
Abstract
Aims: Left atrial appendage occlusion (LAAO) is a stroke prevention therapy for patients with non-valvular atrial fibrillation (AF). This study reports one-year outcomes from patients enrolled in the prospective global Amulet registry.
Methods and results: A total of 1,088 patients were recruited, aged 75±9 years; 65% of patients were male. The CHA2DS2-VASc and HAS-BLED scores were 4.2±1.6 and 3.3±1.1, respectively. Eighty-three percent (83%) of patients had contraindications to anticoagulation (OAC); 72% had a history of major bleeding. An AMPLATZER Amulet LAA occluder was successfully implanted in 99% of cases. Transoesophageal echocardiography one to three months after implant showed no residual flow or flow <3 mm in 98.4%. The observed ischaemic stroke rate was 2.9%/year. Device-related thrombus was noted in 1.7% of patients. There were ten cases between 0 and 90 days and eight cases between 91 and 365 days. Patients discharged without OAC (>80%), in particular those on single aspirin therapy, did not appear to have a higher risk of device-related thrombus. In the first year, major bleeding occurred at an annualised rate of 10.3%. All-cause mortality was 8.4% at one year.
Conclusions: In the global prospective Amulet registry of patients at high risk of stroke and bleeding, the annualised ischaemic stroke rate was 2.9%. The LAA was sealed in 98.4% after one to three months and device-related thrombus was observed in 1.7% of cases with only a minority of all patients on anticoagulation treatment.
Abbreviations
AF: atrial fibrillation
APT: antiplatelet therapy
BARC: Bleeding Academic Research Consortium
CEC: clinical events committee
DRT: device-related thrombus
LAA: left atrial appendage
LAAO: left atrial appendage occlusion
NOAC: non-vitamin K antagonist oral anticoagulant
OAC: oral anticoagulation
TOE: transoesophageal echocardiography
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia and is associated with an increased risk of ischaemic stroke, at least in part due to cardiac thrombus formation, predominantly in the left atrial appendage (LAA)1. Percutaneous catheter-based LAA occlusion (LAAO) solutions have been developed to mitigate the risk of stroke in appropriate patients1. These offer the potential to reduce the risk of stroke without the use of long-term oral anticoagulation (OAC) in selected patients with non-valvular AF, as randomised controlled trials suggest that LAAO is non-inferior to warfarin therapy2,3.
The two most common catheter-based devices for LAAO are the AMPLATZER™ Amulet™ LAA occluder (Abbott, St. Paul, MN, USA) and WATCHMAN™ (Boston Scientific, Marlborough, MA, USA). Both devices have shown high implant success rates, either in randomised controlled trials3 or in large registries4,5. A recent publication of one-year WATCHMAN registry data (EWOLUTION trial) describes an 84% and 48% reduction in the risk of ischaemic stroke and major bleeding, respectively, relative to predicted rates based on baseline CHA2DS2-VASc and HAS-BLED scores6. In a retrospective study of 1,001 patients implanted with the AMPLATZER™ Cardiac Plug (ACP; Abbott), a previous generation of the Amulet device, the risk of systemic thromboembolism was reduced by 59% and the risk of major bleeding by 61% in patients successfully implanted (97.3%) and followed for an average of 13 months7. These studies provide one-year follow-up to LAAO in real-world clinical practice; however, a prospective study with echocardiographic core laboratory analysis and independent adjudication of strokes and major bleeds is lacking.
The current global study evaluates the use of the AMPLATZER Amulet device in a real-world prospective manner including an all-comer population. We present the one-year follow-up results of pre-specified clinical events adjudicated by an independent committee and characterise the utilisation of antithrombotic medication during follow-up.
Methods
The design and methods of this study have been described in detail previously5,8; additional information is available in Supplementary Appendix 1. Briefly, echocardiograms were evaluated by a core laboratory (MedStar Health Research Institute, Hyattsville, MD, USA) and serious adverse events (SAEs) were evaluated by an independent clinical events committee (CEC) comprised of experts in interventional cardiology, electrophysiology, and neurology. The CEC determined if the event was device- and/or procedure-related. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and are described in Supplementary Appendix 1.
