Abstract
Aims: To investigate the safety, feasibility, and efficacy of left atrial appendage occlusion (LAAO) with the AMPLATZER Cardiac Plug (ACP) for stroke prevention in patients with atrial fibrillation (AF).
Methods and results: Data from consecutive patients treated in 22 centres were collected. A total of 1,047 patients were included in the study. Procedural success was 97.3%. There were 52 (4.97%) periprocedural major adverse events. Follow-up was complete in 1,001/1,019 (98.2%) of successfully implanted patients (average 13 months, total 1,349 patient-years). One-year all-cause mortality was 4.2%. No death at follow-up was reported as device-related. There were nine strokes (0.9%) and nine transient ischaemic attacks (0.9%) during follow-up. The annual rate of systemic thromboembolism was 2.3% (31/1,349 patient-years), which is a 59% risk reduction. There were 15 major bleedings (1.5%) during follow-up. The annual rate of major bleeding was 2.1% (28/1,349 patient-years), which is a 61% risk reduction. Patients with single LAAO on aspirin monotherapy or no therapy and longer follow-up had fewer cerebral and fewer bleeding events.
Conclusions: In this multicentre study, LAAO with the ACP showed high procedural success and a favourable outcome for the prevention of AF-related thromboembolism. Modification in antithrombotic therapy after LAAO may result in reduction of bleeding events.
Introduction
Left atrial appendage occlusion (LAAO) is considered a valuable alternative to oral anticoagulation (OAC) therapy for stroke prevention in patients with non-valvular atrial fibrillation (AF)1-10. Evidence supporting LAAO mainly comes from the PROTECT AF and PREVAIL trials, the only major randomised studies published to date1-4. Five-year results of PROTECT AF showed superiority of the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA) in mortality and stroke reduction compared to optimal medical treatment with warfarin4. The AMPLATZER™ Cardiac Plug (ACP; St. Jude Medical, St. Paul, MN, USA) is a self-expanding device, based on the well-established Amplatzer occluder technology and specifically designed for LAAO, which has demonstrated favourable feasibility and safety results in observational studies5-8. The aim of this study was to investigate the safety, feasibility, and efficacy of LAAO with the ACP for stroke prevention in a real-world patient population with AF.
Methods
The study included consecutive patients with non-valvular AF who underwent LAAO with the ACP in 22 centres, between December 2008 and November 2013. Prospectively collected data from each centre were transferred to a dedicated database and analysed retrospectively. Initial centre selection was based on the number of patients treated (>25 patients), the duration of follow-up (>1 year), and the availability and quality of data (>95% complete data). Subsequently, more centres with less experience (<25 patients) were included until completion of >1,000 implants. All treated patients were included in the database without roll-in subjects. The collected variables were grouped into 10 categories: demographics, baseline characteristics, indications for LAAO, CHA2DS2-VASc score, HAS-BLED score, baseline antithrombotic medication, procedure, periprocedural adverse events, clinical follow-up, and transoesophageal echocardiography (TEE) follow-up. Details regarding LAAO procedure and special features of the ACP device have been published elsewhere5.
Definitions – study design
PROCEDURAL SUCCESS
Procedural success was defined as successful implantation of the ACP in the left atrial appendage (LAA).
PERIPROCEDURAL ADVERSE EVENTS
Reporting of periprocedural adverse events (occurring during zero-seven days post procedure or before hospital discharge, whichever was latest) was based on the VARC criteria11 and included death, myocardial infarction, stroke, transient ischaemic attack (TIA), systemic embolisation, air embolisation, device embolisation, significant pericardial effusion or cardiac tamponade, and major bleeding (requiring surgery or transfusion). The definition of major adverse events (MAEs) was the following: acute (zero-seven days) occurrence of death, stroke (ischaemic or haemorrhagic), systemic embolism and procedure or device-related complications requiring major cardiovascular or endovascular intervention. This definition was used in order to be able to compare the study results with previous reports3.
CLINICAL FOLLOW-UP
Clinical follow-up included all implanted patients and was carried out by patient visits or phone contact, per centre or operator protocol. Reporting of adverse events during follow-up was based on the VARC criteria11 and included death (cardiovascular or non-cardiovascular), stroke, TIA, systemic embolism, and major bleeding. All centres provided a summary for every reported major adverse event. Antithrombotic medication was recorded at the admission date, and at last follow-up visit. The recommendation by the device manufacturer following LAAO was to prescribe acetylsalicylic acid (ASA) 80-100 mg and clopidogrel 75 mg daily for one to three months and then only ASA 80-100 mg for at least another three months. However, the choice and the duration of antithrombotic therapy were individualised depending on the patient history, indication for LAAO, and physician preference.
