vein limbus (at 0°) and from the mitral annulus to a point 1-2 cm from the limbus (45°, 90°, and 135°). The ostium of the LAA usually has an oval shape. It is recommended to use the diameter of the longest axis (generally superoinferior). The depth of the LAA is measured from the ostium line to the apex of the LAA. The Watchman device can be used if the maximum LAA ostium is >17 or <31 mm, the ACP if it is <28 mm (for larger diameters, deeper placement may be an alternative or the new Amulet device may be used for landing zones up to 31 mm). For both, the Watchman and ACP devices, sizing tables are available. In general, the device size should at least be 10-20% larger than the measured diameter, although some operators may prefer up to 30%. If the depth of the LAA is smaller than the width of the ostium, placement of a Watchman device may result in unstable position with unacceptable device protrusion into the LA. For further information regarding the sizes and choice of the device, refer to the section Implantation techniques and to Table 2.
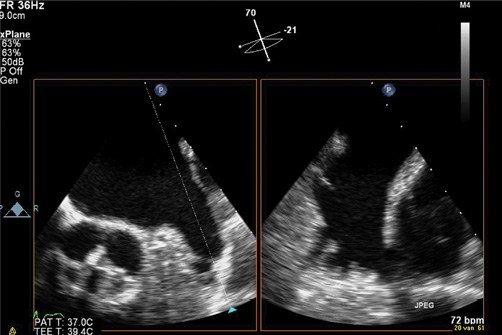
Figure 4. Three-dimensional TOE with live x-plane imaging allows simultaneous display of the LAA from two views. Measurement of LAA ostium diameter (A) and LAA depth (B) is crucial for selecting the appropriate device size.

PROCEDURAL IMAGING TO GUIDE LEFT ATRIAL APPENDAGE OCCLUSION
Real-time visualization of the LAA for device positioning and deployment is a key for successful implantation. As long as the total procedure time can be kept short, deep sedation may not be necessary. However, the majority of centres perform the procedure in general anaesthesia with 2D TOE in combination with X-ray guidance, while some operators close LAAs under fluoroscopic guidance alone to facilitate the logistics (less personnel and no sedation or intubation required).41,78,79 Personnel performing TOE during LAA occlusion should be well trained and familiar with the procedure and the required measurements for the type of device used. Limited data are available on LAA occlusion using ICE, but this may allow the procedure to be performed under local anaesthesia.80 Reports showed superior visualization of the LAA with the ICE probe positioned in the LA or pulmonary artery compared with a position in the right atrium or coronary sinus. To place the ICE probe in the LA in the absence of a PFO, a second transseptal passage is required. Left atrial appendage occlusion with ICE should only be performed by operators with experience in ICE catheter handling and interpretation of ICE images.
Only 3D TOE can provide a real-time full view of the LAA and importantly, the shape of the LAA ostium. A recent report demonstrated that 3D TOE-derived measurements of LAA orifice area were closely related with CT measurements. In this study, 2D TOE significantly underestimated LAA dimension and orifice size, as compared with 3D TOE.81 Future studies demonstrating the feasibility and accuracy of 3D TOE during LAA occlusion procedures are required.
Transoesophageal echocardiography or ICE also facilitate transseptal puncture. Most operators prefer an inferoposterior, others a mid to superior and posterior puncture. This illustrates how variable the anatomy is. A puncture site superior and anterior is usually suboptimal. Therefore, LA access via a PFO (which results in a superior and anterior path) is avoided by most operators. After positioning a sheath or pigtail catheter in the LAA, selective contrast injection under fluoroscopy in RAO caudal and cranial (10°-30°) projection gives excellent views of the LAA not overlapping the LA. Final decision on device size is based on information collected with both echocardiography and fluoroscopy. Real-time TOE provides direct information on the position of the delivery sheath in the LAA and helps during device deployment. Following successful device deployment, the pericardium is evaluated for effusion. Some experts recommend another echocardiography (TTE usually) to confirm device position and exclude pericardial effusion prior to discharge. We are not aware of any information on the yield of such an examination. Rare device embolizations upon first mobilization of the patient with change of the heart position have been observed.
FOLLOW-UP IMAGING
Transoesophageal echocardiography is the most revealing technique. Alternatively, post-procedural imaging to assess device position, peridevice residual flow in the LAA, and thrombus formation on the device consists of chest X-ray (position only) or CT. Magnetic resonance imaging is hampered by device artefacts. The timing of a follow-up TOE varies between institutions. Most operators use early or follow-up echocardiographic findings, i.e. the absence of large residual flow into the LAA or thrombus, as a guide for prescribing antithrombotic drugs. In the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial, serial TOE imaging was performed at 45 days, 6 months, and 1 year following implant.45 The logic behind the timing of the first two examinations was based on changes in medications subsequent to the examination, i.e. warfarin and clopidogrel were discontinued if TOE showed the absence of thrombus, occlusion of the LAA, or residual peridevice flow of <5 mm width (assessed by colour Doppler) at 45 days and 6 months. A sizable series with the ACP used a regimen of clopidogrel for 1 and acetylsalicylic acid (ASA) for 3 to 6 months.82 Follow-up TOE was performed at variable times between 3 and 6 months following implantation.
For patients treated with coumadin and antiaggregants according to the PROTECT AF protocol, it is prudent to follow the imaging protocol of the trial, as it serves as a guide to changes in medications. In other patients who are treated with antiaggregants only, it makes sense to image prior to cessation of clopidogrel and again if ASA cessation is planned. It is recommended to perform a TOE at 45 days to 6 months after implantation, since most adverse events including device dislodgement and thrombi were so far documented at the 45-day TOE. The value of additional TOE investigations at later time points is unclear. In case of a new embolic event, a repeat TOE is indicated as is the case if a TOE demonstrated a significant leak or a thrombus on the device (see chapter on Anticoagulation).
Residual peridevice flow is a common echocardiographic finding in patients treated with the Watchman device. There is concern that this could potentially lead to thromboembolic events, since new thrombi may form in the distal LAA pouch as a result of low flow velocities. In 41% of patients of the PROTECT AF study, this was observed during TOE at 45 days.83 This decreased to 32% at the 1-year follow-up. The majority of patients had flow jet widths of 1-3 mm. Of note, in patients with peridevice flow who discontinued warfarin, the clinical outcome was not affected. Amplatzer devices have less residual flow because the disc of the device typically covers the entire LAA ostium (pacifier principle). For further recommendations on antithrombotic treatment in patients with peridevice flow, we refer to the section on Anticoagulation.