Results
PATIENTS
One thousand and eighty-eight patients were enrolled at 61 centres from June 2015 to September 2016. Mean enrolment per centre was 17.8±16 patients (Supplementary Appendix 2). Successful implants occurred in 1,078 (99%) patients (a patient initially reported5 as receiving an ACP device was corrected later as having received an Amulet device). The 10 patients with unsuccessful implants were withdrawn per protocol; no clinical events were observed prior to withdrawal. There were 950 patients who completed the one-year follow-up (Figure 1) of the expected 987 visits, resulting in a follow-up rate of 96.3%. Baseline clinical characteristics comparing patients who did vs. those who did not complete one year of follow-up are shown in Supplementary Table 1. The mean follow-up duration was 11.1±2.6 months. Thirty-four patients were withdrawn (including 10 unsuccessful implants) and 13 additional patients did not complete the one-year visit. Baseline clinical characteristics of all enrolled patients are shown in Table 1. Patients, on average, were at high risk for stroke with a CHA2DS2-VASc score of 4.2±1.6 and for bleeding with a HAS-BLED score of 3.3±1.1, corresponding to an annual stroke risk of 6.7% and an annual major bleeding risk of 6.7%. Supplementary Table 2 lists the CEC-adjudicated procedure- or device-related SAEs up to one year of follow-up.
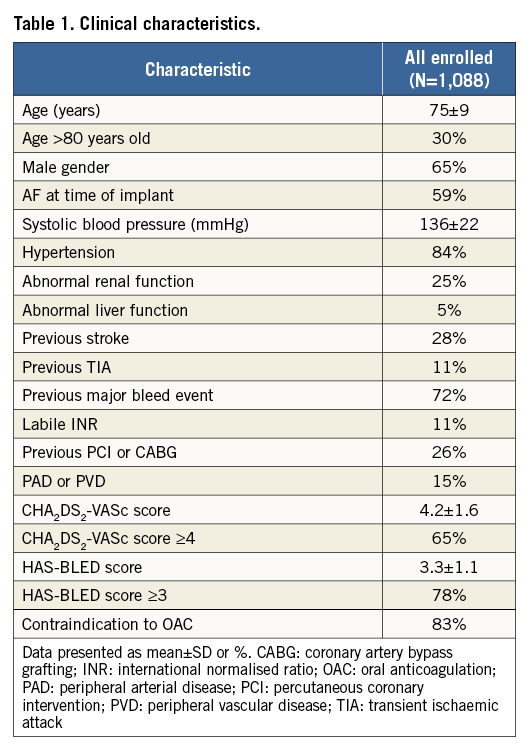
Figure 1. Flow chart of patients up to one year. Patients were considered enrolled and included in the current analysis if an implant was attempted. The follow-up rate up to one year was 96.3%. *88 patients died within one year of implant. An additional three patients did not complete the one-year follow-up visit as they died more than one year after implant but within the one-year follow-up visit window.
ECHOCARDIOGRAPHY
The core laboratory analysed 779 procedural and 763 one- to three-month follow-up studies for residual flow. At implant, 99.3% of patients achieved adequate LAA occlusion, with 89.4% having no residual flow and 9.9% having small residual flow <3 mm. At follow-up, 98.4% of echocardiograms showed either no flow or flow <3 mm.
MEDICATION REGIMEN
Table 2 and Figure 2 summarise medication use after LAAO. Most patients (80.1%) were discharged on antiplatelets alone, and a minority (11.2%) on anticoagulants. OAC was used infrequently over follow-up with 11.2% being discharged on OAC, and only 6%, 5.7%, and 6% of patients on OAC at the 1-3-month, 6-month, and 12-month assessments, respectively, reflecting the high bleeding risk of the population. Conversely, the proportion of patients without antithrombotic therapy increased from 7.5% at one to three months, to 14.5% at six months, and to 18.9% at 12 months.
