
Abstract
Aims: Percutaneous left atrial appendage occlusion (LAAO) may be considered for stroke prophylaxis in patients with non-valvular atrial fibrillation (NVAF). Data on device implantation safety and feasibility and long-term follow-up are limited.
Methods and results: LAAO was performed using the AMPLATZER Cardiac Plug (ACP) device in 134 NVAF patients with long-term OAC contraindication, with median (interquartile range) CHA2DS2-VASc and HAS-BLED scores of four (3-5) and three (2-3.75), respectively. Follow-up data were collected over a mean follow-up period of 680 days (range: 42 days to 4.3 years) comprising a total implant experience of 238 patient-years. Device implantation was successful in 95.5% of the procedures and associated with a rate of major procedural complications of 2.2%. At the most recent follow-up, almost all patients were receiving antiplatelet therapy. Ischaemic stroke was observed at an annual rate of 0.8% and the annual rate of any thromboembolic (TE) event was 2.5%. Major bleeding during follow-up occurred at an annual rate of 1.3%.
Conclusions: LAAO is a safe and effective stroke prevention therapy in a high-risk NVAF cohort, both at implantation and over longer follow-up periods. The long-term assessed ischaemic stroke rate in patients treated with LAAO is markedly reduced compared to the expected rate based on the patients’ risk scores.
Introduction
Oral anticoagulation therapy (OAC) with warfarin1 or novel anticoagulants2-4 is the current standard of care for stroke prevention in patients with non-valvular atrial fibrillation (NVAF). Nevertheless, patients with contraindications to OAC, a high bleeding risk, or embolism despite OAC may be considered for percutaneous left atrial appendage occlusion (LAAO)5. This device-based therapy is aimed at circulatory exclusion of the left atrial appendage (LAA), the major source of cardiac thromboembolism (TE) in NVAF patients6.
In a randomised trial, LAAO has been demonstrated to provide non-inferior stroke prevention as compared to warfarin7. In addition, a number of non-randomised studies have reported a stroke rate reduction associated with LAAO, compared to the expected stroke rate, based on the patients’ risk score8-11. However, data regarding longer-term follow-up of patients treated with LAAO are limited with respect to the number of patients and the follow-up duration. We report on the cumulative experience from two Italian centres obtained from a relatively large cohort treated with LAAO using the ACP device and followed for up to four years.
Methods
Between January 2009 and December 2012, all consecutive patients with paroxysmal, persistent or permanent NVAF at high risk for ischaemic stroke (CHADS2 or CHA2DS2-VASc ≥1) and not suitable for long-term OAC were considered for LAAO. The institutional review board of both centres approved this retrospective study.
Patients were implanted with the AMPLATZER Cardiac Plug device (St. Jude Medical, St. Paul, MN, USA). This device, specifically designed for percutaneous LAAO, consists of a lobe and a disc, constructed from a nitinol mesh and polyester patch, connected by a flexible waist. Percutaneous implantation is facilitated by a dedicated delivery system. The lobe is implanted within the LAA and has stabilising wires to retain its position. The disc seals the orifice of the LAA. The appropriate device size is selected from eight different lobe diameters available, based on the internal LAA diameter. Depending on the lobe diameter, the disc diameter is 4 mm or 6 mm larger than the lobe.
Prior to the device implantation, imaging investigation was performed to exclude left atrial (LA) and LAA thrombus, to explore the relevant anatomy and to determine the appropriate device size. Imaging modalities used for these examinations included pre-procedural transoesophageal echocardiography (TEE) and/or cardiac computed tomography (CCT). Intracardiac echocardiography (ICE) and/or TEE and angiography were used for the LAAO procedure guidance. Whenever possible, devices were implanted using local anaesthesia only, under ICE and/or angiographic guidance12. Profound sedation with spontaneous breathing was applied in combination with TEE, while implantation under general anaesthesia was considered only as a last option. The device was implanted applying standard right heart catheterisation techniques and transseptal left atrial access, using the dedicated delivery system. Implantation techniques and the use of procedural imaging are described elsewhere13. Subsequently, the device was released from its delivery system. If indicated, the device was repositioned or exchanged for a differently sized device. Eventually, LAA sealing was evaluated using angiography and/or echocardiography. Transthoracic echocardiography and chest X-ray were performed within 24 hours from the procedure. All the patients were hospitalised for at least 24 hours after the procedure.