Information is scarce about the role of imaging in Lariat device implantation and follow-up. Transoesophageal echocardiography is used to verify LAA occlusion during the procedure and for follow-up, but no clear protocol has been put forward.
Suggested standards for operators and centres and required information for registries and studies
REQUIREMENTS FOR OPERATOR AND CENTRES
TRAINING AND KNOWLEDGE OF PHYSICIANS PERFORMING THE PROCEDURE
Extensive knowledge of cardiac anatomy, particularly of the LA and LAA and the surrounding structures is required for operators who embark on LAA occlusion. Operators must be acquainted with transseptal puncture techniques and with pericardiocentesis as a prerequisite to start LAA occlusion training. Experience with other procedures performed transseptally for an interventional cardiologist or with LA ablation procedures for an electrophysiologist should be required to be sufficiently cognizant of LA anatomy and the anatomical position of LAA in relation to the surrounding structures.84-86 Operators must be aware of the several LAA anatomical variations, in terms of size, angulation, and mobility. The procedural skill is also based upon the operator’s ability to put LAA morphology in relation to the technique and the outcome of the procedure. Left atrial appendage anatomy plays a key role for the selection of the most adequate occlusion device. The success of the procedure is closely related to the level of knowledge and experience of each individual belonging to the team, including the echocardiographer supporting the procedure. The learning curve has to be expected rather flat in light of the intricacy of the procedure.
A key factor for procedural success is a structured training process before becoming an independent operator. The training process, currently provided by the device manufacturer, includes basic principles, specific device features, and the performance of the procedure. We believe that a training process for implantation of a specific device should include the following:
(1) Theoretical course, often taken on line, teaching anatomy, clinical data, and the theory of device implantation including interactive cases. This training stage should also include critical issues, such as patient and device selection, possible complications, and detection and management of major adverse events including pericardiocentesis.
(2) Practical training including bench training with handling of the equipment, and attending live cases at experienced centres, or interactive during congresses or online. The addition of hands-on simulator training on several virtual cases is helpful but should not replace attendance at real cases.
(3) During their first procedures, operators are to be properly proctored at their sites by experienced operators.
Importantly, in addition to the implanters, echocardiographers involved in patient evaluation for the procedure and echocardiographic support during procedures should be specifically trained for the respective aspects of the procedure.
CENTRE AND LABORATORY REQUIREMENTS
Since the procedure is usually performed under general anaesthesia and TOE guidance, an anaesthesiologist and an experienced echocardiographer who had specific training in supporting LAA occlusion procedures should be part of the procedural team. Nursing and technical personnel should also be familiar with every procedural step, well accustomed to interventional techniques, and prepared to manage adverse events and emergency situations. Site readiness for the procedure necessitates not only a knowledgeable operator but a thorough team understanding of the procedure and of the individual role of each member of the team. According to current practice in house cardiovascular surgery in centres performing LAA occlusion procedures is not deemed mandatory but arrangements for rapid transfer to a centre with available cardiac surgery should be available, with a maximum time of 60 min to reach the operating room.
DATA COLLECTION FOR REGISTRIES AND STUDIES
In light of the worldwide increasing number of LAA occlusion procedures, there is a need to collect extensive information regarding the number of procedures, criteria of patient selection, acute and long-term clinical outcome, and the occurrence of any type of complications.87 Initial clinical data are available from the PROTECT AF45 study and Continued Access Protocol (CAP) study analysis88,89 using the Watchman device. They are, in part, offering randomized comparisons to warfarin, whereas data about success and complications rates with the ACP device are entirely based on registries with indirect comparison to OAC.90 Due to the fact that not all patients with AF and contraindications to OAC or with serious untoward effects with OAC are suitable for LAA occlusion, there is a need for extensive data to guide patient selection. In this regard, studies should be including multiple centres with proven experience of each operator with at least 10 proctored and 10 main operator procedures. While new studies are being designed, there is also a need for large registries of LAA occlusion. To create large registries, defined inclusion criteria among the different centres (e.g. CHA2DS2-VASC and HAS-BLED scores) are the basis. This allows assessment of acute and long-term clinical outcome with respect to several issues, such as successful implantation, periprocedural or late complications, rate of neurological cardiovascular events, and safety of the approach. For the purpose of uniformity and ability to compare and group together results of different registries and studies, Table 3 lists recommended parameters to be collected in LAA occlusion registries.

Clinical results
PLAATO
Several observational human studies using the no longer available PLAATO device have been published.38,43,91-93 In all reports, antiplatelet therapy only was used after implantation. Invariably, the postprocedural stroke incidence was lower than projected by the CHADS2 score for patients treated with ASA only. According to data from three studies including a total of 359 patients (mean follow-up of 9.6 months, 9.8 months, and 24 months, respectively), the annualized stroke risks were 2.3, 2.2, and 0.7%, respectively, substantially lower than the risks predicted based on the CHADS2 score (6.6, 6.3, and 4.9%, respectively).43,92,93 At long-term follow-up (61 patients, mean follow-up of 5 years), the annual stroke or transient ischaemic attack rate (3.8%) remained lower than predicted by the CHADS2 score (6.6%).92 Including data from 364 patients (three studies) with attempted or successful PLAATO implantation, procedural mortality and the incidence of pericardial effusion requiring drainage, device embolization, and periprocedural stroke were 1, 2.5, and 0.5% respectively.43,92,93 Hence, though associated with a small risk of major periprocedural events, the PLAATO device appeared to be effective in reducing the stroke risk equally or better than OAC in patients with AF when compared with historical controls of equivalent stroke risk in a non-randomized fashion.
WATCHMAN
Table 4 summarizes current published and presented clinical experience with the Watchman device.