EFFICACY FOR PREVENTION OF STROKE, TIA, AND SYSTEMIC EMBOLISM
Device efficacy to prevent stroke, TIA, and systemic embolism was tested by comparing the actual event rate at follow-up with the predicted event rate by the CHA2DS2-VASc score12. Individual patient annual risk was recorded and the average annual risk for the whole study population was calculated. The total number of thromboembolic events (defined as stroke, TIA, and systemic embolism) during both the periprocedural and follow-up periods was divided by the total patient-years of follow-up and was multiplied by 100 in order to get the actual annual rate of thromboembolism. Thromboembolism reduction was calculated as follows: (estimated % - actual % event rate)/estimated % event rate.
IMPACT ON BLEEDING
LAAO with the ACP was accompanied by modifications in antithrombotic medication and was expected to reduce bleeding events. Bleeding reduction was assessed in analogy to stroke reduction. The total number of major bleeding events (periprocedural and at follow-up) per year was compared with the events predicted by the HAS-BLED score13: (estimated % - actual % event rate)/estimated % event rate.
SUBGROUP ANALYSES
The impact of combined interventions, antithrombotic therapy and duration of follow-up was further analysed.
TEE FOLLOW-UP
Implanted patients underwent a TEE at follow-up per centre or operator protocol. Colour Doppler was used in order to assess peri-device leaks in multiple views. Leaks were categorised according to the width of the colour jet as follows: trivial (<1 mm), mild (1-3 mm), or significant (>3 mm). Complete LAAO was defined as the absence of significant (>3 mm) leak at last TEE. All TEEs underwent careful interrogation for device thrombosis.
PRIMARY ENDPOINT
The primary endpoint was device efficacy to prevent stroke, TIA, and systemic embolism. There was no control group. Therefore, efficacy was tested by comparing the actual event rate at follow-up with the predicted event rate by the CHA2DS2-VASc score.
Statistical analysis
Continuous variables are presented as means (±SD) and categorical variables are presented as frequencies and percentages. In case of skewed distribution, variables are presented as medians (interquartile range). Continuous variables were tested by using the independent samples t-test and categorical variables by using the Fisher’s exact test. A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS 20.0 software (IBM Corp., Armonk, NY, USA).
Results
A total of 1,053 consecutive patients from 22 centres were included in the database; six patients were excluded from the analysis due to incomplete data (no adverse events occurred in these patients). Therefore, the study population consisted of 1,047 patients. Baseline patient characteristics are summarised in Table 1. The mean age was 75±8 years, and 576 patients (55%) were >75 years old. Permanent AF was present in 594 patients (57%), whereas 404 (39%) had a history of stroke/TIA. The mean CHADS2 score was 2.8±1.3 and the mean CHA2DS2-VASc score was 4.5±1.6. Based on the CHA2DS2-VASc score, the expected annual risk for thromboembolism was 5.7%. The mean HAS-BLED score was 3.1±1.2. A score of ≥3 was present in 742 patients (72%). The annual risk of major bleeding was 5.4±3.8%. Enrolment per centre varied substantially (Figure 1). The main indication for LAAO (Figure 2) was previous major bleeding (47%), followed by high risk for bleeding (35%) and coronary stenting mandating triple therapy (22%). In 16% of patients, one of the indications was a stroke on warfarin (or phenprocoumon). The composite of previous bleeding (major or minor) and high bleeding risk was 73%.
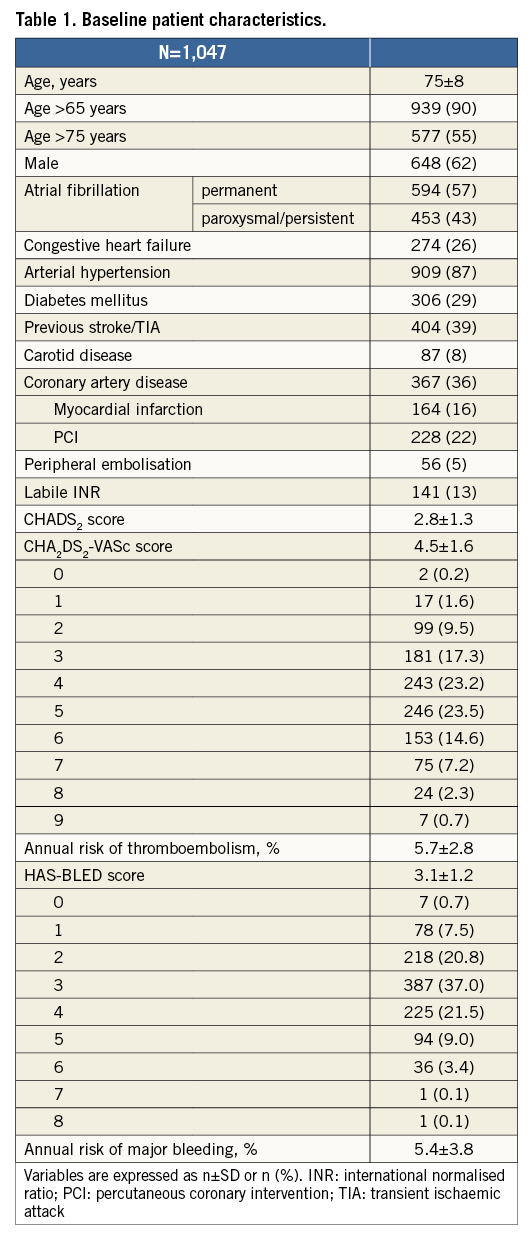
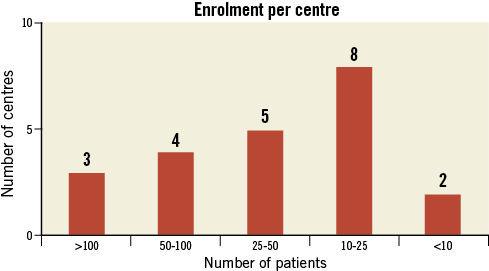
Figure 1. Distribution of participating centres according to the number of patients enrolled in the study.