Technical success was defined as the successful deployment and implantation of the device within the LAA. Procedural success was defined as technical success and implantation without major procedure-related complications, including major pericardial effusion/tamponade, stroke, systemic embolism with end-organ damage, major bleeding (except tamponade/effusion) and device embolisation. Pericardial effusion/tamponade and bleeding were considered significant if they required drainage, transfusion with ≥2 units of packed red blood cells or surgical intervention.
Follow-up was performed by clinical visits or telephone follow-up at one, six and 12 months and yearly thereafter with particular attention to mortality, thromboembolic events, bleeding events, current antithrombotic medication and repeated hospitalisations. In case of an event, hospital chart reviews were performed for a more detailed assessment. Post-procedural antithrombotic therapy was tailored to the patient’s individual risk profile. As a rule, short-term dual antiplatelet therapy (one to three months) and subsequent indefinite single antiplatelet therapy were prescribed after successful device implantation. At six months post implantation, cardiac imaging was performed using TEE and/or CCT to confirm stable device position, to exclude device-related thrombus and to assess residual leaks. A leak was classified as minor, moderate or major when the jet, observed by TEE, was <1 mm, between 1 and 3 mm, or >3 mm, respectively.
Continuous data are expressed as mean±standard deviation or median (25th to 75th percentiles) depending on distribution of the data. Technical and procedural success rates were calculated as percentages of the total number of patients. The procedural complication rate was determined as a percentage of the number of attempted implantations. For proportions, numbers and percentages were used. Comparisons between observed and expected rates of thromboembolic and bleeding events were assessed using binomial tests. Statistical analysis was performed using SPSS Version 19 (IBM Corp., Armonk, NY, USA).
Results
PATIENTS
A total of 134 consecutive patients were included in the study. Mean age was 76.6±7.6 years and 51 patients were aged 80 years or older. Additional clinical characteristics are provided in Table 1.
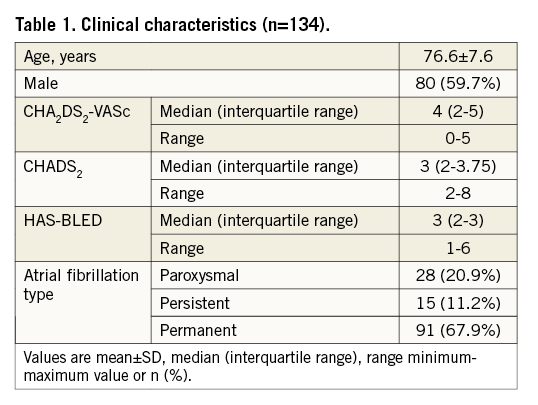
The median (interquartile range) CHA2DS2-VASc score was four (3-5) and the median HAS-BLED score was three (2-3.75) (Figure 1), thus representing a high-risk cohort with respect to stroke and bleeding. Ninety-two patients (68.7%) had both a CHA2DS2-VASc score and a HAS-BLED score of three or more. The most common reasons to consider LAAO as a stroke prophylaxis included major and minor bleedings, 40% and 25% respectively, occurring in 11% of the patients while on OAC, with 56% of the major bleeds being due to intracranial bleeding. Other reasons for LAAO were thromboembolic events while on Coumadin in 7% of the patients, and labile INRs and the need for triple antithrombotic therapy in combination with drug-eluting stents in 6% and 7%, respectively. In the remaining 15% of the patients the indication was a miscellany of perceived high bleeding risk as assessed by the HAS-BLED score, risk of falls and poor medication compliance. Approximately one quarter of the patients presented with renal insufficiency, which potentially complicates OAC management. At the time of admission for the LAAO procedure, 16% of the patients were on warfarin and none was treated with novel anticoagulants. Aspirin, clopidogrel, and low molecular weight heparin was used by 40%, 17% and 37% of the patients, respectively (6% were on dual antiplatelet therapy). Thirty-six of the patients had both CCT and TEE prior to implantation, seven patients had only CCT, and the rest of the patients (n=91) had TEE imaging prior to the procedure.
