
Abstract
Aims: The aim of this study was to determine the opinion of the scientific community regarding percutaneous left atrial appendage closure (LAAC). The main focus of the survey was on concerns and expectations regarding the safety and efficacy profile of LAAC in clinical practice and on current and future clinical perspectives.
Methods and results: A voluntary web-based survey was distributed by the European Association of Percutaneous Coronary Interventions (EAPCI) to all individuals registered on the EuroIntervention mailing list (n=21,800). A total of 724 physicians responded to the survey, of whom 31.8% had first operator experience with LAAC. Exclusive use of the Amulet (34.4%) or WATCHMAN (30.3%) was similar, but the former was the most frequently used device in Europe. The majority of respondents (59.3%) deemed LAAC to be as effective as, but safer than oral anticoagulants (OAC) in reducing stroke risk. Periprocedural complications (40.3%) and cost (28.8%) were the major concerns. Most practitioners did not consider novel oral anticoagulants (NOACs) to be a deterrent for performing LAAC procedures. Moreover, a history of serious haemorrhage was not deemed necessary to justify LAAC for 59.8% of physicians.
Conclusions: The results of this survey reveal a high level of confidence in percutaneous LAAC amongst surveyed interventional cardiologists, with the majority believing it to be as effective as OAC in terms of stroke prevention and safer in terms of bleeding risk.
Introduction
Atrial fibrillation (AF) is a major health issue due to its growing prevalence and potentially devastating complications, the most important of these being cardioembolic stroke1. The absence of atrial systole in the presence of AF results in stasis of blood in the left atrium, and, in particular, the tubular left atrial appendage (LAA). This structure, which is a remnant of the embryonic left atrium, has trabeculated walls which, in the absence of sinus rhythm, further increase the risk of thrombus formation. Over the past few decades, vitamin K antagonists (VKA) have been regarded as the treatment of choice for stroke prevention in AF patients, despite the considerable associated bleeding risk2. More recently, novel oral anticoagulants (NOACs) with more predictable pharmacokinetic and pharmacodynamic profiles have been approved for the prevention of thromboembolic events in patients with AF. The superior safety and non-inferior efficacy profiles of these agents compared with VKAs have been proven in randomised trials3-5, and their use in clinical practice is growing. Despite this, some patients have an unacceptable bleeding risk to justify oral anticoagulation (OAC) and remain at considerable risk of thromboembolism.
The role of the LAA in systemic thromboembolism in AF patients is well established6,7. This has led to the development of surgical and, more recently, percutaneous strategies for effective amputatation or obliteration of the LAA to prevent stroke. There are a number of commercially available percutaneous transcatheter LAA closure (LAAC) devices, with WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) and AMPLATZER™ Amulet™ (St. Jude Medical, St. Paul, MN, USA) accounting for the majority of devices used worldwide. Both of these devices received CE mark approval in Europe and have been granted a class IIb indication with level of evidence B for percutaneous LAAC in patients at high stroke risk and contraindications to long-term OAC in European guidelines8.
The safety and efficacy of the WATCHMAN device were tested in two randomised trials, namely the WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation (PROTECT AF) trial9 and the Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial10. Following these pivotal trials, the WATCHMAN device was granted approval by the Food and Drug Administration (FDA) for use in the United States of America with limited labelling: “patients that are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2- VASc scores and are recommended for anticoagulation therapy”; “are deemed by their physicians to be suitable for warfarin”; and “have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin”11. The Amulet device is not currently FDA-approved. Despite a lack of randomised clinical trial data, however, it is widely used in some jurisdictions – particularly in Europe – facilitating publication of registry data12-14.
The aim of the current study was to determine the opinions of the scientific community regarding percutaneous LAAC. In particular, the study aimed to determine operators’ experience with the procedure, their concerns regarding the risk/benefit profile of LAAC, and their opinions on current and future clinical perspectives.