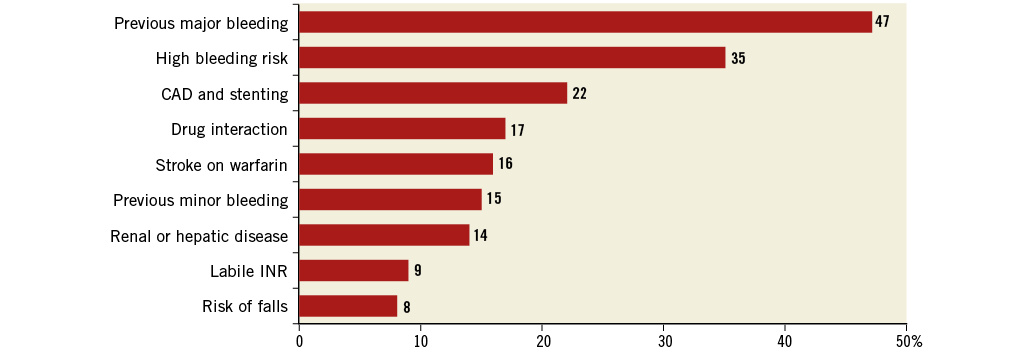
Figure 2. Indications for LAAO (for some patients >1 indication was reported). CAD: coronary artery disease; INR: international normalised ratio
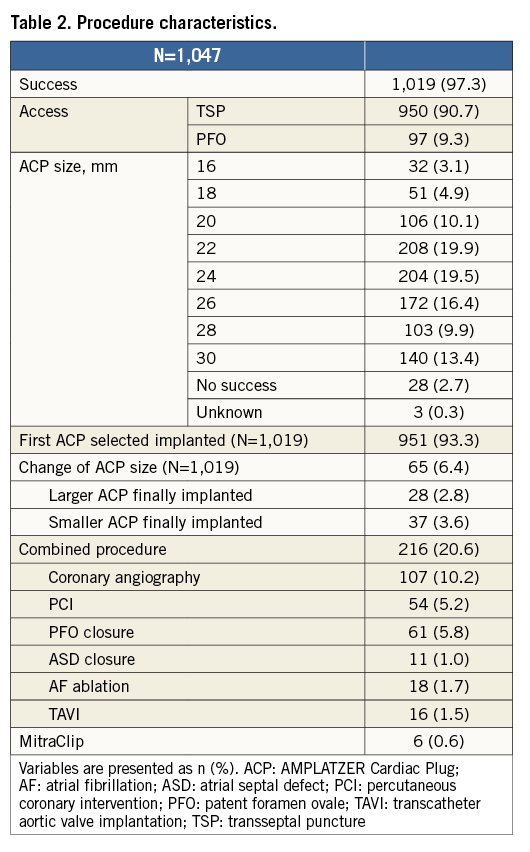
Procedure
Procedural success was achieved in 1,019/1,047 patients (97.3%) (Table 2). In 950 patients (90.7%) a transseptal puncture was used; in the remaining patients the ACP was delivered through a patent foramen ovale. The most commonly used ACP device sizes were 22 mm and 24 mm. In 951 patients (93.3%) the first ACP device tried was the final implanted device. The final implanted devices were a larger device in 28 (2.8%) and a smaller device in 37 (3.6%) patients. In 216 patients (20.6%), LAAO was combined with another procedure, most commonly coronary angiography (Table 2).
