
Stroke remains a major cause of death and disability. Despite the recent advances in acute stroke treatment, the most effective way to avoid stroke-related mortality and disability is not treatment, but prevention. As most severe ischaemic strokes are related to cardioembolism in patients with atrial fibrillation (AF), the cornerstone of successful stroke prevention is oral anticoagulation (OAC). Long-term OAC carries an inherent risk of bleeding complications. The longer OAC is used, the higher is the cumulative risk of bleeding. In most patients with AF, OAC should be given permanently unless severe bleeding occurs. Thus, alternative approaches are being tested, avoiding the need for long-term OAC in patients with AF. Among these, percutaneous left atrial appendage closure (LAAC) is the most promising. It was tested in a few randomised controlled trials, where it was non-inferior to OAC1,2,3.
In this issue of EuroIntervention, Gloekler et al4 present very interesting results of the retrospective APPLY study, using propensity score matching to compare 500 consecutive patients undergoing LAAC with 500 matched patients with AF.
Despite significant methodologic limitations (retrospective design, two leading high-volume centres enrolled patients into the LAAC group and only one centre enrolled patients to the control group, higher proportion of patients with coronary artery disease and thus with the need for combination therapy with antiplatelets plus anticoagulants, 21.2% rate of OAC non-users in the control group), this study is extremely interesting as it is the first study showing potential benefit of LAAC over long-term OAC in reducing a composite endpoint of cardiovascular (CV) death, stroke and systemic embolism. The key factor in order to understand this benefit is in Figures 3A, 3C, 3D and especially 4A (Figures from Gloekler et al4) as it shows the most important messages: (1) the risk of periprocedural complications results in no benefit from LAAC during the first year of follow-up, but (2) at two years the benefit occurs and with longer follow-up the amount of the benefit increases. This is logical, as periprocedural complications occur only during the periprocedural phase, but bleeding complications in patients on long-term OAC increase continuously with longer follow-up.
Thus, the practical message from the APPLY study could be as follows: LAAC should be considered for patients with AF having a high risk of stroke and simultaneously a high risk of bleeding, in whom life expectancy is at least three years or longer. The longer the life expectancy is, the more such patients may benefit from LAAC.
In agreement with the APPLY study, and also in previous randomised controlled trials (RCT) comparing LAAC with warfarin2, CV mortality with LAAC tended to be lower than with OAC. However, the annual incidence of stroke/transient ischaemic attack (TIA) on OAC (62% on vitamin K antagonists [VKA]) in the APPLY study (3.2%) was higher compared to the VKA arms of previous large NOAC trials (~2% per year), and therefore requires special attention. Until now, LAAC has been tested against OAC for non-inferiority only. If superiority of LAAC over OAC were documented, it would present further progress in stroke prevention in AF. Despite its positive observation, the APPLY study could serve as a hypothesis-generating study; an RCT to confirm this finding would be necessary. Assuming the annual incidence of the primary endpoint of 5.6% with LAAC and 7.8% with OAC in the APPLY study, >4,000 patients (>2,000 in each arm) would have to be enrolled in such a trial. Unfortunately, no such large trial is ongoing. Nevertheless, the majority of OAC patients in APPLY were on VKA. The risk of CV death or cardioembolic events for patients on NOAC would differ to that for patients on warfarin which would further affect the sample size of the population needed to test the superiority of LAAC versus OAC.
In the APPLY study, safety events (composite of bleeding + procedure-related complications) were similar. However, when procedural bleeds are excluded (~50% of all safety events), the incidence of bleeding events was significantly reduced with LAAC compared to OAC. It expresses the hope of LAAC, i.e., to maintain the protection from cardioembolic events similar to that provided by OAC and to reduce bleeding events. Bleeding events associated with OAC treatment cannot be considered negligible. Bleeding was the main reason for OAC discontinuation (resulting in no protection from stroke) in all large NOAC trials, e.g., in ARISTOTLE, bleeding tended to result in increased mortality.
