
Abstract
Aims: Several left atrial appendage (LAA) closure systems are available. Due to differences in device design, the results of specific occluders derived from trials cannot simply be generalised to all devices. The present analysis sought to assess two contemporary LAA closure devices in clinical practice.
Methods and results: The work represents a non-randomised sub-analysis of the prospective, multicentre, Left-Atrium-Appendage Occluder Register - GErmany (LAARGE) registry. The WATCHMAN (group 1) and the AMPLATZER Cardiac Plug (ACP) or Amulet occluder (group 2) were assessed. A total of 641 patients at 38 centres were enrolled. Of these, 278 (43%) and 340 (53%) patients received the WATCHMAN and ACP/Amulet occluder, respectively. High technical success was achieved with a slight difference between the groups (96% in group 1 vs 99% in group 2; p=0.007). Procedural safety did not differ (98% in group 1 vs 97% in group 2; p=0.55). The Kaplan-Meier estimated one-year composite of death or stroke was 12.0% and 12.9%, respectively (p=0.79).
Conclusions: Both the WATCHMAN and the ACP/Amulet occluder provide excellent procedural results with comparable implantation success and no differences with respect to procedural safety and long-term effectiveness.
Introduction
Percutaneous left atrial appendage (LAA) closure offers an alternative option for stroke prevention in selected patients with non-valvular atrial fibrillation. The two most frequently used devices are the WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) and the AMPLATZER™ Cardiac Plug (ACP) or the new-generation Amulet™ occluder (St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA). The most solid evidence in stroke prevention is provided by the WATCHMAN occluder proving non-inferiority of safety and even superiority regarding the composite efficacy endpoint of stroke, systemic embolism and cardiovascular death compared to warfarin after a follow-up of four years1. However, these results cannot be generalised to all closure systems since the available occluders differ with respect to device design and implantation technique. Unfortunately, there are no large-scale trials comparing these two devices. Therefore, the present study aimed to compare the WATCHMAN with the ACP/Amulet occluder regarding technical success, procedural safety and long-term outcome.
Methods
STUDY DESIGN
Patients with non-valvular atrial fibrillation and need for oral anticoagulation undergoing LAA closure were included in the present prospective, multicentre, Left-Atrium-Appendage Occluder Register - GErmany (LAARGE) registry (clinicaltrials.gov: NCT02230748). Participating centres were encouraged to include all consecutive patients to avoid recruitment bias. Patients were enrolled prior to implantation. No specific inclusion or exclusion criteria were used in order to maintain the “real-world” character of the study population. Indication for LAA closure was at the discretion of the respective physicians. Centres with different levels of expertise in the field of percutaneous LAA closure participated in the study. However, every operator underwent an obligatory training provided by the proctor programme of the respective company prior to study participation. Due to the non-randomised study design, operators were responsible for device selection. For the current analysis, only patients who underwent LAA closure with either the WATCHMAN (group 1) or ACP/Amulet (group 2) device were included.
In case of documented stroke, major bleeding, or systemic embolism, medical documents were requested from the respective site and centrally assessed and verified by an independent clinical events committee of the present registry. Prior to participation all patients gave written informed consent. The study was approved by the ethics committees of the participating centres and was performed in accordance with the Declaration of Helsinki.
DEVICE DESCRIPTION
The WATCHMAN, the ACP and the Amulet devices have been described in detail previously2,3. Briefly, the WATCHMAN device is a single-lobe occluder consisting of a self-expanding nitinol frame structure which tapers towards the distal end and is closed and covered at its proximal end by permeable polyester polyethylene fabric. The ACP and its redesigned new-generation version, the Amulet occluder, have a two-piece polyester covered nitinol mesh construction with a distal lobe serving as anchor within the LAA body and a proximal disc closing the LAA orifice.
FOLLOW-UP
Follow-up was performed by the Stiftung Institut für Herzinfarktforschung (IHF) using telephone calls based on standardised questionnaires 12 months after enrolment. If patients could not be contacted, they were traced with the help of local municipal authorities. In addition to the central follow-up, the participating hospitals had the possibility to report cases of death, adverse events, clinical visits and echocardiographic exams.