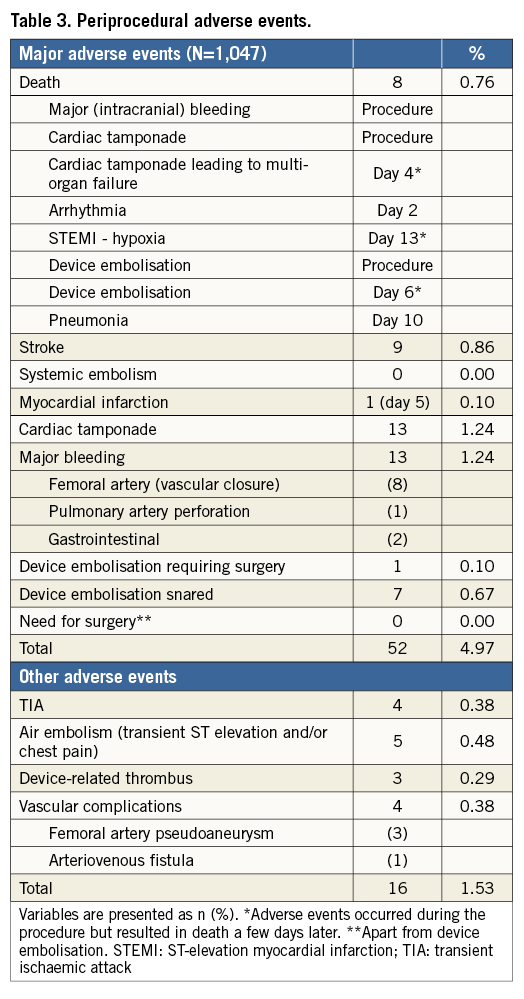
PERIPROCEDURAL ADVERSE EVENTS
A total of 52 periprocedural MAEs (4.97%) was reported (Table 3). There were eight procedure-related deaths, nine strokes, and 13 cardiac tamponades. Eight of 13 major bleeding events were related to femoral artery access, not a necessary feature of LAA closure. All air embolism events were transient, without need for treatment or sequelae. Device embolisations that were snared had no further sequelae.
Follow-up
Clinical follow-up was complete in 1,001/1,019 of successfully implanted patients (98.2%). Average follow-up was 13 months (IQ range six-25 months), resulting in a total of 1,349 patient-years. Table 4 summarises the changes in antithrombotic medication between baseline and last follow-up. ASA monotherapy increased from 31% to 64%, and warfarin (or phenprocoumon) monotherapy decreased from 16% to 1.6%. The average duration of dual antiplatelet therapy was 3.8 months. One-year all-cause mortality was 4.3% (Figure 3). A total of 63 deaths was reported at follow-up (17 due to cardiovascular causes). None was reported as being related to the device. There were nine strokes (0.9%) and nine TIAs (0.9%) at follow-up. The mean CHA2DS2-VASc score of the 1,001 successfully implanted patients with complete follow-up was 4.4±1.6 (annual risk for thromboembolism 5.6%). The annual rate of systemic thromboembolism in the study (periprocedural and follow-up) was 2.3% (31/1,349 patient-years), which translates into a 59.1% risk reduction (Figure 4). There were 15 major bleedings (1.5%) at follow-up. The mean HAS-BLED score of the 1,001 successfully implanted patients with complete follow-up was 3.1±1.2 (annual risk for bleeding 5.3%). The annual rate of major bleeding in the study (periprocedural and follow-up) was 2.1% (28/1,349 patient-years), which is a 61.0% risk reduction (Figure 4).
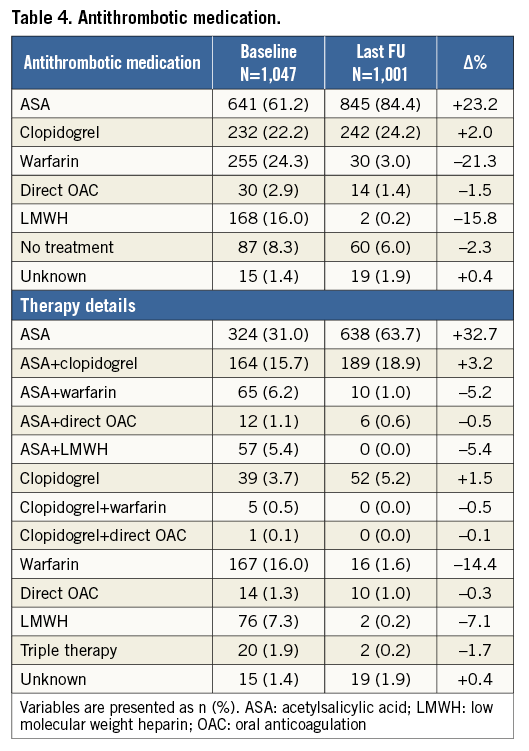
Figure 3. Kaplan-Meier cumulative survival curve of the study population.