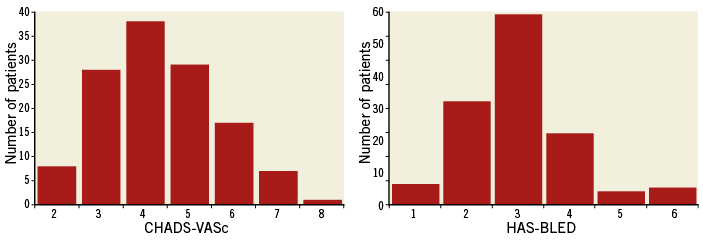
Figure 1. Distribution of CHA2DS2-VASc and HAS-BLED scores. Median (interquartile range) CHA2DS2-VASc score was 4.0 (3-5) and median (interquartile range) HAS-BLED score was 3.0 (2-3.75).
DEVICE IMPLANTATION
Device implantation was attempted in 133 patients. In one patient, implantation was not attempted because of an unsuitable LAA anatomy, suggested by pre-procedural TEE and confirmed during the procedure by angiography. This patient had two very proximal LAA lobes: one was small and very close to the ostium and the other was very wide and acutely bent (“chicken wing” morphology). The landing zone of the appendage was wider than the manufacturer’s recommended dimensions (landing zone, including the small proximal lobe, measuring 36-40 mm) and shallow. We therefore decided not to proceed with the implantation. Successful device implantation was achieved in 128 patients, resulting in a technical success rate of 95.5%. Failure to implant was consistently related to an unfavourable LAA anatomy, characterised by multiple proximal lobes and a shallow landing zone, inadequate for correct anchoring of the device. Additional procedural details are provided in Table 2.
The procedure was completed successfully without major complications in 125 patients, representing a procedural success rate of 93.3%. Procedural complications are listed in Table 3. In none of the cases did the complication result in a failure to implant the device. Three patients experienced major procedure-related complications, resulting in a rate of major procedural complications of 2.2%. In one patient, an important pericardial effusion, though not causing tamponade, was treated by percutaneous drainage on day two. Two cardiac tamponades occurred, comprising one case of probable LAA perforation resolved by percutaneous drainage and a tamponade associated with pulmonary artery laceration, which was treated surgically14. There were no procedural strokes or device embolisations. Six patients experienced minor procedural complications, including TIA, non-significant pericardial effusion and minor bleeding. No complications related to TEE occurred.
FOLLOW-UP
Follow-up data, available from all 128 successfully implanted patients, comprise a total of 238 patient-years, with a mean follow-up duration of 680±351 days (range: 19 days to 4.3 years). Sixty-four patients were followed for more than two years and in 96 patients at least one year of follow-up was available. At the most recently documented follow-up, 75.7% of patients were taking aspirin and 14.0% were on clopidogrel (2.3% of patients were on dual antiplatelet therapy). Only 1.5% of the patients were treated with warfarin and none of the patients was using novel anticoagulants. Fourteen patients (10.9%) were not receiving any antithrombotic therapy.
During follow-up, 28 patients experienced a total of 33 events, of which eight were fatal (Table 4). Two cardiovascular (CV) related deaths occurred. One patient, with known extensive coronary artery disease, died from ST-elevated myocardial infarction two years after the LAA occlusion procedure. Sudden cardiac death, one year after device implantation, occurred in another patient with dilated cardiomyopathy and a previously implanted cardioverter-defibrillator. Six patients died from non-CV-related causes, including hepatic insufficiency, lung cancer, bladder carcinoma, accident-related head trauma (one of each) and pneumonia (n=2). All deaths were confirmed to be unrelated to the implanted device and the implantation procedure.