ENDPOINT DEFINITIONS
Technical success was defined as successful delivery and release of the occluder in the LAA with a subsequent stable position without peri-device leaks >5 mm and without device-related complications based on the Munich consensus paper4. Procedural safety was defined as absence of death, stroke, myocardial infarction, severe bleeding (defined as haemodynamically unstable, need for transfusion, surgical treatment or intracranial bleeding), pericardial effusion requiring surgical or percutaneous treatment, access-site complications (defined as arteriovenous fistula, pseudoaneurysm or retroperitoneal bleeding), haemo-/pneumothorax requiring surgical treatment, peri-device leak >5 mm and device embolisation requiring percutaneous treatment in a separate intervention or requiring surgical treatment.
STATISTICAL ANALYSIS
Continuous variables were expressed as median with interquartile range (IQR), the risk scores as means with standard deviation. Categorical variables were expressed as absolute numbers and percentages. The chi-square test was used for comparison of categorical variables generally, and Fisher’s exact test for rates of hospital complications. For continuous variables, the Mann-Whitney-Wilcoxon test was used. The documentation of baseline characteristics and hospital complications is 99% complete. Otherwise, the size of the sample base is presented as the denominator of rates. The descriptive statistics are based on the available cases. Adjusted odds ratios with 95% confidence intervals were calculated