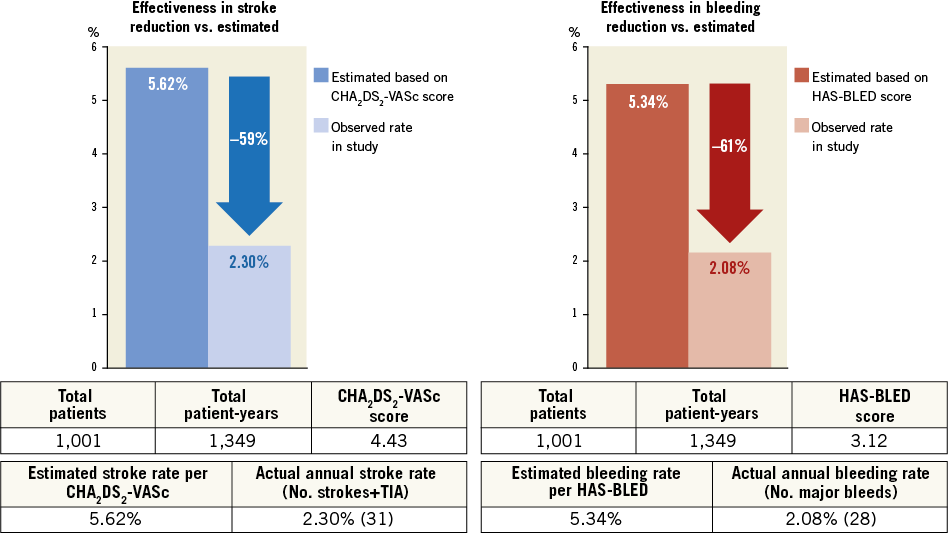
Figure 4. Effectiveness of LAAO in reduction of thromboembolism and bleeding based on annual rate predicted by CHA2DS2-VASc score and HAS-BLED score, respectively. Both periprocedural and follow-up events are included in the analysis.
TEE FOLLOW-UP
A total of 632/1,001 of successfully implanted patients with complete clinical follow-up (63%) had a TEE at seven (IQR 3-11) months after the index procedure. A peri-device leak was found in 73 patients (11.6%). The leak was trivial, mild, and significant in 27 (4.3%), 34 (5.4%), and 12 (1.9%) patients, respectively. Therefore, complete closure was achieved in 620/632 patients (98.1%). Finding of a major leak was not followed by reintroduction of OAC therapy, with the exception of one patient. A thrombus related to the device was observed in 28/632 patients (4.4%) and was treated according to the centre preference (brief period of OAC or LMWH till thrombus resolved, DAPT for life or no treatment due to very high bleeding risk). The size, shape, or mobility of thrombus is the subject of further analysis. Peri-device leaks and device-related thrombus did not correlate with any adverse event at follow-up, i.e., no strokes or TIAs occurred in patients with thrombus or incomplete closure at follow-up.
SUBGROUP ANALYSES
Patients who received ASA monotherapy or no treatment at last follow-up after a successful lone LAAO procedure (Group 1, n=627) were compared to the other patients (Group 2, n=374) (Table 5). Baseline characteristics were similar between groups, with the exception of history of coronary artery disease and previous stenting which was more frequent in Group 2. Although the stroke risk was similar, there were more periprocedural strokes in Group 2, so the overall stroke risk reduction was higher for Group 1 (74.6% vs. 23.6%, p<0.05). With respect to major bleeding, periprocedural events were similar, but Group 2 had more bleedings at follow-up despite the relatively lower HAS-BLED score. Therefore, major bleeding reduction was significantly higher for Group 1 (76.5% vs. 16.5%, p<0.001).
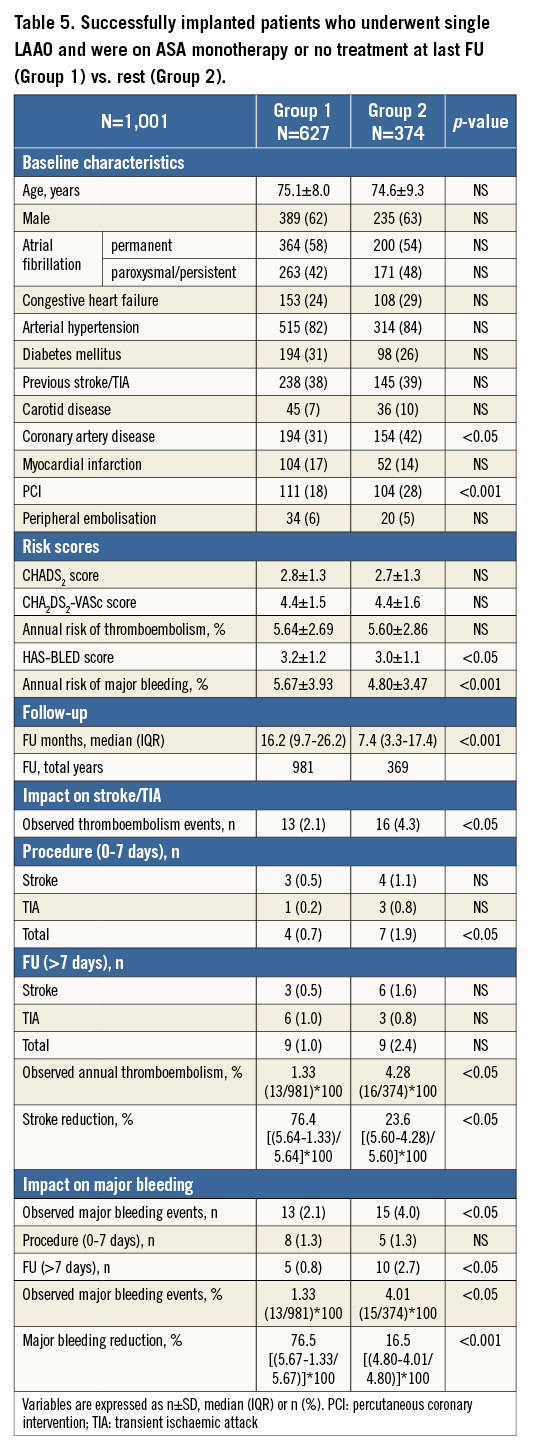
Group 1 was further analysed with respect to the duration of follow-up. Patients with ≥1-year follow-up (Group A, n=432) were compared to patients with <1-year follow-up (Group B, n=195). Baseline characteristics, stroke and bleeding risk were similar (Table 6). The frequency of cerebral events was also similar but the events/year ratio was lower for patients with ≥1-year follow-up (Figure 5). Consequently, the overall stroke risk reduction was higher for these patients. With respect to major bleeding, there was no difference in the periprocedural events, but patients with <1-year follow-up had more events during follow-up. Therefore, for this subgroup of patients (who were typically on dual antiplatelet therapy) there was no bleeding risk reduction.