Methods
This was a voluntary web-based survey undertaken by the European Association of Percutaneous Coronary Interventions (EAPCI). The survey was conducted using a free web-based survey tool (SurveyMonkey, Palo Alto, CA, USA) and comprised multiple-choice questions, including the possibility of adding further comments if desired. It was not mandatory to reply to the entire survey. The sample population comprised the mailing list of EuroIntervention – the official journal of the EAPCI. Overall, a total of 21,800 individuals were invited to participate. The invitation to participate in the survey was sent on 18 January 2016, and a reminder was issued on 8 February 2016. The data are reported as percentages and compared using the chi-square test. A two-sided p-value <0.05 was considered significant. The results were stratified by age (i.e., <40 vs. 40 to 50 vs. >50 years), jurisdiction of practice (i.e., European vs. non-European), experience with LAAC (procedural experience as first operator versus no prior direct procedural involvement), level of interventional cardiology experience (i.e., interventional cardiologist with more than 10 vs. 5 to 10 vs. <5 years of experience vs. non-interventional cardiologist). All analyses were performed with STATA, version 13.0 (StataCorp LP, College Station, TX, USA).
Results
CHARACTERISTICS OF RESPONDENTS
Of the 21,800 invitations sent, a total of 724 (3.3%) recipients responded to the survey. Among these, 604 (83%) provided personal and professional information with respect to age, medical qualification and country of practice (Online Table 1). Participation in the survey was global, with the majority of respondents being European (54.3%) or Asian (22.7%). Leading contributing countries were Italy and Germany, with 55 responders each. The median age of respondents was 46 years. Most of the participants were interventional cardiologists at various stages of their career, with the majority (49%) having >10 years of experience, followed by non-interventional cardiologists and cardiologists in training (8.4% and 4.6%, respectively). A small proportion – accounting for 7.1% of respondents – categorised their profession as “other”. Characteristics of respondents are detailed in Table 1, Online Figure 1, and Online Figure 2.
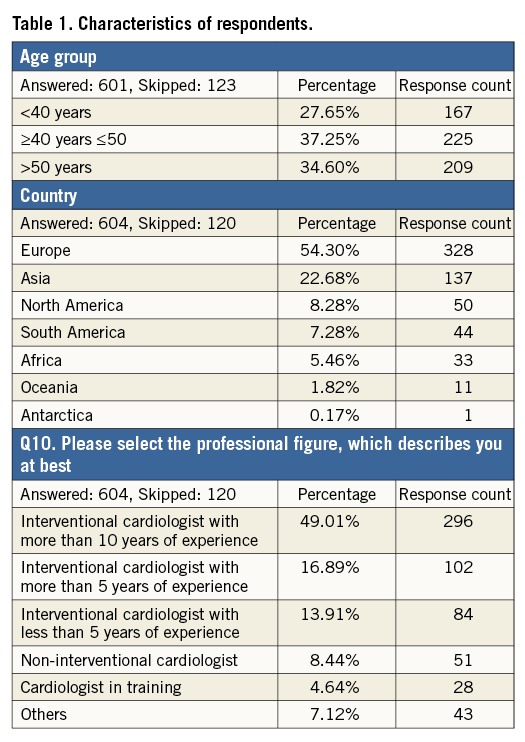
Q1. Do you have experience in percutaneous LAAC?