in multivariable logistic regression models. Event-free survival during one year after the implantation procedure was displayed by Kaplan-Meier curves and compared between groups by the log-rank test. All p-values were calculated using two-tailed tests; statistical significance was defined at p<0.05. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
STUDY POPULATION
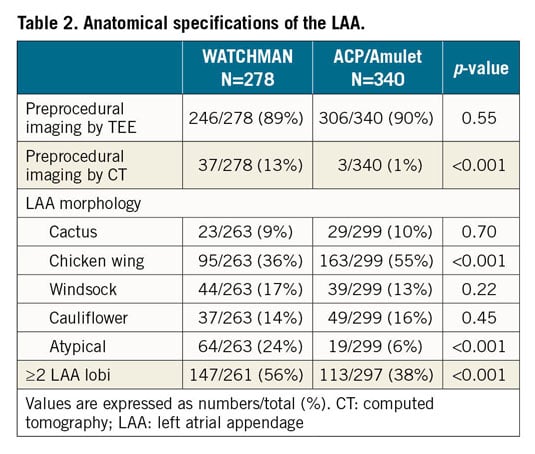
From July 2014 until January 2016, a total of 641 patients at 38 centres were enrolled into the present registry. The present analysis is based on 278 (43%) and 340 (53%) patients, who underwent LAA closure with the WATCHMAN and ACP/Amulet occluder, respectively. In the ACP/Amulet group, 177 (52%) patients received the ACP and 163 (48%) received the Amulet device. Baseline characteristics are listed in Table 1. Anatomical LAA specifications are described in Table 2.
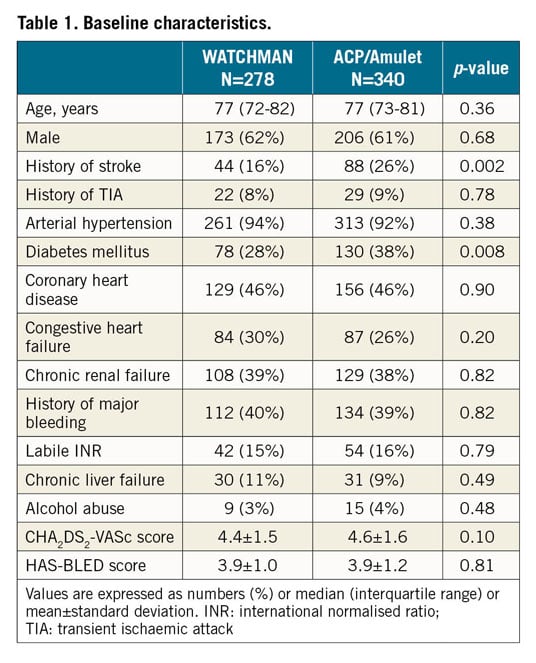
TECHNICAL SUCCESS AND PROCEDURAL DETAILS
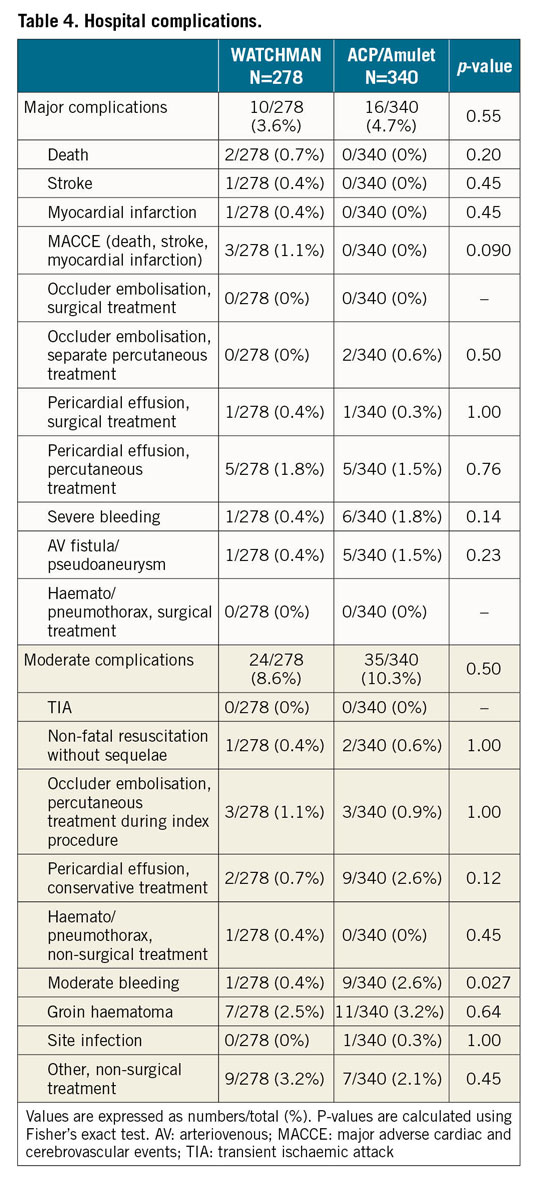
Overall, stable device anchorage was achieved with slightly higher success using the ACP/Amulet device (96% in group 1 vs 99% in group 2; p=0.007) (Figure 1). Furthermore, no significant differences regarding the individual rates of device embolisation, device thrombus and peri-device leak ≥5 mm were noted (Table 3, Table 4). All device embolisations were treated percutaneously and no surgical retrieval was necessary. In two patients, occluder embolisation required percutaneous treatment in a separate intervention. The remaining procedural details are outlined in Table 3. Larger device sizes were used in patients receiving the WACTHMAN occluder (Supplementary Table 1). Post-procedural and one-year antithrombotic treatment is displayed in Supplementary Table 2.