First, clinical experience with the device was published in 2007.44 Thereafter, high-risk patients have been enroled into trials and registries providing data from 1139 patients with over 1500 patient-years of follow-up. The results demonstrate the safety and efficacy of this device in preventing thromboembolic events compared with warfarin therapy.45,66,88,89,94-96
The Watchman device is the only LAA occlusion device that has been evaluated in prospective, controlled, randomized trials examining its efficacy and safety, e.g. in 707 patients with non-valvular AF.45 The PROTECT AF study was designed to assess the non-inferiority of the device against chronic warfarin therapy. The first publication included follow-up of 1065 patient-years,45 but data on 1500 patient-years are currently available97 of whom 87% discontinued OAC at 45 days and 94% after 2 years of follow-up. Although there was a higher rate of adverse safety events in the intervention group than in the control group, due mainly to periprocedural complications (pericardial effusion and procedural stroke typically related to air embolism), most events were without long-term sequelae. Safety events in the Watchman group occurred primarily on the day of the procedure, while the event rate was lower than that of the control group after the periprocedural period. Importantly, when follow-up was extended from 600 to 1500 patient-years, there was a 46% reduction in relative risk from 2.85 to 1.53. Longer follow-up results were recently presented with a further decrease in primary event rate and, for the first time, a survival benefit for the Watchman group when compared with the control warfarin group.96
The effect of increased operator experience is demonstrated in the CAP registry with shorter implant time, higher implant success rate, lower complication rates, and higher warfarin discontinuation rate.88
The Randomized Trial of LAA Closure vs. Warfarin for Stroke/Thromboembolic Prevention in Patients with Nonvalvular Atrial Fibrillation (PREVAIL) study66 was designed similarly to strengthen the results of the PROTECT AF trial in more patients at somewhat higher risk treated by centres with variable experience. Its preliminary results, presented but not yet published, demonstrated low early and long-term primary and safety event rates.
In the PROTECT AF study (average CHADS2 score 2.2 and CHA2-DS2-VASc score 3.4), all patients were treated with warfarin for 45 days after device implantation to facilitate device endothelialization. Warfarin was stopped if TOE examination (performed after 45 days, 6 months, and 1 year) showed either complete occlusion of the LAA or if there was residual peridevice flow of <5 mm in width. However, recent data support the safety and efficacy of LAA occlusion in patients with contraindications to even temporary anticoagulation treated with antiplatelet therapy only after device implantation.95
Peridevice flow is common after Watchman implantation. In a retrospective analysis evaluating the clinical impact of incomplete LAA sealing in patients undergoing percutaneous LAA occlusion with the Watchman device, some degree of peridevice flow was reported in 47% at 45 days and 33% at 12 months. However, the overwhelming majority of leaks were small. Peridevice flow >3 mm was seen in only 12% of patients at 12 months. Most importantly, compared to patients with complete occlusion there was no difference in thromboembolic events in those with any peridevice flow regardless of whether or not anticoagulation was continued. Hence, small amounts of residual flow do not appear to impact safety and clinical efficacy of Watchman implantation.83 Given the small number of patients with large residual leaks, however, the safety of discontinuation of anticoagulation under these circumstances remains unclear.
Gangireddy et al.89 performed an analysis of the net clinical benefit (difference between the annualized rate of serious events in the Watchman group and the rate in the warfarin group, assigning different weight to the events according to severity) of Watchman implantation in PROTECT AF and CAP. This analysis demonstrated an increased net clinical benefit with higher CHADS2 scores, especially when the Watchman device was used for secondary prevention in patients with previous events.89 The device was also effective in improving quality of life compared with warfarin therapy.97 It was cost-effective when compared with warfarin but only marginally so when compared with dabigatran.98
Two ongoing studies are evaluating the device in several hundred additional patients.
AMPLATZER CARDIAC PLUG AND OTHER AMPLATZER DEVICES
Amplatzer devices implanted into the human atria look back at more than a 20-year history in over a million patients. They have a low propensity for device thrombosis (<1%) in patients with sinus rhythm. This track record together with their ease of use led to their utilization for percutaneous LAA occlusion41 only a few months after the first percutaneous LAA occlusion procedure was performed with the PLAATO device.38 With the PLAATO device withdrawn, the Amplatzer devices have the longest clinical follow-up of currently available LAA occluders.79
NON-DEDICATED AMPLATZER DEVICES
Experience with ASD occlusion permitted the use of Amplatzer devices for LAA occlusion under fluoroscopic guidance only.41,79 However, the lack of retaining hooks and suboptimal sheath configurations resulted in a high embolization rate (6% in the aforementioned studies). Most embolized devices were retrieved percutaneously, but in some cases surgical removal (combined with LAA occlusion) was carried out. None of the available Amplatzer devices designed for occlusion of atrial or ventricular septal defects, patent ductus arteriosus, or vascular shunts proved adequate for LAA occlusion. Therefore, a specific mould was developed consisting of a hooked lobe, a thin connector, and a proximal disc. The latter is a remnant of the initial double-disc devices and comes in handy to cover the orifice of the LAA (pacifier principle). The clinical outcome in patients with technically successful Amplatzer LAA occlusion was rewarding with 0.5 events per 100 patient-years compared with the expected 5.5 events without anticoagulation or 1.8 events with anticoagulation according to the CHADS2 score.99 All patients in this series were discharged on antiplatelet therapy only. These results have to be put into perspective of a rate of 1% no device implanted and 4% device embolization. Interestingly and somewhat surprisingly, there were no device embolizations in patients with sinus rhythm at the time of implantation.