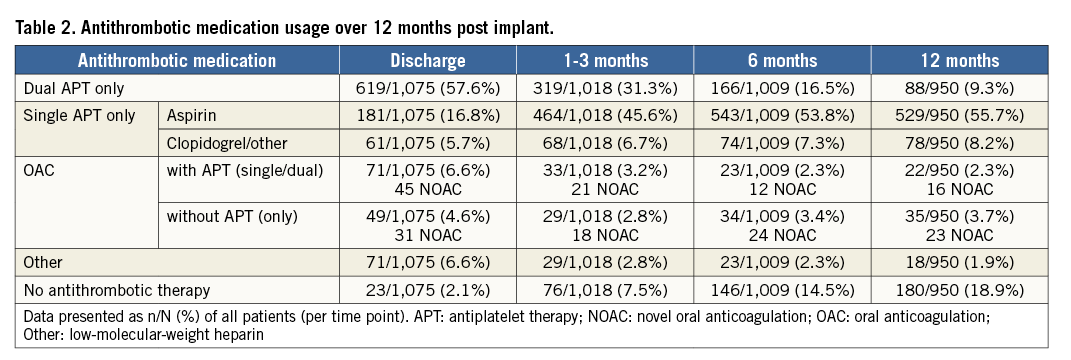
Figure 2. Utilisation of antithrombotic therapy up to one year. Patients were seldom prescribed OAC following LAAO. Patients were most often discharged on dual APT, with the use of aspirin only being most common during follow-up. The proportion of patients on no antithrombotic medication increased during follow-up.
STROKE
The CEC adjudicated ischaemic stroke, TIA, or systemic embolism. Twenty-nine events were observed in 27 patients, corresponding to a 2.9%/year annualised rate of ischaemic stroke. TIA occurred in nine patients and there were no systemic embolisms, resulting in a rate of 0.9%/year. Of the 29 ischaemic strokes, 16 occurred while patients were on aspirin monotherapy, nine while patients were on DAPT, and four while patients were on OAC. Figure 3A shows freedom from this composite endpoint during follow-up. Five patients with an ischaemic stroke died within the first year of follow-up and all deaths were due to the stroke.
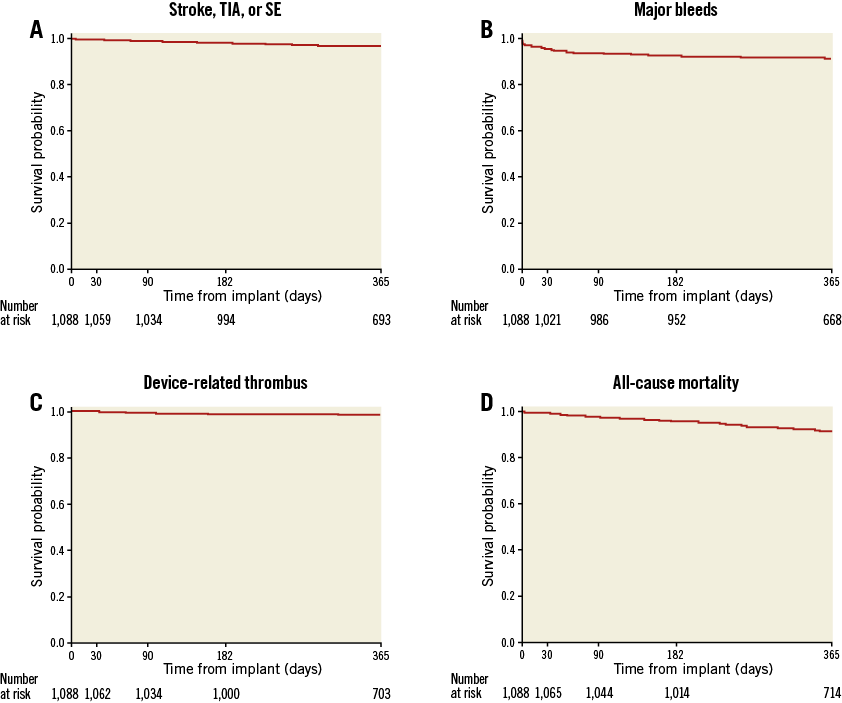
Figure 3. Kaplan-Meier graphs over one year of follow-up. The rates of stroke, TIA, systemic embolism (A), device-related thrombus (C) and all-cause death (D) were steady throughout follow-up. Conversely, major bleeding events (B) occurred at a greater rate in the first 30 days following implant as compared to the remainder of the one-year follow-up.