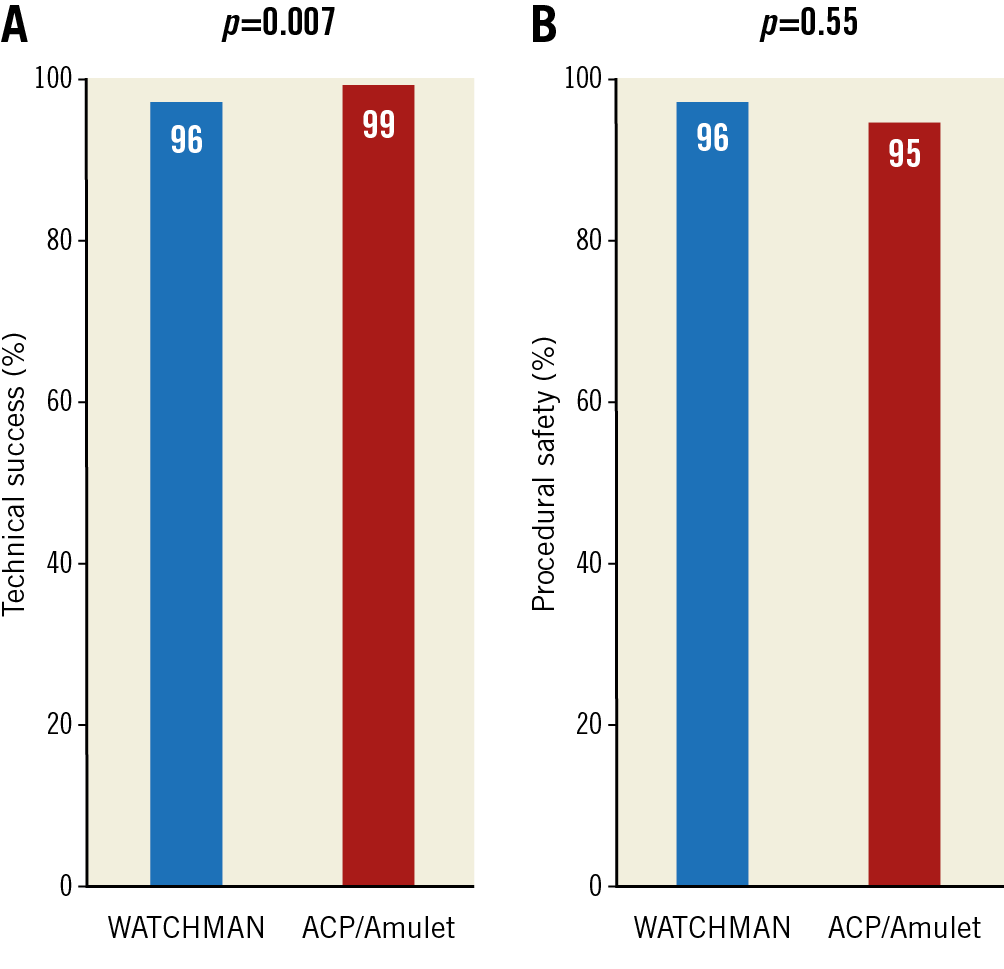
Figure 1. WATCHMAN versus ACP/Amulet with respect to technical success (A) and procedural safety (B).
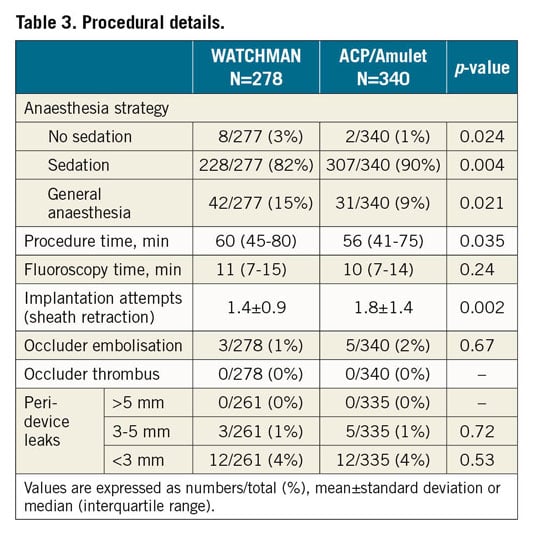
PROCEDURAL SAFETY
With regard to procedure-related safety, no difference was detected between the groups (96% in group 1 vs 95% in group 2; p=0.55) (Figure 1). Also, after adjustment for morphology with more than one lobus, use of a specific LAA occluder was not associated with procedure-related safety (odds ratio for WATCHMAN versus ACP/Amulet use 0.71 [95% confidence interval 0.31-1.61]; p=0.69). The rate of major and moderate complications was low with similar event rates among the devices (Table 4). Pericardial effusions were treated percutaneously in 5 out of 6 cases with need for intervention in the WATCHMAN group and in 5 out of 6 cases with need for intervention in the ACP/Amulet group. Surgical drainage was necessary once per group. In these two patients, LAA perforation occurred during occluder implantation with subsequent tamponade and cardiogenic shock. Thoracotomy was performed without further complications and patients were discharged without sequelae. Overall, there was improved technical success and procedural safety compared with previous studies with both the WATCHMAN and the ACP/Amulet occluder (Figure 2).