AMPLATZER CARDIAC PLUG
Since 2008, the dedicated ACP device with lobe sizes of 16-30 mm diameter (disc diameter slightly larger) and a dedicated double curve sheath with a modified pusher cable have been used almost exclusively. Initial registry data reflected the technical improvements with a reduction of the embolization rate to ~2%48,68,79,99-105 (Table 5). Pericardial effusion leading to cardiac tamponade requiring interventions occurred in ~2% as did neurological events. These figures are comparable with those obtained with the PLAATO43 or the Watchman devices.45,88 In contrast to the Watchman device, there are hardly any anatomical contraindications (with the exception of visible mobile thrombus) for an attempt at LAA occlusion with an ACP. Technical success in the first 200 registry patients was 97% and a relevant thrombus on the device during follow-up TOE was seen in ~3%. The complete occlusion rate at 6-month TOE was 99%. This is considerably higher than what was achieved with the Watchman device. The difference can be explained by the ACP disc occluding the mouth of the LAA in addition to the plug in the neck (feature shared with the Watchman device). Long-term follow-up data are lacking but design and material of the ACP are so close to that of non-dedicated Amplatzer devices that clinical outcomes can be expected to be superimposable to those mentioned in the paragraph above and perhaps even competitive to non vitamin K oral anticoagulants (NOACs).79
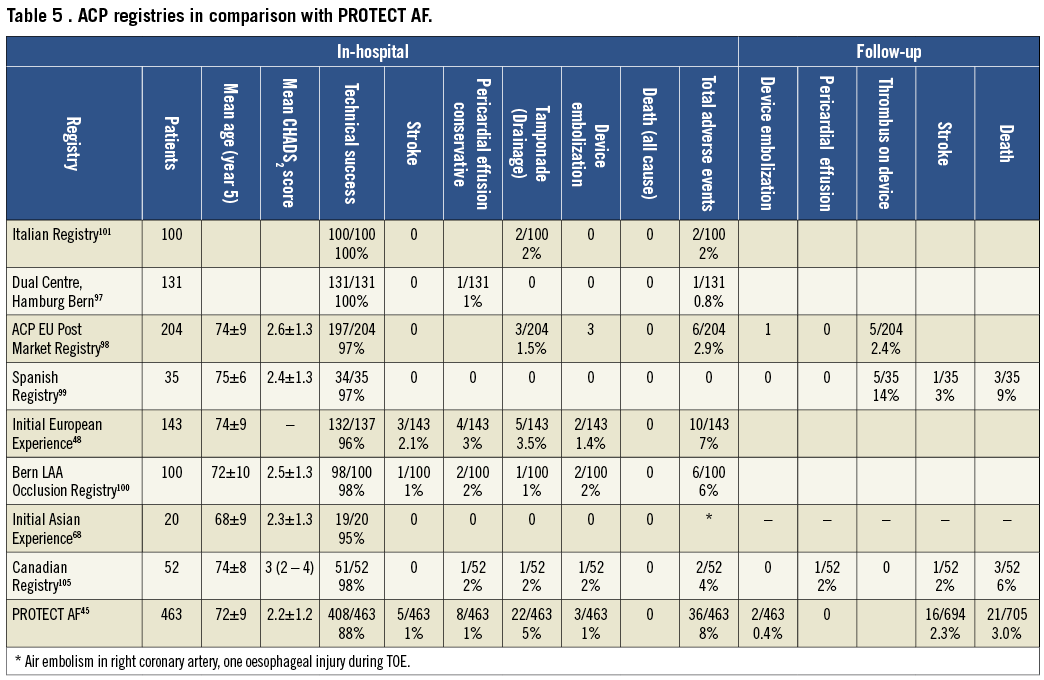
Even with the user-friendly Amplatzer technique, LAA occlusion remains challenging and the learning curve is everything but steep, much in contrast to that of occlusion of the PFO. Notwithstanding, with growing experience the results improve. Technical modifications (more and stronger hooks, deeper lobe) and the tendency to implant larger devices more deeply should lead to improved technical results. Generally ACPs are implanted without OAC thereafter. The observed thrombosis rate still leaves room for improvement so that a brief period of (N)OACs in patients without a contraindication may be part of future protocols.
LARIAT TECHNIQUE
There are but preliminary data on the use of Lariat technique. A recently published series described the results in 89 patients with 96% implant success, with 3 access related complications. Long-term follow-up revealed severe pericarditis, late stroke, and sudden death in two patients each and late pericardial effusion in one patient.106
Indications for left atrial appendage occlusion
The recommended indications for the use of LAA occluders are summarized in Figure 5. In accordance with ESC guidelines, we use the CHA2DS2-VASc risk score (>2) as the threshold value for LAA occlusion, despite the fact that some of the evidence (mainly related to the Watchman device) is based on CHADS2 scores >1.107 We believe that both these definitions may be used.
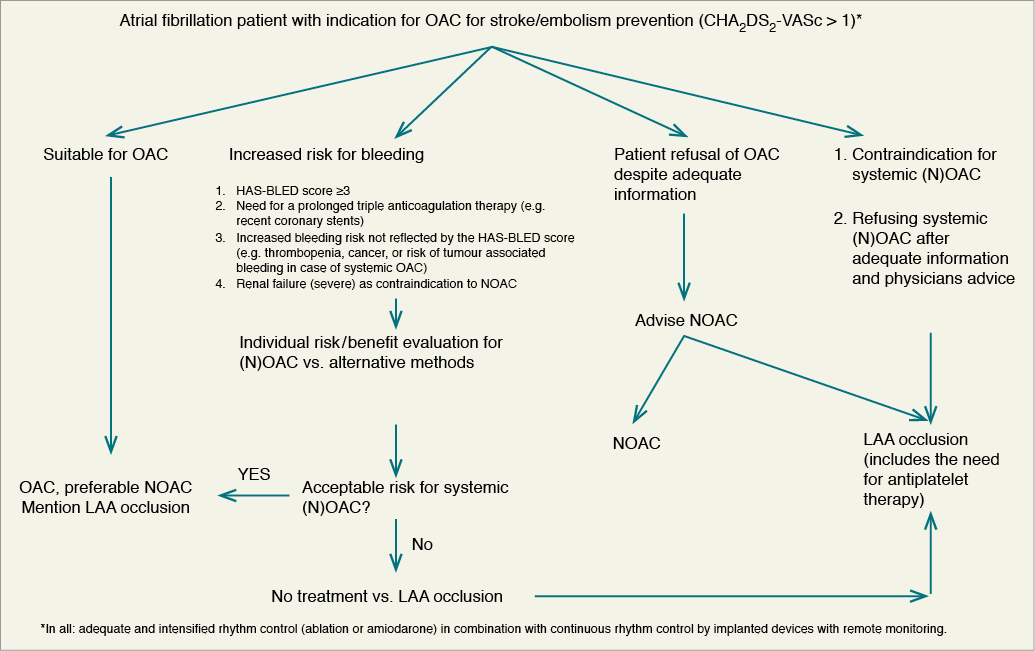
Figure 5. Algorithm of stroke protection in atrial fibrillation. LAA, left atrial appendage; NOAC, novel (non-Vitamin K antagonist) oral anticoagulant; OAC, oral anticoagulant.