Severe procedure-associated adverse events occurred in 25 of 500 patients (5%) in the APPLY study. Despite the initial decline of procedural complications since the PROTECT-AF study, the current event rate of ~5% in recent RCTs and registries is similar and without further decrease (Figure 1). Given the overall similar rate of stroke/TIA after LAAC as with OAC in all RCTs and large registries (despite the limitation of the absence of a really large RCT, as was performed with NOAC), the safety of LAAC is of paramount importance and seems to represent the major limitation to further expansion of the procedure in the general population.
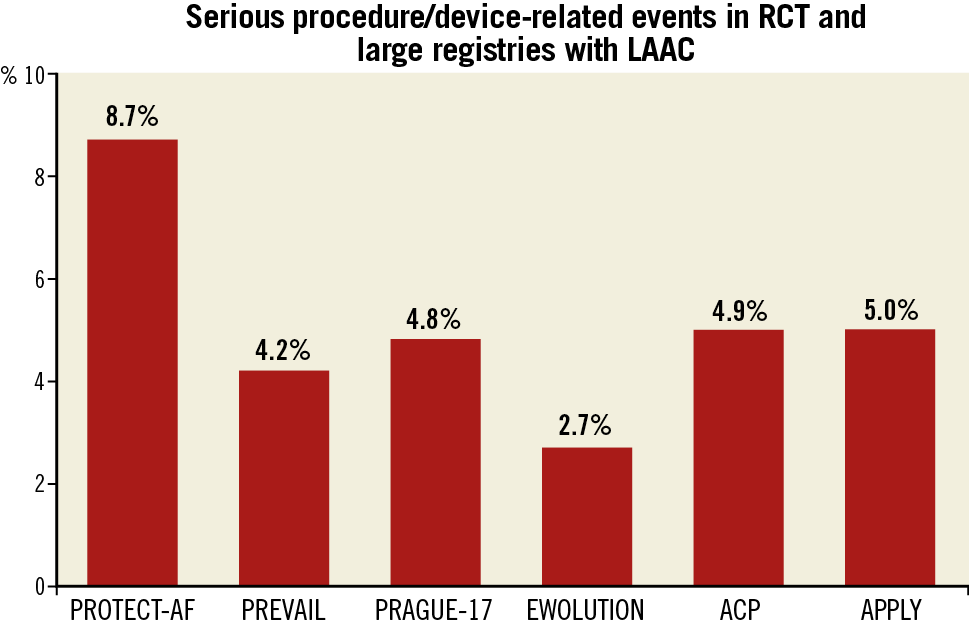
Figure 1. Complication rates in LAAC trials. LAAC: left atrial appendage closure; RCT: randomised controlled trials
Recently, several RCTs comparing LAAC with OAC have been initiated (CATALYST, ASAP-TOO, CLOSURE, STROKECLOSE, OCCLUSION); however, some of them will probably be stopped prematurely due to slow enrolment (ASAP-TOO, STROKECLOSE – personal communication), and the results of the largest will not be available for some time (e.g., CATALYST – in five years). Data from large registries on the efficacy and safety of LAAC will remain the major source of information about LAAC for several years. Therefore, the APPLY investigators should be acknowledged for their effort. Despite the above-mentioned study limitations and despite these (and other) unresolved questions, we found the APPLY study results encouraging, paving the way to a broader use of LAAC in high-risk patients.
Nevertheless, a lot of clinical research is needed to clarify the remaining questions. Can these results be extrapolated to other (than leading academic high-volume) centres? Are these results (achieved in patients mostly using VKA as OAC) applicable also to patients on long-term NOACs, with an expected lower risk of bleeding complications? Are these results applicable to patients with a moderate risk of stroke and bleeding? Is it safe to avoid OAC in LAAC patients in the long term (5-10 years with AF and without OAC)?
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