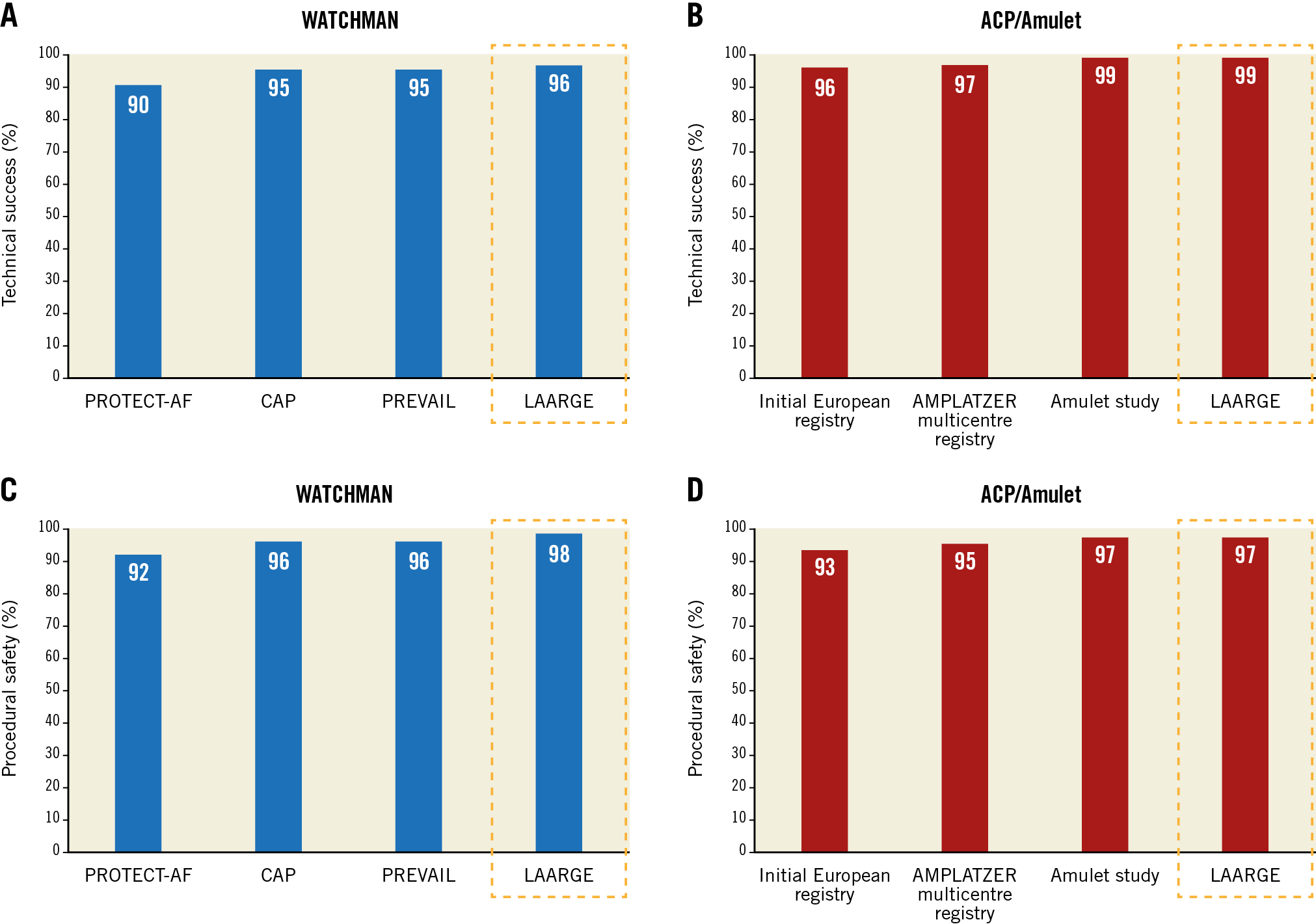
Figure 2. Technical success (A & B) and procedural safety (C & D) of the WATCHMAN and the ACP/Amulet device across different studies. With respect to the WATCHMAN device, data were reported from the PROTECT-AF trial (enrolment from 2005 until 2008)11, CAP registry (enrolment from 2008 until 2010)11, and PREVAIL trial (enrolment from 2010 until 2012)10. Concerning the ACP/Amulet, device data were reported from the initial European experience (enrolment from 2008 until 2009)12, ACP multicentre registry (enrolment from 2008 until 2013)13, and the AMULET study (enrolment from 2015 until 2016)17. Similar to the previous studies, pericardial effusion requiring percutaneous intervention was not counted in the procedural safety endpoint in the LAARGE data set in this Figure for better comparability.