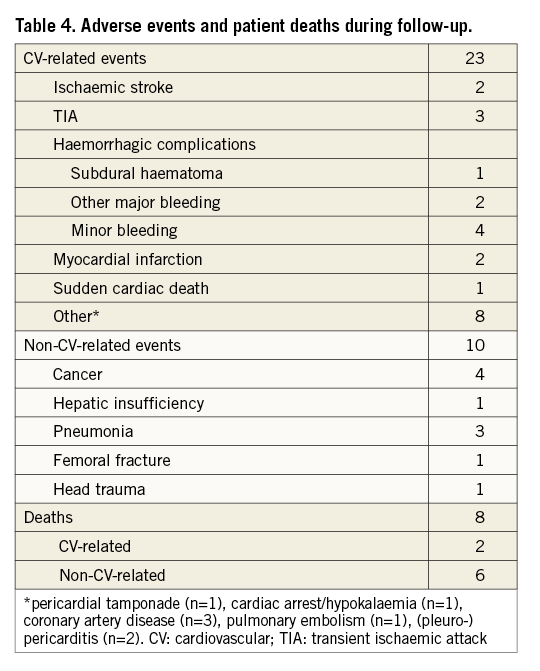
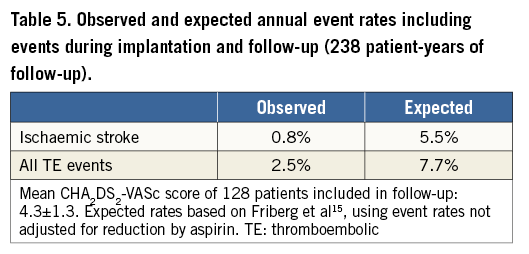
Five ischaemic events occurred during follow-up, all of them being cerebral. Two male patients, aged 77 and 84 years, suffered a non-fatal stroke, 27 months and 18 months after device implantation, respectively. Three other patients experienced a TIA. Four of the five patients who experienced ischaemic complications during follow-up had a CHA2DS2-VASc score higher than five and three were aged 80 years or older. Overall event rates are summarised and compared with expected rates in Table 5.
Intracranial bleeding (subdural haematoma) occurred in a 70-year-old male patient one month after the implantation procedure while on aspirin (100 mg daily), clopidogrel (75 mg daily) and fondaparinux (7.5 mg daily), an unduly high dose of triple antithrombotic therapy following an erroneous prescription by the patient’s general practitioner. The patient was treated with surgical drainage and recovered without significant consequences. Two other patients experienced a major extracranial bleeding, both of gastroenteric origin, resulting in an overall rate of major bleeding of 2.3%, corresponding to an annual rate of major bleeding of 1.3%.
TEE imaging was performed on 67 patients with a mean implant duration at imaging of 9.7±7.8 months. A single major residual leak was observed, and non-significant leaks were found in six patients (four minor and two moderate leaks). In one patient, TEE at 20 weeks after device implantation revealed a device-related thrombus while the patient was on aspirin. Additional antithrombotic therapy with subcutaneous injections of fondaparinux (7.5 mg daily) was initiated, which resolved the thrombus as confirmed by TEE one month later. Fifty-three patients were subjected to CCT at a mean follow-up period of 8.2±7.0 months after device implantation. Thirty-one patients had both TEE and CCT follow-up. Information with regard to device position and thrombus provided by CCT was similar to that obtained by TEE. However, there is no standardised method to quantify residual leakage based on CCT observations.
Discussion
The combination of a high risk for both ischaemic stroke and bleeding represents a true challenge for OAC in NVAF patients. While warfarin has been demonstrated to reduce the stroke risk by approximately 60%1, it is also associated with a considerable rate of major haemorrhagic complications, especially in patients over 80 years of age15. Novel OAC drugs have been shown to provide similar or better stroke prevention than warfarin at reduced, but still clinically relevant, bleeding rates2-4. This dilemma has encouraged physicians to consider LAAO for stroke prevention in NVAF patients who are at high risk for bleeding. The patients in our study comprised a typical cohort with respect to this treatment dilemma, given the high-risk scores for both TE and bleeding and the high proportion of elderly patients. In addition, our cohort was characterised by many comorbidities, representing potential treatment complications and resulting in CV and non-CV-related events not directly related to NVAF.