MAJOR BLEEDING
The annualised major bleeding (BARC ≥3) rate was 10.3%/year, with 103 events occurring in 87 patients. The severity was major (BARC type 3a=62) or life-threatening or disabling (BARC 3b=28; 3c=6; 5=7), with one of the seven fatal bleeding (BARC type 5) events adjudicated as being procedure- or device-related (cardiac perforation day of procedure). Figure 3B shows that the majority of events were observed early in follow-up. Thirty events (30/103=29%) were periprocedural (≤7 days).
Thirty-two major bleeding events were adjudicated as procedure- or device-related (3.2%/year). Five of the procedure- or device-related major bleeding events occurred >7 days after the procedure. These included three pericardial effusions or tamponades, occurring on days 11, 23, and 34, a gastrointestinal (GI) bleed on day 13, and a vascular pseudoaneurysm on day 17. In addition, fifteen vascular access-related complications occurred with onset ≤7 days after the procedure.
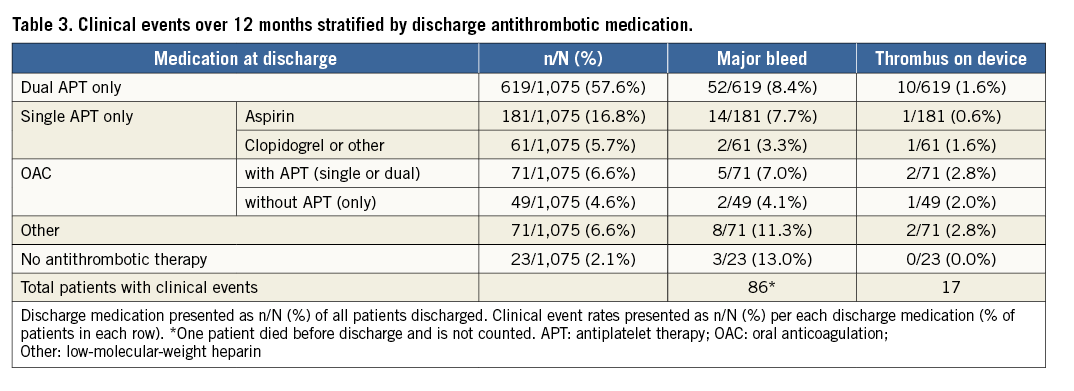
Major bleeding events, in relation to antithrombotic therapy at discharge, are listed in Table 3. The rates were 13% for those discharged without any antithrombotic therapy, 8.4% for those discharged on dual APT, 7.7% for those discharged on aspirin only, 7.0% for those discharged on OAC with APT (single/dual), 4.1% for those discharged on OAC without APT, and 3.3% for those discharged on clopidogrel or other single APT.
DEVICE-RELATED THROMBUS (DRT)
The DRT rate was 1.7%/year, with 18 events occurring in 17 patients (Figure 3C). Ten DRT were discovered between 0 and 90 days and eight between 91 and 365 days. Of the 17 patients who experienced a DRT, three experienced an ischaemic stroke within the year of follow-up. In the first patient, DRT was confirmed on the same day as the ischaemic stroke, which occurred on day 161. The other two patients did not undergo surveillance for DRT during the hospitalisation for stroke, therefore the presence or absence of DRT at the time of stroke is unknown. In one of these two patients, DRT was observed 243 days after the stroke. In the second patient, DRT was confirmed 24 days prior to the stroke, no DRT was reported 37 days after the stroke, and a second DRT was observed 113 days after the stroke. This patient did not have an additional stroke over the remaining 181 days of follow-up after the second thrombus. The core laboratory reported no peri-device leaks on the one- to three-month transoesophageal echocardiography (TOE) in any of the patients who experienced both DRT and stroke. Table 3 details the proportion of patients with observed DRT based on discharge antithrombotic regimen.