DEVICE SELECTION
In 13 of the participating centres only WATCHMAN devices were implanted and in 17 centres only ACP/Amulet occluders were implanted. In the remaining eight centres using both devices, a total of 98 patients undergoing LAA closure with the WATCHMAN and 82 patients with the ACP/Amulet occluder were recruited. LAA anatomy was found to be the only clinically relevant variable that differed between the devices in this subset of patients treated in centres using both devices (chicken wing 33% in group 1 vs 60% in group 2; p<0.001, and atypical LAA 41% in group 1 vs 13% in group 2; p<0.001).
Furthermore, we assessed whether procedural performance was improved when operators had the ability to choose between different devices adapted to the LAA anatomy. As shown in Table 5, no differences in technical success or procedural safety were observed between centres using only one device compared to centres using both devices.
LONG-TERM FOLLOW-UP
Follow-up was available in 98% of WATCHMAN patients and in 99% of patients receiving the ACP/Amulet (p=0.75), with a median follow-up time of 12.4 (12.1-13.2) months and 12.5 (12.1-13.3) months, respectively (p=0.12). The Kaplan-Meier estimated one-year composite of death or stroke (Figure 3A) and the composite of death, stroke or systemic embolism (Figure 3B) were not significantly different between the device groups. The observed one-year stroke rate in survivors was 0.9% (2/234) versus 1.5% (4/263) for the entire follow-up time (p=0.50) and 0.4% (1/234) versus 1.5% (4/263) when excluding procedural strokes (p=0.38) with the respective devices. Complications at one year excluding the hospital period are displayed in Supplementary Table 3.
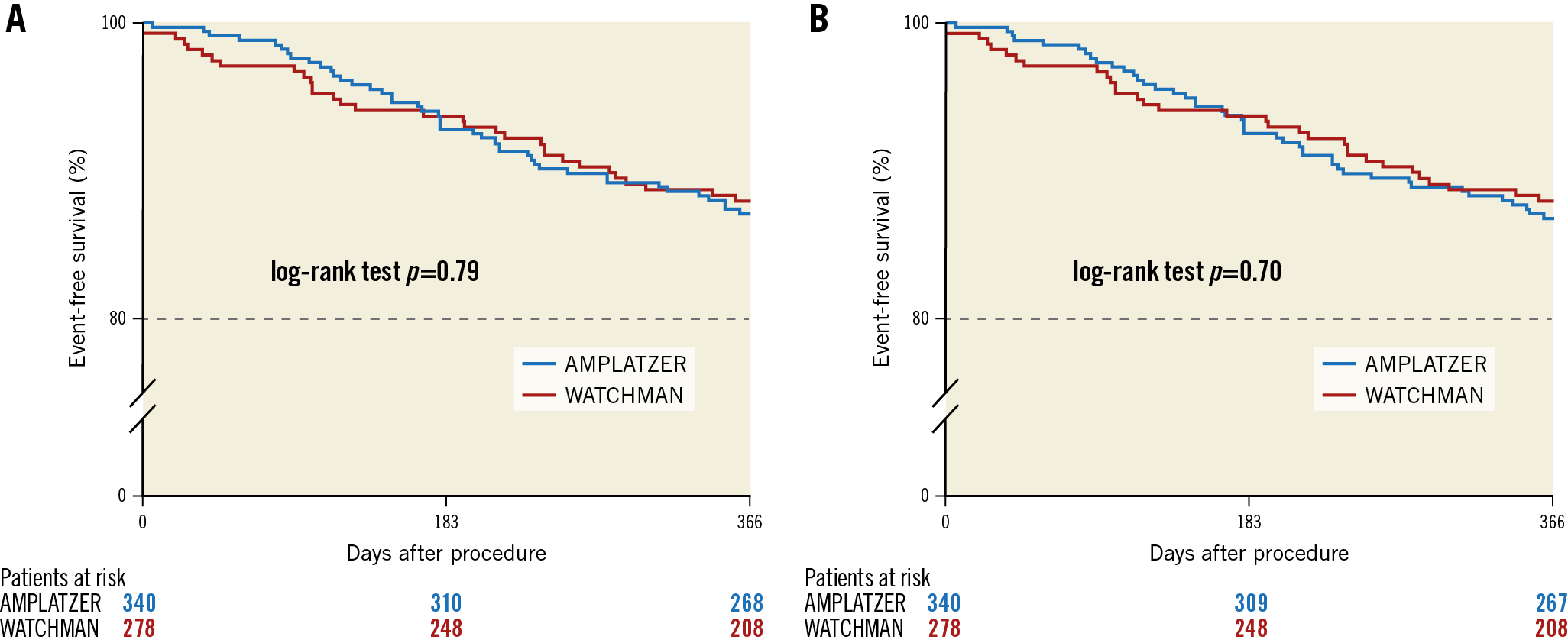
Figure 3. One-year composite of death or stroke (A) and death, stroke, or systemic embolism (B). Event-free survival from death or stroke was 88.0% with the WATCHMAN and 87.1% with the ACP/Amulet (A). Event-free survival from death, stroke or systemic embolism was 88.0% with the WATCHMAN and 86.8% with the ACP/Amulet (B).