PROCEDURE
We were able to implant the device successfully in 95.5% of the patients considered for LAAO, and failure to implant was consistently due to anatomical factors. Successful implantation without major complications was achieved in 93.3% of the cases. The most frequent major procedural complications included pericardial effusion and cardiac tamponade, of which one was associated with laceration of the pulmonary artery by a stabilising hook of the device exiting the LAA wall14. This illustrates the fact that transseptal puncture and catheter manipulations within the LAA remain critical aspects in the implantation procedure. Recently reported technical procedural success rates achieved with the same device as applied in our study range between 95.2% and 100%8-11,16,17. In these studies major procedural complications occurred at rates between 0% and 7.5% and included similar complications to those observed during our study, such as TIA, tamponade and major bleeding. We did not observe any embolisations at a cost of a 4.5% rate of technical failures. Other authors11 have reported case series with the device delivered to almost all of the patients but with a considerable rate of device embolisation (mostly occurring with non-dedicated devices in the series by Nietlispach et al, but occurring also with the ACP device in 1.6% of the cases). Since no predictors of device embolisation after correct ACP positioning are known, we prefer, on the basis of the implanting physician’s experience, to behave conservatively towards unfavourable anatomy appendages (such as the ones occurring in the five failed patients, with very proximal lobes and a shallow landing zone), where the device shows instability at implant or at the pre-release tug test. Closure of the appendage with more than one device of the same or different type has been reported and appears to be feasible11. Different types of appendage might require specific implant techniques18. Nevertheless, it might still be prudent to abstain from implantation in certain anatomies, such as the one we encountered in the one case not attempted.
FOLLOW-UP
Follow-up data were collected from all 128 patients in whom the device was successfully implanted, yielding accumulative follow-up experience of 238 patient-years. To our knowledge, this is the most extensive follow-up experience gathered with the ACP device so far9-11. Over a mean follow-up duration of more than two years, ischaemic stroke was observed at an annual rate of 0.8%. Based on a recent large cohort of AF patients not receiving warfarin19, the expected annual stroke rate for our population would be 5.9%, considering the aspirin use, with an estimated stroke rate reduction of 86%. If we also took into consideration the use of clopidogrel, although mostly only prescribed for one to three months after the procedure, we should discount the 5.9% rate by 32% (the stroke risk reduction provided by clopidogrel). The expected stroke rate would then be 4.0% per year with a still important, 80% stroke rate risk reduction. In a Spanish single-centre study9, an annual thromboembolism rate of 1.6% was found, while the CHADS2 predicted rate was 4.8%. A Canadian multicentre study10 reported observed and predicted TE rates of 2.3% and 5.0% per year, respectively. In the single-centre 10-year experience study from Switzerland11, the authors reported on 152 patients who underwent LAA occlusion with a number of non-dedicated and dedicated Amplatzer devices and showed an overall TE events rate of 1.3% in a population with a mean CHA2DS2-VASc score of 3.4±1.7. These data are consistent with our results, indicating the effectiveness of LAAO in the prevention of ischaemic stroke in AF patients not suitable for long-term OAC. Categorisation of TE events per risk score category (Figure 2) showed that in our study cohort prevention of TE events was achieved over the entire risk spectrum, and predominantly in patients with a CHA2DS2-VASc score of 4.0 (no patients with events versus an expected annual TE rate of 5.5%). Moreover, in the subgroup of patients followed for more than two years, 63 out of 64 AF patients were free of ischaemic stroke for more than two years after device implantation.
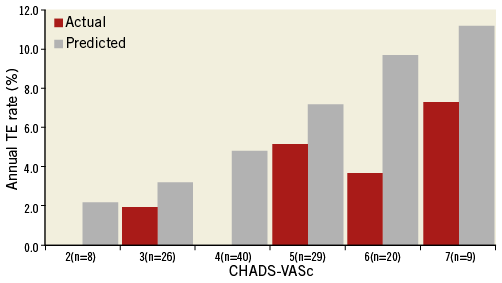
Figure 2. Actual and predicted annual rate of TE events per CHA2DS2-VASc category (number of patients indicated per category). The actual TE rates include all TE events which occurred during the study. Strokes occurred in the categories with risk scores 3.0 and 5.0 (one each). The predicted rates are based on the CHA2DS2-VASc indicated TE rate according to Friberg et al19. (CHA2DS2-VASc score of 8.0 not shown because of the small number of patients).