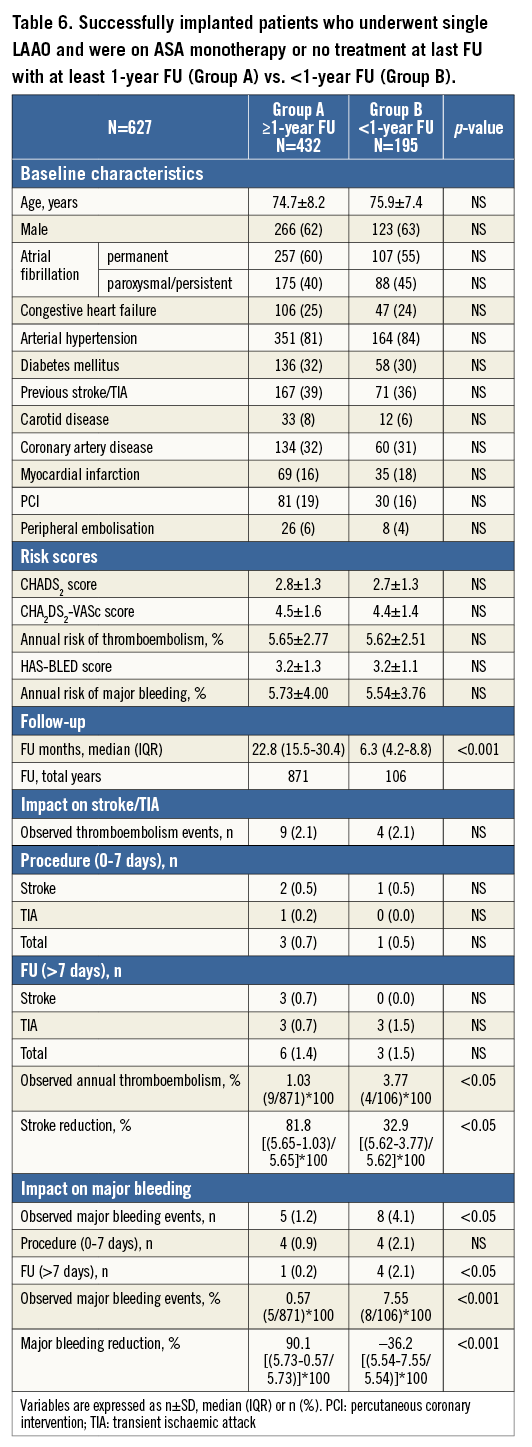
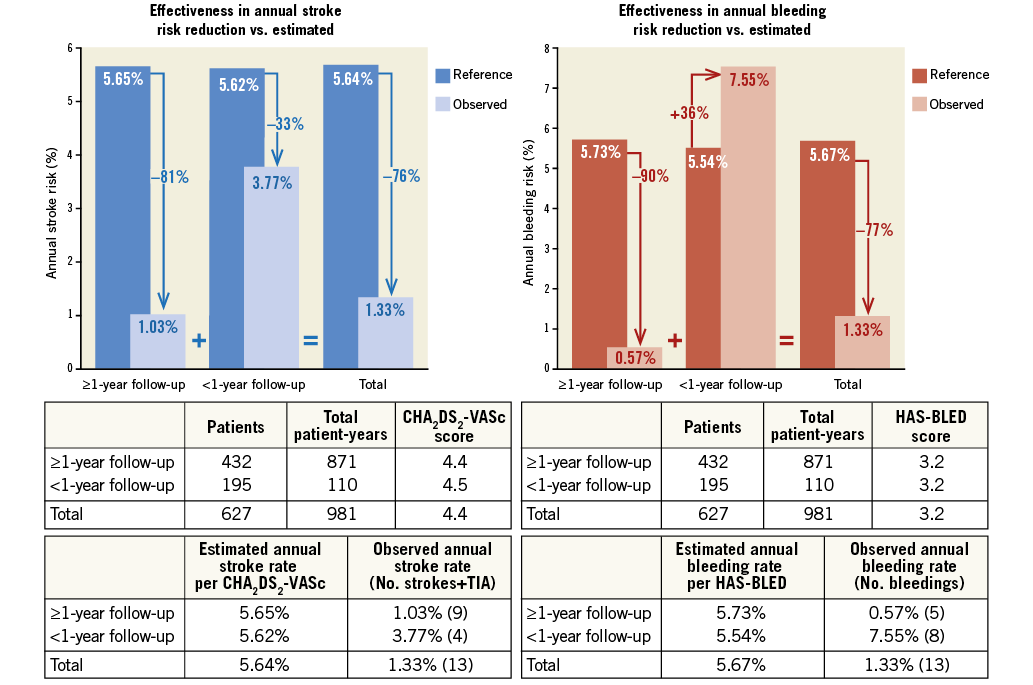
Figure 5. Subgroup analysis of patients who underwent single LAAO and were on ASA monotherapy or no therapy at last follow-up. Patients were divided into two groups based on the duration of follow-up. Both periprocedural and follow-up events are included in the analysis. ASA: acetylsalicylic acid
Discussion
The main finding of this large, multicentre, all-comers study was that LAAO with the ACP showed a favourable outcome for the prevention of AF-related thromboembolism, with a high procedural success rate and a moderate number of periprocedural complications. Moreover, this study showed that reduction of antithrombotic therapy after LAAO resulted in fewer than expected bleeding events.