Discussion
The present analysis is the first large-scale multicentre study comparing the WATCHMAN with the ACP/Amulet occluder. The large number of patients provides adequate statistical power to obtain reliable results. Additionally, the multicentre design and participation of centres with different levels of expertise enables transferability of the data to daily clinical practice.
TECHNICAL SUCCESS
The study clearly showed that both the WATCHMAN and the ACP/Amulet provide high implantation success. The crude numbers show a numerically small, but statistically significant, signal favouring ACP/Amulet which may or may not persist in light of the changes made to the new-generation WATCHMAN FLX™ occluder, which received its CE mark this year. Equivalent technical success of the two devices was already observed in a small retrospective study5. However, no other direct comparison is available regarding the effectiveness and safety for the two occluder systems.
Leaks >5 mm and acute device thrombus, both conditions precluding discontinuation of anticoagulation and therefore criteria for technical success according to the Munich consensus document used in this analysis, were completely avoided in the present study. Both the intra-operator learning curve as shown in single-centre studies6 as well as experience gained over the years in terms of a “group learning effect” as shown in Figure 1 have contributed to an excellent procedural performance with both devices nowadays. It has been shown repeatedly that implantation technique has a strong impact on immediate technical success and long-term safety7,8. When assessing previous trials, technical success improved continuously across the studies, especially with the WATCHMAN device. The other large real-world registry assessing the WATCHMAN occluder (the EWOLUTION study) reported a slightly higher technical success with a rate of 98% compared to 96% in the present study9. The significantly shorter procedure time seen in the ACP/Amulet group in the present analysis may indicate that occluder implantation is less demanding with this device type. However, more implantation attempts were necessary with the ACP/Amulet device than with the WATCHMAN. It remains unclear whether this is the consequence of less preprocedural imaging, especially by computed tomography (CT), in the present ACP/Amulet group or whether the ACP/Amulet implantation is not technically simpler than with the WATCHMAN, as sometimes assumed.
PROCEDURAL SAFETY
The present data confirmed that procedural safety was not influenced by device selection. Furthermore, no differences were detected between the occluders with respect to the individual safety endpoints. The stroke rate was as low as 0.4% in the WATCHMAN group and even 0% in the ACP/Amulet group. This reflects a profound improvement, since stroke occurred in 0.9% and 0.7% in the previous WATCHMAN trials10,11 and in 2.1% and 0.9% in the previous ACP/Amulet experience12,13. The risk of pericardial effusion requiring intervention was the same with both devices. Similar to the stroke risk, an incremental reduction of pericardial effusions was achieved compared to early trials from 5.2%11 to 1.8% with the WATCHMAN and from 3.5%12 to 1.8% with the ACP/Amulet occluder, respectively. Avoiding bleeding events is the main rationale of LAA closure as an alternative to anticoagulation. However, due to the implantation procedure and the antithrombotic therapy during the endothelialisation period following LAA closure, the bleeding risk is particularly high in the initial phase. Approximately one half of major bleeding (4.9% in a pooled analysis of PROTECT-AF and PREVAIL) occurs during the procedure14. Therefore, reduction of procedural bleeding events is key to achieving long-term superiority of LAA closure over anticoagulation. In the present analysis, severe bleeding was reduced to 1.1% without significant differences between the devices. Overall, both occluders showed an improved safety profile when compared to their initial performance in previous studies.