DEATH
Eighty-eight patients (8.4%) died during the first year following implantation. Deaths were categorised as cardiovascular (n=53) or non-cardiovascular (n=35). Six patients died within 30 days of implantation, three of whom died within three days (immediate procedural mortality), one from cardiac perforation, one from cardiorespiratory arrest, and one from an unknown cause which was conservatively considered to be cardiovascular. The remaining deaths were distributed uniformly over the follow-up period (Figure 3D). In patients with death attributable to cardiovascular cause, two were adjudicated as related to the device. The first patient underwent an uncomplicated procedure but developed hypotension two hours afterwards. A pericardial effusion was present, with suspicion of ongoing cardiac bleeding despite draining 1 litre of blood. Chest exploration revealed a 3 mm perforation at the LAA ostium, which was closed using a pericardial patch. The patient expired two days later, secondary to cardiogenic shock. A TOE performed 314 days after implant in the second patient revealed multiple DRTs and high-grade mitral valve insufficiency. Open chest surgery was performed, removing the vegetations and the Amulet device, amputating the LAA, and reconstructing both the mitral and tricuspid valves. A complicated post-surgical course occurred, with additional surgeries required for pericardial effusion and wound healing disorder. The patient passed away 365 days after LAAO implant.
Discussion
In this global study we report one-year follow-up of a large group of patients undergoing LAAO using the AMPLATZER Amulet device. Unlike previous observational LAAO studies, clinical events were adjudicated by a CEC and sealing of the LAA was examined by a core laboratory6,7. In the present study, the ischaemic stroke rate was reduced as compared to the expected rate without OAC by CHA2DS2-VASc score. The Amulet device resulted in a high degree of LAA sealing on echocardiographic follow-up and DRT formation was observed in 1.7% of cases despite a substantial proportion of patients on single antiplatelet therapy.
The ACP device showed feasibility, safety, and efficacy of percutaneous LAA closure without requiring patients to be on OAC after implant7,9. This current study reports results of the second-generation AMPLATZER LAAO device. In a population with a high risk of stroke (CHA2DS2-VASc 4.2±1.6), the annual rate of ischaemic stroke was 2.9%/year, a 57% reduction as compared to the predicted event rate. Although this stroke rate is greater than that reported in the EWOLUTION registry6 (1.1%/year) or ACP study7 (0.9%/year), comparisons must be made cautiously. Firstly, the post-implant medication regimen in EWOLUTION differed from that in the current study as patients in EWOLUTION were discharged on OAC more often (27% vs. 11.2%) and on single APT less often (7% vs. 22.5%). Recently, a DAPT subgroup analysis of EWOLUTION reported DRT in 4.0% of patients, 1.4%/year rate of ischaemic stroke, and 2.5%/year major bleeding rate up to one year in 605 patients discharged on DAPT, with discontinuation of DAPT in 85% of patients within one year10. Despite a comparable proportion of patients contraindicated to OAC (83.8% vs. 83%) between the EWOLUTION subgroup and the current study, the HAS-BLED median score of 2.0 was lower than the mean (3.3±1.1) and median (3) of the current study. In the 619 patients discharged on DAPT in the current study, the rates for major bleeds and DRT, as adjudicated by the CEC, over the first year were 8.4%/year and 1.6%/year, respectively (Table 3), highlighting the need for further investigation of the optimal post-Amulet device antithrombotic regimen. Although the subgroup sizes are too small to draw conclusions, we observed higher major bleeding rates in patients discharged on aspirin alone or no antithrombotic medications as compared to those on OAC (Table 3). As the protocol recommended patients take aspirin for six months post implant and clopidogrel per standard of care, patients receiving aspirin monotherapy or no antithrombotic medications may have had the highest bleeding risk while patients prescribed OAC probably had lower risks of bleeding.
Our rate of ischaemic stroke is slightly greater (2.9%/year vs. 2.3%/year) and the DRT rate is comparable (1.7% vs. 1.9% of patients) to those of the 107 patients in whom Korsholm and colleagues prescribed aspirin alone (85% of patients) following ACP or Amulet device implant, followed for two years11. Both the cohort described by Korsholm and colleagues and the current study population had a high proportion of patients (26.3% vs. 18.9%) without any antithrombotic use 12 months after implantation of an ACP or Amulet LAAO device. In the current study, patients discharged on single aspirin therapy do not appear to have an increased rate of DRT relative to those discharged on more aggressive antithrombotic regimens (Table 3).