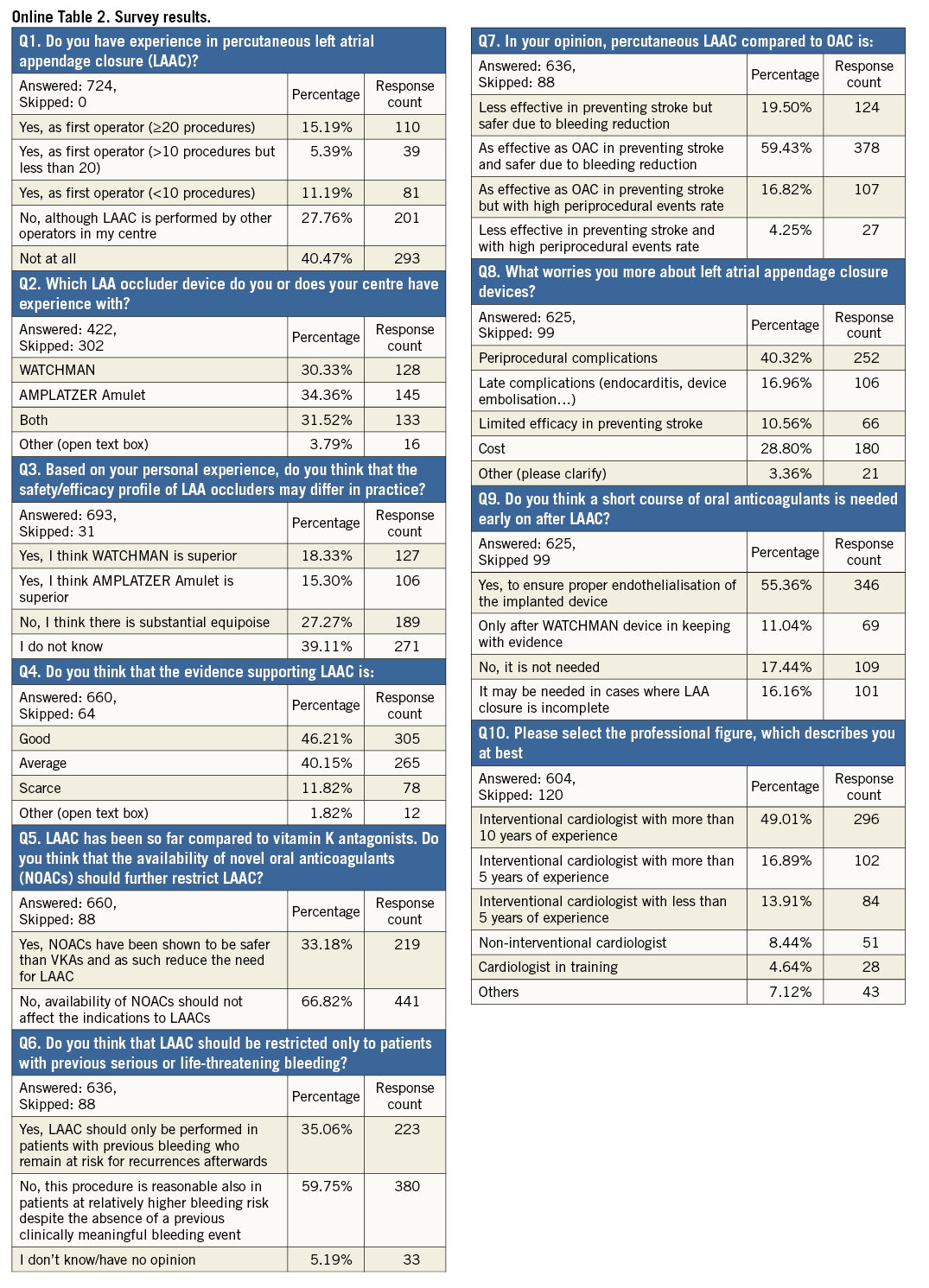
The majority of responders replied yes to this question, with 31.7% having performed LAAC as first operator and 27.8% working in centres offering percutaneous LAAC; 40.5% of participants declared no experience with LAAC (Figure 1, Online Table 2). Procedural expertise varied across age groups, with 22.2% of first operators younger than 40 years compared with 40.7% among more senior (>50 years old) responders (p<0.0001).
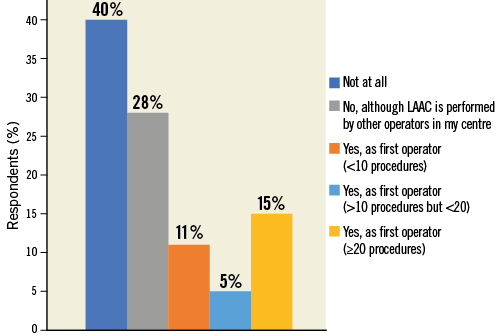
Figure 1. Question 1: Do you have experience in percutaneous left atrial appendage closure (LAAC)?