The ACP device has a proximal disc and a distal lobe, which has a crown of six pairs of stabilising wires5. The ACP lobe is deployed in the LAA “neck” in a depth 10-15 mm from the LAA ostium and has two roles: to stabilise the device and to occlude the LAA like a plug. The ACP disc acts like a second sealing layer as it is pulled by the ACP lobe and waist against the LAA ostium, occluding the LAA from the atrial side in a way termed the “pacifier principle”13. This dual layer occlusion may explain the relatively high rate of complete LAAO reported in this study despite a more strict definition used (cut-off of 3 mm instead of 5 mm leak for complete LAAO used in other studies)1,5.
The clinical implications of incomplete LAAO are currently unknown, especially in patients who are not taking OACs or platelet aggregation inhibitors14-16. This study confirms the results of previous reports which considered peri-device leaks benign and clinically irrelevant; however, it is too early to draw any definite conclusions14. Peri-device leaks and device-related thrombus are considered potentially harmful. In this study, in keeping with previous reports, there was no association between device thrombosis and stroke. Again, the event rate is too small to conduct a robust analysis with this follow-up duration.
In this study, there was variation in experience of participating centres, ranging from <10 to >100 cases per centre. In addition, most of the centres included patients treated at the beginning of their LAAO programmes. In large-volume centres, the learning curves of several different implanters have to be accounted for. Therefore, this study reflects the results of LAAO performed by physicians with different levels of experience and includes every case. In view of this, and taking into account that LAAO is quite a demanding procedure, the overall success rate is remarkable. One possible reason may be the ACP design, which allows proximal LAAO avoiding the challenging anatomic variations of LAA distal parts (i.e., multiple lobes, bends, etc.).
Nevertheless, similar to other studies, complications did occur - most commonly cardiac tamponade, major bleeding, and stroke. Cardiac tamponade can occur during transseptal puncture, when exchanging wires and sheaths or after device deployment. Out of ten device embolisations, two resulted in death (despite surgery), one required surgery, and the remaining seven were snared without sequelae (in 3/7 another ACP was successfully implanted). A total of six additional deaths was reported. Two of these were not related to LAAO; they were included in the report because they occurred in the periprocedural period. It should be underlined that the study population comprised old patients with many comorbidities, and that in some cases a combined procedure was carried out.
The indication for LAAO in this all-comers study varied substantially. The main indication was previous bleeding on OAC or high bleeding risk (73% of patients in total). However, a significant number of patients underwent LAAO in order to avoid triple anticoagulation after PCI, whereas some patients were treated for secondary prevention due to a previous stroke on warfarin. Despite differences in indications, the study population had a relatively higher stroke risk in comparison to previous reports with the WATCHMAN device (CHADS2 score 2.8 versus 2.2, 2.5, and 2.6 in PROTECT AF, CAP Registry, and PREVAIL, respectively)1-3. Age, one of the main risk factors for stroke was similar (75±8 versus 72±9, 74±8, and 74±7 years). However, in this cohort the frequency of a previous stroke was 39% whereas in the aforementioned studies it ranged from 18 to 30%.