AS ALTERNATIVE TO ORAL ANTICOAGULATION WHEN ORAL ANTICOAGULATION IS POSSIBLE
Although this population constitutes a small minority of LAA occlusion recipients today, this is the only indication that is currently based on randomized controlled data and was recently recommended by an FDA panel for approval. Contrary to FDA opinion and emerging report on the cost-effectiveness of LAA occlusion,98,108 the British National Health Service Commissioning Board ruled that the cost-effectiveness and clinical effectiveness of the device are not established enough yet, and therefore the device is not funded in the UK.109
When patients are eligible for OAC and do not exhibit an increased risk for bleeding, it is the consensus of the writing committee that the option of LAA occlusion should be mentioned to the patient while OAC currently remains the standard of therapy. Left atrial appendage occlusion should not be presented as superior treatment at this stage. Instead, the advantages and disadvantages of both treatments should be explained in detail emphasizing that randomized data currently are limited to two studies with a single device comparing it with a single agent (warfarin), an oral VKA.45,66 As far as devices other than Watchman are concerned, they are based exclusively on observational studies. Long-term outcome after LAA occlusion (taking into account periprocedural adverse events) was shown equivalent or according to the 4-year results of the PROTECT AF study even superior (in terms of stroke prevention and survival) to anticoagulation with warfarin. Yet, serious complications related to the procedure itself (including, but not limited to, the risk of death, stroke, and emergency surgery) occur. Finally, patients should be educated that NOACs are available that, compared with oral VKAs, has at least equivalent and probably improved efficacy. All tested NOACs compounds have a lower rate of intracranial and some also of overall risk for haemorrhage and they are free of the logistical challenges associated with surveillance of therapeutic levels. It should be emphasized that none of them has so far been compared with LAA occluder devices while there are ample data to show that they are equal or better than VKAs. Hence, they should be considered and discussed as an important preventive treatment alternative with much more supporting evidence than LAA occluders. Ultimately, the decision should be made by a well-informed patient in collaboration with the treating physician(s). It should be mentioned that for this indication the only device that has evidence-based support for its use is the Watchman device, whereas other devices were not systematically studied in a randomized controlled fashion.
AS REPLACEMENT FOR ANTICOAGULATION WHEN ANTICOAGULATION IS NOT POSSIBLE
PATIENTS WITH A CONTRAINDICATION TO ANTICOAGULATION
Patients with a high thromboembolic risk (CHA2DS2-VASc score of >2) but contraindication to oral and systemic anticoagulation (e.g. history of a significant bleeding event such as intracranial or life-threatening bleeding, the source of which cannot be eliminated) represent the most accepted clinical indication for LAA occlusion, albeit by having to extrapolate the results of the PROTECT AF study to that specific cohort.110 So far, no randomized data targeting this specific group of patients are available. Hence, our statement is based on expert consensus. This is the result of several observational studies and registries (described above in the sections dedicated to the Watchman and Amplatzer devices) suggesting that occlusion is safe and effective despite the absence of even temporary (N)OACs. It should be noted that dual antiplatelet therapy (DAT) is generally indicated for 1-6 months, not infrequently followed by lifelong single antiplatelet therapy. It needs to be mentioned that DAT generates a major bleeding risk comparable to that of warfarin.111 However, DAT exposure following LAA occluder implantation is only short time, thus reducing the cumulative risk of major bleeding events. Even when single-centre experience is reporting a favourable outcome after termination of any antiplatelet therapy, the majority of patients are exposed to a long-lasting single antiplatelet therapy after occluder implantation, again having the disadvantage of inducing a major and intracranial bleeding risks while, e.g. on ASA, similar to those with warfarin when stratified by the HAS-BLED score.112 In patients who cannot receive any antiplatelet agent, transepicardial LAA ligation, e.g. with the Lariat technique can be considered.
PATIENTS WITH AN INCREASED BLEEDING RISK UNDER SYSTEMIC ANTICOAGULATION
As depicted in the flow chart (Figure 5), we see the following three patient groups as possible candidates for LAA occlusion as the result of an individual risk benefit evaluation recognizing that the primarily recommended strategy is the use of OAC:
(1) In general, patients with an increased HAS-BLED score should be individually evaluated as to whether systemic OAC subjects them to an unacceptable bleeding risk and whether this high risk can be sufficiently reduced by the use of appropriately dosed NOACs (discussed below) shown to be associated with a lower bleeding risk than VKAs. Those in whom VKAs or NOACs are still considered to pose an unacceptable bleeding risk, but who remain at high stroke risk (CHA2DS2-VASc score of >2), should be considered for LAA occlusion. More detailed information to perform an individual risk evaluation were discussed by Friberg et al.112 and Oleson et al.113
(2) Triple anticoagulant therapy causes a significant rise in bleeding risk.114-116 Hence, in patients with the need for a prolonged period of triple anticoagulant therapy as a result of severe coronary artery disease treated with one or more stents and AF with a high thromboembolic risk (CHA2DS2-VASc score of >2) should be considered for percutaneous LAA occlusion.
(3) In clinical practice on a case-by-case basis, some patients with high bleeding risk who are not well characterized by the HAS-BLED score (e.g. patients with cancer or chronic inflammatory bowel disease) but have a high risk of bleeding with OAC may also be considered for LAA occlusion provided even NOACs be deemed to be associated with an unacceptable bleeding risk.
(4) In patients with end-stage renal failure, high stroke risk, and high bleeding risk, the implantation of an LAA occluder is a debatable alternative. In those patients, all NOACs are contraindicated at a creatinine clearance <15 mL/min. The benefit of VKA or NOACs in renal failure with creatinine clearance <15-30 mL/min is questioned due to elevated bleeding risks. The use of VKAs in patients with renal failure is controversially discussed due to an increase in tissue calcification and enhanced atherosclerosis.