Twice as many European, as compared to non-European, respondents declared having first operator LAAC procedural experience (44.8% vs. 22.1%, p<0.0001), and non-European participants more frequently reported working in centres where LAAC is not an established treatment option (European vs. non-European: 24.4% vs. 52.9%, p<0.0001) (Figure 2, Online Figure 3).
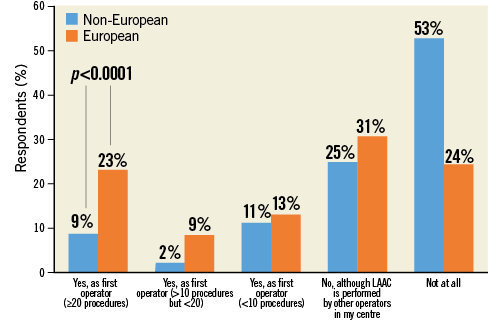
Figure 2. Question 1 stratified by region.
Q2. Which LAA occluder device do you or does your centre have experience with?
This question surveyed personal or centre-specific experience with different LAAC devices: in particular, respondents had to choose among WATCHMAN, Amulet, both or other devices (using a free text field). Overall experience with Amulet and WATCHMAN was similar at 34.4% and 30.3%, respectively. Approximately one third of respondents declared having experience with both devices. Among the small proportion of those who chose “other devices” (3.8%), the most frequently indicated choice was the AMPLATZER Cardiac Plug (St. Jude Medical), i.e., the first-generation AMPLATZER™ LAAC device (Figure 3, Online Table 1). Not surprisingly, device penetration varied across countries, with the use of Amulet (resulting by combining declared experience with Amulet device only or “both”) comprising 22.8% for non-European respondents versus 56.7% among European respondents (p<0.0001).
Figure 3. Question 2: Which LAA occluder device do you or does your centre have experience with?
Q3. Based on your personal experience, do you think that the safety/efficacy profile of LAA occluders may differ in practice?
The majority of respondents selected the “I do not know” answer (39.1%), followed by those who considered the two available devices equivalent (27.3%). Approximately 18% and 15% of participants stated a preference for WATCHMAN or Amulet, respectively (Figure 4, Online Table 1). There was a significant relationship (p<0.0001) between perceived device superiority and prior procedural experience in that WATCHMAN and Amulet were considered superior by those operators disclosing selective prior experience with the WATCHMAN and Amulet device, respectively (Figure 5). European respondents were more likely than their non-European colleagues to judge the clinical performance of these two devices as equivalent (European vs. non-European: 62.7% vs. 37.3%, p=0.008). There was no detectable age effect for this answer.
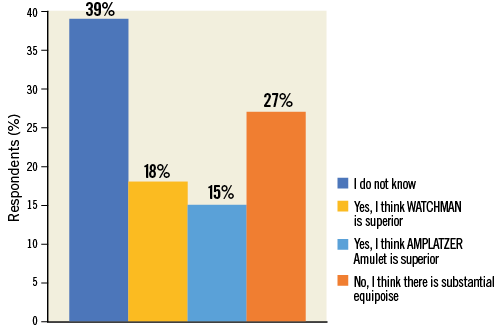
Figure 4. Question 3: Based on your personal experience, do you think that the safety/efficacy profile of LAA occluders may differ in practice?
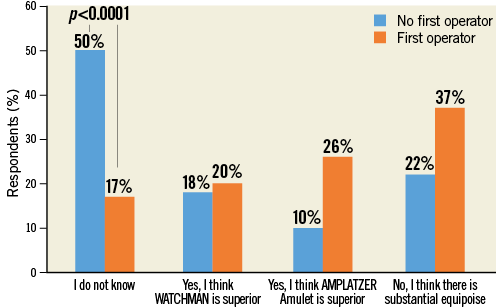
Figure 5. Question 3: stratified by experience as operator.