With 88% of the patients receiving implant-indicated antiplatelet therapy, major bleeding during follow-up occurred at an annual rate of 1.3%, which closely resembles the bleeding rate reported for large cohorts taking aspirin. For instance, in the ACTIVE-A study20, the same rate of major bleeding (1.3%) was found in the control arm, including 3,782 patients on aspirin with a mean age of 71 years. Higher aspirin-associated bleeding rates have been reported for octogenarians21. With a mean HAS-BLED score of 3.0 in our study cohort, major bleeding during follow-up was expected at an annual rate of 3.1%19. The overall therapy-related major bleeding rate, which accounts for follow-up events as well as for tamponade and effusion during device implantation, was 2.6%. This rate represents a beneficial outcome of LAAO compared to warfarin14 and novel OAC medication2-4, especially considering the age and bleeding risk of our patients. Nevertheless, in view of the high bleeding risk of patients typically considered for LAAO, further attempts to reduce the incidence of bleeding complications are warranted, including procedural measures to prevent bleeding as well as a reduced intensity or elimination of indefinite post-implant antiplatelet therapy.
Device-related observations during TEE included a single major residual leak (1.4%) and one case of device-related thrombus formation (1.4%). Up to the most recent follow-up, both of these occurrences did not impact on the safety or the effectiveness of the therapy. The true occurrence of peri-device leaks and device thrombosis might have been underestimated by the fact that routinely only one TEE examination was performed per patient and in just 52% of the patients. The CT scan, performed in 22 other patients, showed no thrombus formation and no massive leaks (free flow in the LAA). The former imaging technique is still not completely standardised for the evaluation of percutaneous LAAO results but provides valuable preliminary information.
Study limitations
This was an observational, retrospective, non-randomised study, and comparisons of safety and effectiveness between LAAO and other therapies for stroke prophylaxis were made on the basis of risks assessed by the CHA2DS2-VASc and HAS-BLED schemes. Post-implant antithrombotic therapy was not standardised but tailored to the patient’s individual situation.
Although our observations may require confirmation from a randomised and more uniformly treated cohort, we consider our therapeutic approach consistent with the clinical LAAO practice. The imaging follow-up was performed in 69% of the patients; thus, the real rates of post-implant leaks and thrombosis might have been underestimated.
Conclusions
Results from this observational study indicate that LAAO is a safe and effective therapy for stroke prevention in NVAF patients who are at high risk for ischaemic stroke and bleeding and are not suitable for long-term OAC. The stroke rate assessed over a period of up to four years of follow-up remains lower than the cohort’s risk-predicted stroke rate. Areas for therapeutic advancement include a further reduction in the incidence of procedural complications and a reduced intensity of device-indicated post-implant antithrombotic therapy.
| Impact on daily practice Atrial fibrillation is the most frequent sustained arrhythmia and its prevalence increases with age. The cornerstone of AF therapy is anticoagulation for stroke prevention. However, as many as 30-40% of the eligible patients are not appropriately anticoagulated because of various contraindications. Left atrial appendage occlusion with percutaneous devices is an alternative to warfarin for stroke prophylaxis. Our study confirms the safety and the efficacy of the procedure in a retrospective cohort of 134 patients treated with the AMPLATZER Cardiac Plug device. The long-term follow-up proves a low incidence of stroke and thromboembolic events in non-anticoagulated patients after ACP implantation, comparable to OAC therapy. LAAO is a promising new procedure and long-term follow-up of randomised trials is awaited for its further evaluation. |
Acknowledgements
The authors acknowledge with thanks Bert Albers for his contribution to the manuscript drafting, and Karin Tyack for her linguistic support.
Conflict of interest statement
G. Santoro was a proctor for AGA Medical and for St. Jude Medical. S. Berti is a proctor for St. Jude Medical. The other authors have no conflicts of interest to declare.