Importantly, for all four above groups,we recommend the performance of an individualized risk/benefit analysis for NOACs and to consider LAA occlusion as an alternative to anticoagulation. For this analysis, it should to be taken into account that at least 1-6 months of either OAC or DAT are warranted after LAA occlusion. Thereafter, patients are typically treated with at least one antiplatelet agent. Therefore, the bleeding risk for ASA (as documented, for example, in the Apixaban versus Acetylsalicylic Acid to Prevent Strokes (AVERROES) trial)117 has to be included into the LAA occlusion strategy discussion. However, the notion that, beyond the post-procedural period, indefinite single antiplatelet therapy prevents thromboembolic events related to the device itself is not evidence-based, but merely the result of the assumption that many patients have concomitant risk factors for atherosclerosis and stroke irrespective of AF and any foreign body continues to pose some risk of thrombus formation even beyond expected incorporation into the surrounding tissue.
AS A COMPLEMENT TO ANTICOAGULATION
The combination of LAA occlusion and OAC is discussed and occasionally performed in patients with embolic events despite adequate OAC provided no other plausible cause (e.g. carotid disease, severe mobile aortic arch atheromata) can be identified. The ESC guidelines107 recommended approach is increasing the international normalized ratio (INR) target 2.5-3.5 in this situation, when it occurs while taking warfarin. Another discussed option is the switch from VKA to one of the NOACs.118-121 Adding an antiplatelet agent to OAC is performed in the clinical arena, especially when embolism occurred at elevated INRs or while taking NOACs; however, there are no data available demonstrating a positive effect on embolic events which would support this approach. Left atrial appendage occlusion could be debated as an alternative treatment in those patients, especially when AF-related embolism occurs while taking VKA with documented elevated INRs or switching to NOACs is not possible due to a NOACs contraindication like severe renal impairment.
AS ADJUNCT TO ABLATION OF ATRIAL FIBRILLATION
So far, few data on the combination of LAA occlusion and AF ablation in a single session have been published.122 Additional personal communications about limited single-centre experiences still do not allow a general recommendation. However, as long as no randomized data to support a significant reduction in thromboembolic events after successful ablation are available, in very select cases, this combination seems to be a valuable and practical approach: patients with a significant risk of thromboembolic events (CHA2DS2-VASc score of >2) undergoing an ablation procedure to treat symptomatic AF, who, in addition, have a strict or relative contraindication to (N)OACs, might be acceptable candidates. Under these circumstances, the ablation itself is associated with the risk of transseptal puncture, perhaps general anaesthesia, and anticoagulation and the incremental procedural risk of LAA occlusion is substantially lower than if performed as a standalone procedure even though the overall procedure is becoming longer. However, once again, patient preference after thorough discussion pointing out the absence of data supporting this strategy must be integral part of the decision-making process.
IN THE ERA OF NEW ANTICOAGULANTS
There are no scientific data available directly comparing LAA occlusion to NOACs. Though intracranial haemorrhage may be lower with dabigatran 150 mg orally twice daily121 or rivaroxaban 20 mg orally once daily,120 the overall incidence of major bleeding remains similar to VKAs. Therefore, in the absence of further data, it is the opinion of this consensus panel that, at the aforementioned doses, contraindications to VKAs apply equally to dabigatran and rivaroxaban, and other NOACs (Figure 5). As an exception, low-dose (110 mg orally twice daily) dabigatran or apixaban119 have been associated with lower rates of overall major bleeding as well as intracranial haemorrhage compared with VKAs while maintaining equivalent efficacy in stroke prevention.121 Therefore, consideration of low-dose dabigatran or apixaban may be reasonable in patients at increased bleeding risk provided inclusion criteria for pivotal trials examining dabigatran or apixaban are met. A decision should be made on case-by-case basis carefully evaluating potential bleeding sources and risks and recognizing limitations (among others, the unclear safety in patients with renal dysfunction and the absence of a difference in gastrointestinal haemorrhage). In addition, currently the safety of triple therapy using any of the NOACs compared with warfarin remains to be determined. In fact, the bleeding risk reduction of dabigatran in combination with clopidogrel and ASA was only minor compared with triple therapy including warfarin.121 Though there are clear advantages both in the logistics of administration and surveillance as well as the safety and efficacy with NOACs, a head-to-head comparison to LAA occlusion has not yet been done and final conclusions favouring NOACs over LAA occlusion or vice versa cannot be made.
Anticoagulation
Although LAA occlusion is originally meant as a substitute for chronic OAC among AF patients, the selective application of anticoagulants including antithrombotics and antiplatelets for various procedure and device-related indications remains essential. Since a majority of patients subjected to LAA occlusion are at high risk for bleeding, the anticoagulation regimen should be tailored individually.
BEFORE IMPLANTATION
Mobile thrombi visualized by screening TOE are considered a contraindication to catheter-based LAA occlusion. In such cases, ≥4 weeks of (N)OACs may allow thrombus resolution to be documented on repeated TOE before catheter-based LAA occlusion is attempted.
DURING IMPLANTATION
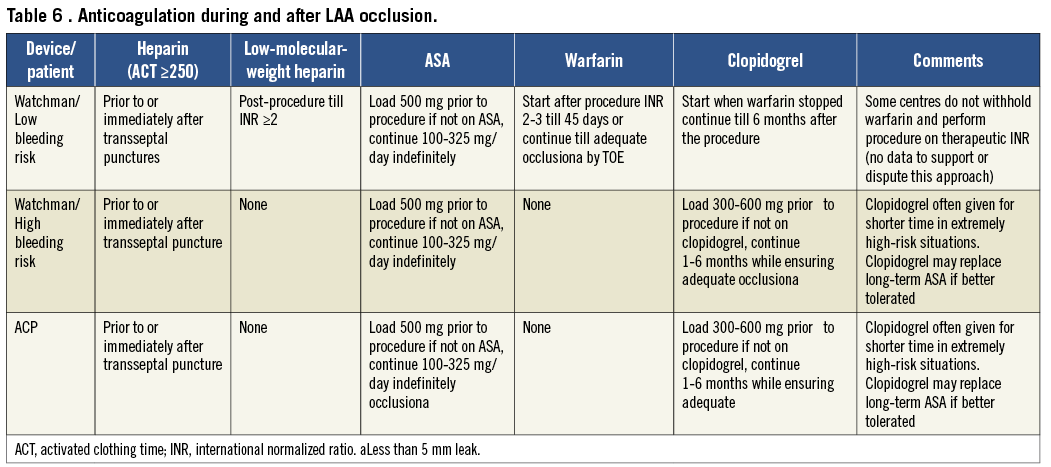
Femoral venous puncture by itself does not necessitate anticoagulant therapy withdrawal. Nevertheless, most operators aim for a normal INR at the time of the procedure and use intravenous antithrombotic agents (mostly unfractionated heparin) during the procedure (Table 6). The antithrombotic protocol of the PROTECT AF study,45 mandated an INR <2.0 at the time of procedure. Acetylsalicylic acid 81-325 mg was begun at least 1 day before the procedure and weight-adjusted heparin (70-100 IU/kg) was administered after transseptal puncture to maintain an ACT >200 s for the duration of the procedure.123 However, some operators perform the procedure while patients are on OAC with a therapeutic INR, an approach that can neither be supported nor condemned by currently available data. Intravenous antithrombotics are generally administered at the latest immediately after traversing the interatrial septum. A weight-adjusted bolus of unfractionated heparin (70-100 IU/kg) is most commonly used, which should maintain an ACT ≥250 s. Left atrial appendage occlusion may be performed as part of a combined procedure for which another antithrombotic is being used, e.g. bivalirudin. This requires no further anticoagulation.
