
Five-year data from randomised trials comparing left atrial appendage (LAA) closure employing the WATCHMAN® device (Boston Scientific, Marlborough, MA, USA) to warfarin for stroke prevention in patients with atrial fibrillation (AFib) have shown non-inferiority and a net benefit for patients; in fact mortality as well as stroke severity was lower in the device group1. However, current ESC guidelines only give a IIb B recommendation for LAA closure in patients with increased bleeding risk. Prospective registry data from Europe now give us new insights into the benefits and risks of this procedure.
Previously, certain anatomies were believed not to be suitable for effective closure and some researchers reported high rates of peri-device leakage during follow-up. In this issue of EuroIntervention, Fastner et al present an interesting subanalysis from the prospective German LAA closure registry (LAARGE) stratifying patients for LAA morphology2.
Stratification to one of five groups defined by LAA morphology was performed by the treating physician employing a combination of imaging modalities. The primary endpoint was the rate of successful closure with no leak >5 mm at hospital discharge. The implanters classified 46% of LAA as chicken wing, and 9% as cactus; the remaining morphologies each accounted for 15% of patients. Overall, there was a high success rate; the atypical anatomy represented the biggest challenge with a success rate of “only” 94%; other morphologies could be closed with a >98% success rate. The chicken wing morphology was closed by an AMPLATZER™ device (St. Jude Medical/Abbott Laboratories, Santa Clara, CA, USA) in 63% of cases; an exemplary case from the editorialist’s practice is depicted in Figure 1. In contrast, 77% of atypical anatomies were closed with a WATCHMAN device (exemplary case in Figure 2). Periprocedural complications were rare and not different among the five morphologies. Around 90% of patients were discharged on dual antiplatelet therapy (DAPT).
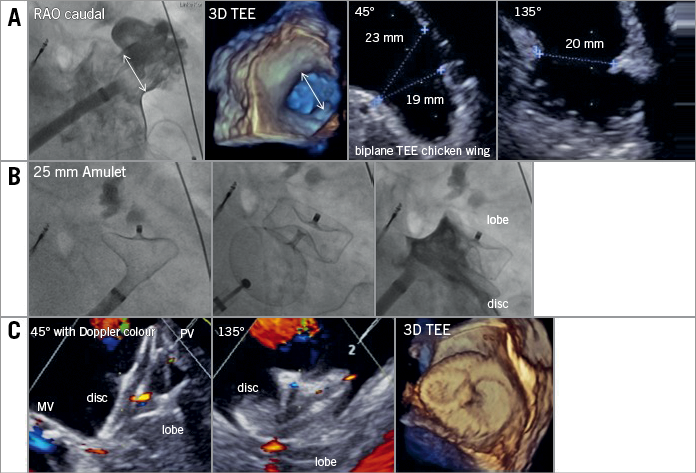
Figure 1. Exemplary closure of a chicken wing LAA anatomy employing an AMPLATZER Amulet device. A) Anatomical assessment of the LAA. The neck is measured to decide on device sizing. B) Step-by-step deployment of device lobe, test for anchoring and final results after disc deployment. C) Echocardiographic result after LAA closure. The lobe anchors and closes the LAA neck while the disc spans between the pulmonary vein ridge (PV) and the annulus of the mitral valve (MV). No colour flow is observed towards the depth of the LAA.
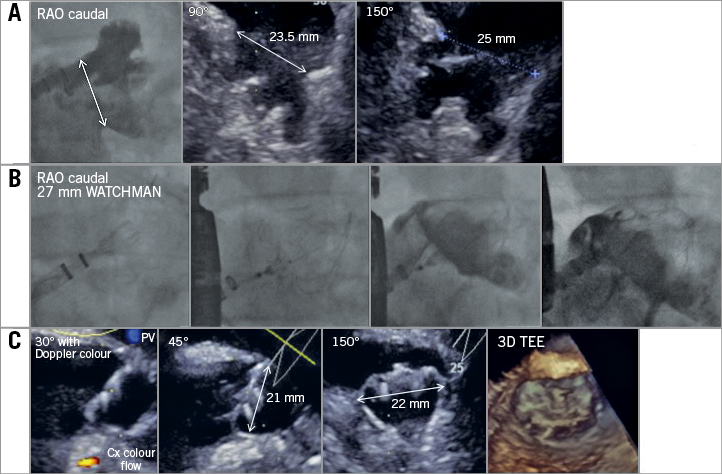
Figure 2. Exemplary closure of an atypical, shallow LAA employing the WATCHMAN device. A) Anatomical assessment of the LAA. Atypical conformation not classifiable as chicken wing, cauliflower, windsock or cactus. Landing zone for the WATCHMAN device clearly identifiable at the ostium of the LAA. B) Stepwise release of the device starting distally in the superior lobe followed by a tug test. Angiographic confirmation of sealing parallel to echocardiographic assessment (C) of leakage, compression (15-30%) and 3D image to assess LA protrusion (“shoulder”). Release of the device and final assessment.