DEVICE SELECTION
The LAA can be closed with one type of occluder in the majority of patients. However, in some anatomical specifications of the LAA, selection of a particular occluder may be an advantage. The present data indicate that, for chicken wing morphology of the LAA, operators rather chose the ACP/Amulet. This particular shape of the LAA may present with an early bending of the LAA, leading to a short landing zone. In such anatomical circumstances, the implantation of the relatively short ACP/Amulet is preferred over the WATCHMAN to avoid mechanical irritation of the LAA wall in the bending area. On the other hand, the ACP/Amulet device needs a stricter orientation of the sheath perpendicular to the LAA ostium compared to the WATCHMAN to obtain an optimal landing zone. This may be an explanation for the more frequent use of the WATCHMAN in atypical shapes of the LAA in the present study. The hypothesis of a potentially better sealing with the proximal disc of the ACP/Amulet leading to lower stroke rates compared to the WATCHMAN device is not supported by the present results.
LONG-TERM FOLLOW-UP
The two devices showed similar long-term results with respect to mortality and thromboembolic events. Regarding the WATCHMAN, stroke rates were comparable between the present analysis (0.7%) and previous trials, e.g., 1.5 per 100 patient-years in PROTECT-AF1, 1.3 per 100 patient-years in the CAP registry15 and an annual rate of 1.1% in the latest EWOLUTION registry16. With respect to the ACP/Amulet, a similar number of strokes was published in the ACP multicentre study reporting an annual rate of 0.9%13, but the stroke risk was higher in the Amulet study with an annual rate of 2.9%17 compared to the present data (1.2%). However, these divergent results should be interpreted with caution due to differences in study methods. A randomised trial assessing both devices using uniform inclusion criteria, endpoints and follow-up is urgently needed. The randomised Amulet IDE trial comparing the Amulet with the WATCHMAN is currently ongoing and will provide more evidence (NCT02879448). However, occluder selection for certain LAA anatomies will not be possible in that trial due to pre-screening and randomisation. This may limit the transferability of the results to daily clinical practice in contrast to the present registry.
Limitations
The main limitation of the present study is the non-randomised design. However, the present device selection dependent on anatomical criteria, personal preference or simply the availability of specific occluders among the respective centres reflects best current daily clinical practice. Since the study methods were inferior to randomised controlled trials (e.g., lack of on-site monitoring), underreporting of clinical events cannot be excluded. Moreover, the comparative evaluation of the devices is limited to a follow-up time of 12 months after the procedure. Therefore, potential differences regarding safety and efficacy beyond this time point need to be assessed in the future. Information regarding transoesophageal echocardiography (TEE) or CT imaging during follow-up was not gathered in this registry. Hence, only clinically relevant device complications and no imaging-based adverse events such as leaks or clinically inapparent device thrombi were assessed. The comparison of centres using both devices versus those using one device may have been influenced by potentially higher operator experience in sites using both devices due to their higher implantation numbers.
Conclusions
The present analysis is a further contribution to the growing body of evidence showing that LAA closure can be performed with remarkable implantation success and procedural safety. Based on the present data, there is a slightly higher rate of technical success with the ACP/Amulet device. No differences with respect to procedural safety and long-term outcome between the WATCHMAN and the ACP/Amulet occluder were observed.
|
Impact on daily practice Both the WATCHMAN and the AMPLATZER Cardiac Plug (ACP) or the new-generation Amulet occluder provide excellent procedural results and similar long-term outcome. According to the present results, device selection may be based on personal experience and preference as well as LAA anatomy. Performance of both devices should be assessed in future randomised studies. |
Funding
The present Left-Atrium-Appendage Occluder Register - GErmany (LAARGE) was conducted independently from industry and only scientifically and financially sponsored by the Stiftung Institut für Herzinfarktforschung (IHF) Ludwigshafen, Germany. For the biometrical analyses of the present work, the IHF was financially supported by an unrestricted grant from Boston Scientific.
Conflict of interest statement
T. Lewalter has received speaker honoraria from St. Jude Medical/Abbott Vascular and Boston Scientific. H. Sievert has received study honoraria, travel expenses, and consulting fees from Access Closure, AGA, Angiomed, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm medical, Recor, Rox Medical, Sadra, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Viacor, Velocimed, and Veryan, and has stock options from Cardiokinetix, Access Closure, Velocimed, CoAptus, Lumen Biomedical, and Coherex. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