AFTER IMPLANTATION
Post-procedural anticoagulation with warfarin is recommended for the Watchman device to avoid thrombus formation on the device until completion of endocardialization, provided there are no contraindications to anticoagulation. The anticoagulation protocol of the PROTECT AF trial (OAC for 6 weeks, DAT for 6 months, and ASA for life) was adopted in the instructions for use of the Watchman device. All patients enroled in the PROTECT AF study had to be eligible for warfarin to enable randomization to either a Watchman device occlusion or chronic warfarin therapy. However, this circumstance is rather atypical for patients considered for LAA occlusion in clinical practice. A considerable proportion of them have had a bleeding complication or have a contraindication to chronic anticoagulation (VKA or NOACs), under which circumstances operators refrain from implementing a drug regimen including an oral antithrombotic and directly prescribe DAT for at least 1 month or until a 6-month TOE follow-up, modifying the anticoagulant therapy upon its result. A satisfactory result on TOE (complete LAA occlusion or small residual shunt <5 mm jet width in the absence of device surface thrombi) justifies withdrawing at least one antiplatelet agent, unless otherwise indicated. Usually the other antiplatelet agent is continued indefinitely, as most patients are elderly with evidence of atherosclerotic disease, although the bleeding risk of ASA even as a standalone therapy must be considered. This treatment rationale of DAT was mainly derived from previous experience with the PLAATO device as well as ASD and PFO device occlusions and was recently confirmed by the results of the ASA-Plavix (ASAP) registry.95 In patients who underwent Watchman implantation in the ASAP registry and received clopidogrel for 6 months and ASA indefinitely without OAC, the ischaemic stroke rate was only 1.7% compared with 2.2% in the PROTECT AF device group. By arbitrary practice, it is usual to load ASA or clopidogrel naïve patients accordingly (Table 6).
The ACP banks on the good record regarding low thrombogenicity of the Amplatzer device family,124 and indicates in its instructions for use DAT only without an oral anticoagulant. The safety and feasibility of this drug regimen was shown in initial registry data for the ACP.48
In a recently published study using the Lariat device among 89 patients, those with a contraindication to warfarin remained off warfarin, while patients with a CHADS2 score of 2 who could tolerate warfarin but had been non-compliant or had labile INR continued warfarin. Warfarin use in patients with a CHADS2 score of 1 was left to the discretion of the referring physician. For patients not on warfarin, ASA was recommended. At 1-year follow-up, 55% of patients were still on warfarin.106
LONG-TERM
Transoesophageal echocardiography follow-up performed after 4-6 months is highly recommended to verify outcome and define the further anticoagulant regimen. In addition, in patients with clear contraindications to warfarin it is often not possible to use warfarin even for short periods of time and they undergo device implantation followed by antiplatelet therapy only. Therefore, consideration should be given to TOE follow-up at 1 month as, theoretically, this is the crucial period for device-associated thrombus formation.
IN CASE OF THROMBUS ON THE DEVICE
Device-associated thrombus was observed in 20 of 478 successfully implanted with a Watchman device (4.2%) in the PROTECT AF trial.88 Of these patients, however, only three had ischaemic strokes (thrombus-associated annualized stroke rate of 0.3/100 patient-years). The remainder were asymptomatic. The device-associated thrombus was mobile in 4 patients (3 pedunculated and 1 laminar) and non-mobile in 10. In the remaining patients, the thrombus was not further characterized. Of the three patients with a device thrombus and a stroke, one occurred in a patient with a mobile, pedunculated thrombus. For the ACP, individual cases of device-related thrombi seen on routine TOE have been described.125 For both devices, OAC for a period of weeks to months effect thrombus resolution in most cases. Therefore, anticoagulant therapy is recommended in all patients with device-associated thrombus regardless of symptoms until thrombus resolution is confirmed by follow-up TOE.
IN CASE OF INCOMPLETE OCCLUSION OF THE LEFT ATRIAL APPENDAGE
Incomplete LAA occlusion could create a thrombus containing pocket allowing emboli to enter the systemic circulation potentially causing strokes. Based on PROTECT AF data, small residual shunts with a jet diameter <5 mm are usually deemed irrelevant and may close spontaneously with time. They do not warrant further drug or device interventions. At 45 days, 14% of the PROTECT AF cohort had residual flow >5 mm, in whom warfarin was continued per protocol. At the 6-month TOE, the prevalence of large (≥5 mm) residual leaks decreased to 8%. Persistent large shunts are becoming rare with increasing operator experience and can usually be avoided by proper initial sizing and implantation techniques. Generally, when all patients with residual shunts are included into one subgroup, the stroke risk is no different compared with patients in whom the LAA is completely occluded regardless of whether or not anticoagulant therapy is continued.83 However, whether persistent large (≥5 mm) shunts deserve long-term (N)OACs or second occlusion attempts using dedicated or non-dedicated occlusion devices remains at the operator’s discretion.