In this study, the impact of LAAO in stroke and bleeding reduction may have been positively or negatively affected by combined interventions and/or DAPT, especially in the initial one to three months of follow-up. The results of the subgroup analyses showed that periprocedural strokes/TIAs were more frequent in patients who underwent a combined intervention with LAAO and were taking any antithrombotic medication other than aspirin (i.e., DAPT, warfarin, etc.) at last follow-up. Therefore, in this “purified” cohort, single LAAO with the ACP performed even better. In the second subgroup analysis, patients with ≥1-year follow-up were tested separately in order to ameliorate the potentially positive (fewer strokes) or negative (more bleedings) impact of more intense antithrombotic treatment that is typically prescribed post procedure. The two groups had a similar frequency of strokes but during a different follow-up period (median 22.8 vs. 6.3 months), so the annual stroke rate was lower and the relative stroke reduction was higher for patients with ≥1-year follow-up. In other words, the benefit of LAAO becomes more apparent with time. With respect to major bleeding, differences between subgroups were even greater. As expected, patients who underwent combined procedures and were under more intense antithrombotic therapy had more major bleeding events, in particular during follow-up. In fact, the analysis demonstrated more benefit for patients with longer follow-up. Therefore, this study showed that bleedings after single LAAO with the ACP should be expected during the initial months after the procedure but thereafter the risk of bleeding decreases significantly.
In the latest update of the AF guidelines, OAC is considered mandatory in all patients with ≥1 risk factor (with the exception of female gender as single risk factor)8. Even in the era of novel OACs, physicians’ (and patients’) adherence to the guidelines is very disappointing17,18. High bleeding risk and/or previous bleeding are the main reasons for not using OAC, especially in the elderly. However, it is also known that the risk for stroke increases with age. This study shows that LAAO results in a stroke reduction rate similar to OAC studies19. At the same time, bleeding events decrease significantly due to less aggressive antithrombotic therapy strategies post LAAO. Of course, any projection of these results in the novel OAC era would be speculative since the use of direct OAC is relatively novel. Nevertheless, LAAO may become a valid alternative to OAC in the future. Specially designed studies should address this matter. Moreover, a number of unexplored topics, such as the role of LAAO in special patient populations (i.e., renal impairment), the clinical impact of peri-device leaks and device thrombosis, and the ideal antithrombotic treatment after LAAO, need further investigation. Finally, it should be underlined that well-designed registries and further randomised studies are mandatory in order for LAAO to be offered to the right patients, meeting the requirements of evidence-based clinical practice.
Limitations
This is a non-randomised, retrospective, observational study, which included many large-volume centres. There was no control group and the use of CHA2DS2-VASc and HAS-BLED scores for comparisons (although validated) is methodologically imperfect. TEE follow-up was not available for all patients. The clinical and TEE results were self-reported and there was no independent adjudication. However, all the important events were discussed within the study group members and a written summary was provided.
Conclusions
In this multicentre all-comers study, LAAO with the ACP had a high procedural success and a moderate number of periprocedural complications. LAAO with the ACP showed a favourable outcome for the prevention of AF-related thromboembolism. Modification in antithrombotic therapy after LAAO may result in a reduction in bleeding events.
Impact on daily practice
The AMPLATZER Cardiac Plug (ACP) is one of the most commonly used occluders for LAAO. However, few data about this device have been published in the literature. This article presents the results of the largest clinical study to date using the ACP from 22 centres with different levels of experience. In this study, LAAO with the ACP had a procedural success rate of 97.1% and a 4.97% rate of major periprocedural adverse events. The study showed a favourable outcome for the prevention of AF-related thromboembolism with an annual reduction of 59% as compared to the rate predicted by the CHA2DS2-VASc score, and a 61% annual reduction in major bleeding events as compared to the rate predicted by the HAS-BLED score. These favourable results need to be confirmed in a randomised clinical study.
Appendix. Collaborators
Werner Budts, MD; University Hospital of Leuven, Leuven, Belgium; Tom Depotter, MD; Cardiovascular Center Aalst, Aalst, Belgium; Eduard Benit, MD; Jessaziekenhuis, Hasselt, Belgium; Francis Stammen, MD; AZ Delta Hospital, Roeselare, Belgium; Steffen Gloekler, MD; University Hospital of Bern, Bern, Switzerland; Roger Eckhardt, MD; CardioVascular Center Frankfurt, Frankfurt, Germany; Michael Koa-Wing, MD; Imperial College Healthcare NHS Trust, London, United Kingdom; Riccardo Proietti, MD; Ospedale Luigi Sacco, Milan, Italy; Milosz Jaguszewski, MD; Universitätsmedizin Göttingen, Göttingen, Germany; Luis Paiva, MD; Coimbra University Hospital Centre, Coimbra, Portugal.
Acknowledgements
The authors would like to thank Mark Hünlich for his contribution in data collection and analysis from Göttingen.
Funding
There was no funding for this study with the exception of an unrestricted writing honorarium provided by St. Jude Medical to A. Tzikas.
Conflict of interest statement
A. Tzikas, H. Sievert, P. Kanagaratnam, W. Schillinger, B. Meier, and J-W. Park are consultants, proctors, and have received research grants from St. Jude Medical. H. Omran, S. Berti, G. Santoro, J. Kefer, J. Nielsen-Kudsk, U. Landmesser, I. Cruz-Gonzalez, F. Nietlispach, A. Aminian, X. Freixa, P. Danna, M. Costa, R. Ibrahim, and W. Budts are consultants and proctors for St. Jude Medical. The other authors have no conflicts of interest to declare.