Despite 60% of patients on single APT or no antithrombotic medications one to three months post implant, the relatively low stroke rate compared to the predicted rate without OAC was probably facilitated by the high degree of adequate occlusion of the LAA. Following placement of the device, 99.3% of procedural and 98.4% of one- to three-month TOE showed adequate sealing. A similarly high degree of LAA closure was obtained in the EWOLUTION (99.7%) and ACP (98.1%) studies6,7. The current study utilised an independent core laboratory, which assessed LAA closure using a threshold of <3 mm for residual flow, while EWOLUTION did not use a core laboratory and used a threshold of 5 mm.
The observed procedure- or device-related major bleeding rate of the current study was 3.2%/year. These major bleeding events were adjudicated by the CEC as resulting from the LAAO procedure including the device or the delivery system and were not restricted to a periprocedural (i.e., ≤7 days from implant) timeline. We observed 18 (1.65%) pericardial effusion or tamponade events adjudicated as procedure- or device-related, a rate comparable to other LAAO observational studies of AMPLATZER devices7,12,13. Although the observed rate is relatively low, improved outcomes should continue to be sought in these high bleeding risk patients, with consideration given to post-procedural monitoring, medication management, and imaging prior to discharge. The overall rate of major bleeding in the current study was higher, at 10.3%/year, than those reported in EWOLUTION (2.6%/year) and in the Tzikas et al ACP study (2.1%/year)6,7. The current study population was predisposed to major bleeding events more than those enrolled in the EWOLUTION or ACP studies. A history of major bleeding was present in 72% of Amulet patients, 47% of ACP, and 31% of WATCHMAN. The mean HAS-BLED scores also predicted higher bleeding rates, being 3.3, 3.1, and 2.3 for the Amulet, ACP, and EWOLUTION registries, respectively. The ACP study reported a 7.6%/year bleeding rate in 195 patients on aspirin monotherapy or no antithrombotic medications with <1 year of follow-up. Conversely, the 432 patients on a similar regimen with ≥1 year of follow-up had a dramatically low annualised bleeding rate of 0.6%/year (90% reduction compared to HAS-BLED score estimated). Continued follow-up for clinical events up to two years in the current study, with 74.6% of patients on aspirin alone or no antithrombotic medications at one year, will evaluate if a similar reduction in the annualised bleeding rate is seen over a longer period of follow-up. Although the 18.9% of patients we observed without any antithrombotic medication at one year may have reduced the risk of bleeding, careful consideration must be given prior to omitting all APT following LAAO. The stagnant atrial blood flow of patients in AF may result in thrombosis over the long term despite adequate LAA sealing, and indications for APT, such as PCI/CABG or PAD/PVD, are present in a number of patients receiving LAAO. For these reasons, most patients may receive aspirin monotherapy long term as we observed in this study. Future studies are needed to investigate the feasibility and safety of stopping all antithrombotic medication during long-term follow-up in appropriate patients after LAAO.
Comparisons of LAAO to non-vitamin K antagonist oral anticoagulation (NOAC) for ischaemic stroke prevention in AF patients at high risk of stroke must involve a risk/benefit analysis focused on the patient’s history of bleeding and risk of bleeding. The current study population consists of 72% with a history of major bleeding and 83% who were contraindicated to OAC, characteristics excluded from NOAC trials. Systemic anticoagulation with NOACs would be contraindicated in many of these patients at present. Five of the seven intracerebral major bleeds we observed were in patients on aspirin monotherapy, while one occurred in a patient on DAPT and the other in a patient on aspirin plus NOAC. There is no safe pharmacological option for significantly reducing the risk of thromboembolic events for many of the high bleeding risk patients enrolled in the current study. Although there are upfront periprocedural risks, as with any cardiac procedure, we believe that there are long-term potential benefits of device-based percutaneous LAA closure for many patients. We observed almost half of the major bleeding events within the first month of implant, while the rate of major bleeding over the next 11.5 months of follow-up was reasonable in a population with a history of major bleeding and contraindication to anticoagulation.
Novel oral anticoagulants are an appropriate clinical approach to reduce the risk of stroke in patients without a history of major bleeding. However, issues with compliance and use in patients with abnormal renal function (25% of the present population) are present in patients appropriately indicated for NOACs. The prospective randomised CLOSURE-AF trial (NCT03463317) will determine the clinical benefit of LAAO directly compared to novel oral anticoagulants (if feasible) in patients at high risk of bleeding or with chronic kidney disease, a population similar to the present study.