Summary, conclusions, and recommendations
Left atrial appendage occlusion as a means to prevent thromboembolism in AF is based on the observation that the majority of thrombi in non-valvular AF form in this cul de sac structure. Left atrial appendage occlusion using interventional techniques has been demonstrated to be equivalent to oral VKAs in reducing thromboembolic events.
CLINICAL EVIDENCE
Current commercially available devices include the ACP and the Watchman and WaveCrest devices as well as the Lariat technique for percutaneous endocardial/epicardial ligation. All have shown efficacy and relative safety in achieving the goal of preventing thromboembolism in AF patients who do not wish or cannot receive OAC. The Watchman device has demonstrated non-inferiority and later superiority when compared with warfarin in a controlled randomized trial (PROTECT AF).45 The results seem to improve with increasing operator experience. Patients who are at high risk or cannot receive OAC have been treated successfully with LAA occlusion followed by antiplatelet therapy only in observational studies and registries with results comparable to the PROTECT AF trial.
STANDARDS OF PERFORMANCE AND DOCUMENTATION
The procedure requires training and knowledge acquired by a structured mentoring process. Moreover, institutions entering this field should have appropriate echocardiography and anaesthesia support and, preferably also surgical backup. Operators must have experience with pericardiocentesis and autotransfusion to manage pericardial haemorrhage and tamponade. Registries are required to document implantation results, complications, and follow-up.
IMAGING
Two-dimensional TOE is currently the standard imaging technique in selection of patients and follow-up assessments and, as a complement to fluoroscopy, in device sizing and selection and procedural guidance. The purpose of follow-up TOE (recommended at, e.g. 45 days, or 3-6 months) is to detect leaks and thrombi on the device. Whether other modalities such as 3D TOE or ICE, and CT or MRI will partially replace 2D TOE in the future for these purposes remains to be determined.
MEDICAL THERAPY
The procedure is performed under full anticoagulation. Standard medical treatment following Watchman device implantation includes a VKA for at least 6 weeks followed by DAT for 6 months and a single antiplatelet drug thereafter. However, recent data suggest that Watchman implantation followed by antiplatelet therapy only (in the absence of OAC) can also be performed safely in patients at high bleeding risk. Most ACPs were implanted with DAT for several weeks to months and a single antiplatelet drug or nothing thereafter. Prolonged (N)OACs are indicated in case of a device-associated thrombus or large (≥5 mm) leak.
INDICATIONS
Despite the results of the PROTECT AF trial, OAC (with VKA or NOACs) remains the standard therapy when there is no special risk or contraindication to (N)OACs. However, the option of LAA occlusion should be discussed with the patient, including risks of the procedure and limited proof of superiority. Patients who refuse (N)OACs after thorough discussion of current data including limitations may be considered for LAA occlusion. The main indication for LAA occlusion today is a relative or absolute contraindication to (N)OACs in patients with AF and a CHADS2 score of ≥1 or CHA2-DS2-VASc score ≥2. It is important to realize that this recommendation is based on observational studies and registries only. With increasing thromboembolic risk, the use of LAA occlusion becomes more attractive. To be a candidate for LAA occlusion, patients should be able to receive at least several weeks of DAT followed in most cases by lifelong single antiplatelet drug therapy. If antiplatelet therapy is not an option, percutaneous endocardial/ epicardial or minimally invasive surgical epicardial LAA occlusion may be alternatives. The use of LAA occlusion as adjunct to AF ablation and as a supplement OAC seems to be reasonable but remains to be explored.
NEED FOR FUTURE RESEARCH
Several issues regarding LAA occlusion remain to be studied. The ACP and the WaveCrest device have yet to be studied in a randomized controlled trial. No device has been adequately studied in a randomized controlled fashion in a population at high risk that cannot receive (N)OACs. None of the devices has been directly compared with N NOACs. These knowledge gaps should stimulate further trials to clarify best practice under these circumstances. An LAA occlusion registry should be established by the European Heart Rhythm Association (EHRA) and European Association of Percutaneous Coronary Interventions (EAPCI) of the ESC to document real-world results of implantation and follow-up and to complete data on indications that are not well represented in current trials.
Acknowledgements
The authors wish to thank the following colleagues for their help in reviewing scientific material and assistance in writing: Stefan Bertog MD, CardioVascular Center Frankfurt, Frankfurt, Germany; Gaetano Fassini MD, Cardiac Arrhythmia Research Center, Centro Cardiologico Monzino, IRCCS, Milan, Italy; Avishay Grupper MD, Sheba Medical Center, Tel Hashomer, Israel.
Conflict of interest statement
A.A.K.: Direct Personal payment: St Jude Medical; Payment to your Institution: St Jude Medical. B.M.: Direct Personal payment: Astra Zeneca, Bayer, Biosensors, BMS, Boehringer-Ingelheim, Lilly St Jude Medical; Payment to your Institution: Abbott Vascular, Biotronik, Boston Scientific, Cordis, Edwards Lifesciences, Guerbet, Medtronic, St Jude Medical. C.T.: Direct Personal payment: BiosenseWebster, Biotronik, Boston Scientific, Medtronic, St Jude Medical. H.S.: Direct Personal payment: none; Payment to your Institution: Access-Closure, AGA medical, Angiomed, Ardian, Arstasis, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiokinetix, Cardiomems, Coherex, Contego, CSI, CVRx, EndoCross, Endotex, Epitek, Ev3, Evalve, FlowCardia, Gardia Medical, GDS, Gore, Guidant, HLT, InSeal, Kensey Nash, Kyoto Medical, Lifetech, Lumen Biomedical, Lutonix, Maya Medical, Medinol, Medtronic, NDC, OAS, Occlutech, Osprey, Ovalis, Pathway Medical, Pendra-Care, Percardia, PFM, Recor, Rox Medical, Sadra Medical, Sentre-Heart, Sorin Group, Spectranetics, SquareOne, Trireme, Trivascular, Veryan, Viacor, Vessix. M.G.: Direct Personal payment: Boston Scientific; Payment to your Institution: Biotronik, Medtronic. T.L.: None.Y.B.: Direct Personal payment: Astra Zeneca, Boehringer-Ingelheim, Medtronic.