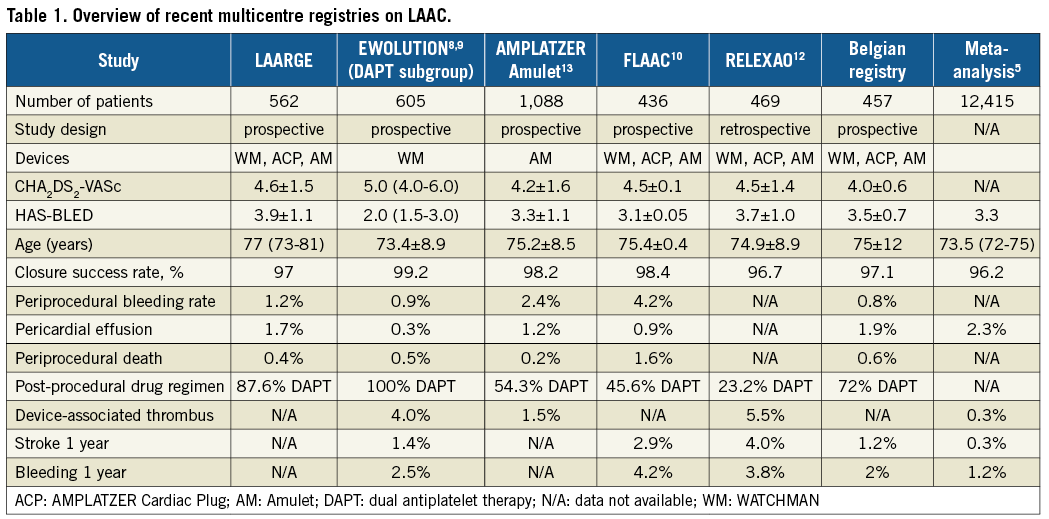
The data add significantly to our understanding of this procedure. The field has certainly matured and implanters together with industry have standardised the procedure irrespective of the large variability in LAA morphology3,4. Table 1 summarises current European multicentre registries including LAARGE, as well as a meta-analysis on all LAA closure studies with >25 patients published between 1999 and 20165. While in general the procedure is safe, Table 1 also shows pericardial effusion and periprocedural bleeding to be the two main issues still responsible for a low periprocedural mortality. This observation should lead to particularly careful procedure planning and minimal manipulation during the intervention in the fragile structure of the LAA. Integrating modern imaging modalities such as cardiac computed tomography (CT) or three-dimensional transoesophageal echocardiography (3D TEE) in the LAA closure workflow might allow further improvement of safety through minimising device manipulation and optimising sizing6,7.
Data on stroke rate following LAA closure in high-risk patients are available from EWOLUTION and two investigator-initiated registries from France (FLAAC) and Belgium8-11. The global AMPLATZER study group is expected to present its one-year results at this year’s EuroPCR meeting; EWOLUTION two-year results will be presented at Heart Rhythm 2018. The majority of patients in these registries were switched to dual antiplatelet therapy following LAA closure for a couple of months. There was a rate of around 3-5% device-associated thrombus not linked to clinical events. A recently published, retrospective registry from France now describes a high rate of device-associated thrombus, particularly with the St. Jude AMPLATZER™ Amulet™ device (25%)12. The presence of thrombus and a history of vascular disease were predictive of stroke/transient ischaemic attack (TIA) during follow-up. DAPT and oral anticoagulation (OAC) at discharge after LAA closure were associated with a substantially lower risk of thrombus on the device; surprisingly, however, 43.7% of patients were discharged without any specific treatment or only single antiplatelet therapy12. These data support the current consensus to initiate either DAPT or (N)OAC therapy at discharge following LAA closure; in case of bleeding, patients can however be switched to single antiplatelet or no therapy as even in the series of Fauchier the total clinical event rate was low (annual event rate of ischaemic stroke 4%).
In summary, LAA closure has emerged as a growing and scientifically active field, as witnessed by the analysis from LAARGE presented in this issue of EuroIntervention. Current data support the notion of LAA closure to complement or even replace oral anticoagulation regarding stroke prevention in AFib patients at increased bleeding risk.
Conflict of interest statement
M. Bergmann received speaker honoraria from Boston Scientific and Abbott.