In a single-centre study, Weise et al reviewed 298 patients at high risk for major bleeding (HAS-BLED 3.5±1.0) who were prescribed dual APT for six weeks after LAAO, which was decreased to single APT in the absence of peri-device flow or device-related thrombus at six-week TOE13. Five different LAAO devices were used in the study, with the Amulet or ACP being implanted in 42% of cases. Major bleeding (BARC ≥3) events occurred in a greater proportion of patients, with Weise and colleagues observing events in 10.7% of patients (32/298), while the current study reports that 8.0% of patients (87/1,088) suffered a BARC ≥3 event. The majority (n=15/25) of non-procedure-related major bleeding events observed by Weise et al occurred early in follow-up (≤50 days), while patients were often (n=13/15) prescribed dual APT. The remaining 10 non-procedure-related events occurred beyond day 50, with most being GI bleeds while on single APT. The results of the current study and that by Weise and colleagues suggest that most major bleeding events following LAAO in patients with a history of major bleeding and at high risk of future events are not related to the device or procedure, and that cessation of intense antithrombotic therapy (OAC or dual APT) following adequate LAAO reduces the frequency of major bleeding events in these patients.
Limitations
The present study is an observational registry with no control group and results should be interpreted accordingly (Supplementary Appendix 3). However, an independent CEC for AE adjudication and core laboratory for echocardiographic evaluation of LAA closure and detection of device thrombus were utilised to provide standardisation of the data reported. Although comparisons of observed stroke and major bleeding rates to predicted values based on historical control data sets using CHA2DS2-VASc and HAS-BLED scores have been utilised in previous LAAO studies6,7,12, they do not take the place of prospective randomised trial data.
Conclusions
In this large prospective registry of patients at increased risk of stroke, the observed ischaemic stroke rate following implantation of the AMPLATZER Amulet device was 2.9% over one year of follow-up. Additionally, in a population at high risk of major bleeding, patients were able to avoid use of dual APT or OAC therapy shortly after implant with a similar level of non-procedure-related bleeding events to those reported in other LAAO registries of similar populations.
| Impact on daily practice In patients with non-valvular AF at high risk of ischaemic stroke and bleeding, implantation of an AMPLATZER Amulet device was associated with a 57% lower risk of stroke over the first year as compared to the predicted rate. This occurred while the majority of patients did not receive OAC. These data build upon previous AMPLATZER Amulet reports showing a high degree of implant success (99%) and TOE confirmation of LAAO (>98%). |
Acknowledgements
The authors thank Ryan Gage, Kyle Brunner, and Hong Zhao from Abbott for their contributions to analysis and review of the data, and Ryan Gage for writing assistance.
Funding
Abbott provided funding.
Conflict of interest statement
U. Landmesser reports personal fees from Abbott outside the submitted work. C. Tondo reports personal fees from Abbott and Boston Scientific outside the submitted work. J. Camm reports personal fees and investigational grants from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer/BMS, Abbott, and Boston Scientific outside the submitted work. H-C. Diener reports receiving honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from Abbott, Achelios, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, MSD, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Portola, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth and Yamanouchi and financial support for research projects provided by AstraZeneca, GSK, Boehringer Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi-Aventis, Syngis and Talecris. The Department of Neurology at the University Duisburg-Essen has received research grants from the German Research Council, German Ministry of Education and Research, European Union, NIH, Bertelsmann Foundation and Heinz-Nixdorf Foundation. H-C. Diener has no ownership interest in and does not own stocks in any pharmaceutical company. V. Paul reports personal fees from Medtronic and Abbott outside the submitted work. B. Schmidt reports personal fees from Abbott during the conduct of the study, and personal fees from Abbott and Boston Scientific outside the submitted work. J.E. Nielsen-Kudsk reports personal fees from Abbott outside the submitted work. D. Hildick-Smith reports personal fees from Abbott outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Results.
Supplementary Appendix 3. Limitations.
Supplementary Table 1. Baseline characteristics comparing patients who completed one-year follow-up versus those who did not.
Supplementary Table 2. CEC-adjudicated procedure- or device-related SAEs up to one year.
To read the full content of this article, please download the PDF.