Q4. Do you think that the evidence supporting LAAC is:
Many responders regarded evidence in support of LAAC as “Good” (46.2%) or at least “Average” (40.1%), and 11.8% of responders considered it “Scarce” (Figure 6, Online Table 1). Prior first operator LAAC experience affected the answers to this question, leading to higher satisfaction rates in favour of available evidence (“Good” choice in 57.3% vs. 40.6% of responses, p<0.0001) (Figure 7). On the other hand, no prior direct first operator experience was more frequently associated with the “Scarce” choice (no first operator vs. first operator: 14.1% vs. 7.3%, p=0.011). There were no differences among age groups or countries.
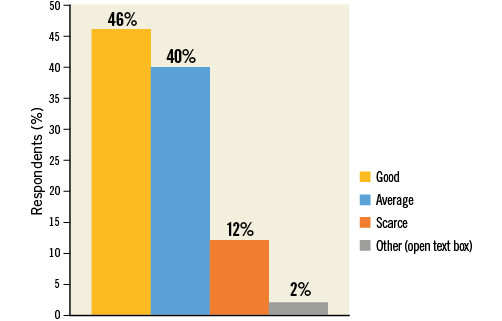
Figure 6. Question 4: Do you think that the evidence supporting LAAC is:
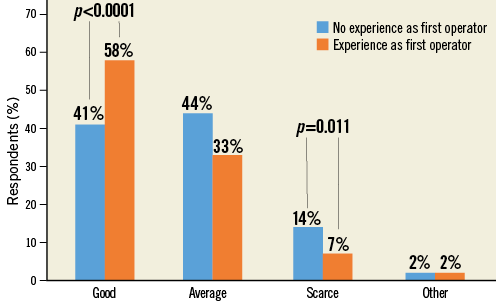
Figure 7. Question 4 stratified by experience as operator.
Q5. LAAC has been so far compared to vitamin K antagonists. Do you think that the availability of NOACs should further restrict LAAC?
The large majority (66.8%) of respondents thought that the growth of LAAC is and will not be restricted by the availability of NOACs (Figure 8, Online Table 1). This answer was consistent with replies obtained for previous questions: responders who deemed quality of evidence for LAAC “Good” or those with prior first operator experience were confident that NOACs should and will not restrict indications for LAAC (“Good” vs. “No good” in Q4: 80% vs. 55.5%, p<0.0001; first operator vs. no first operator: 75.3% vs. 62.6%, p<0.0001).
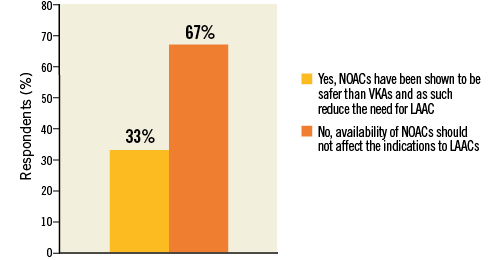
Figure 8. Question 5: LAAC has been so far compared to vitamin K antagonists. Do you think that the availability of non-vitamin K oral anticoagulants (NOACs) should further restrict LAAC?
Q6. Do you think that LAAC should be restricted only to patients with previous serious or life-threatening bleeding?
Almost 60% of participants responded that LAAC is a reasonable treatment option also in patients without history of severe bleeding as long as they are deemed at high bleeding risk (Figure 9, Online Table 1). Those with prior first operator experience and those who judged NOACs’ availability of no concern regarding current or future indications for LAAC were more likely to answer LAAC as bleeding primary prevention treatment option (first operator vs. no first operator: 67.1% vs. 53%, p=0.002; “No, …” vs. “Yes, …” in Q5: 70.5% vs. 38.2%, p<0.0001).
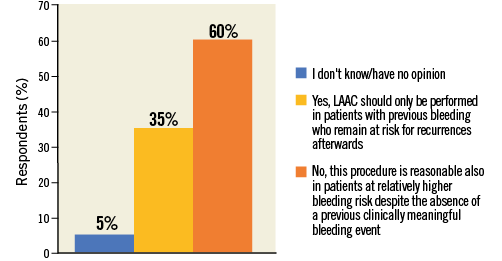
Figure 9. Question 6: Do you think that LAAC should be restricted only to patients with previous serious or life-threatening bleeding?
Q7. In your opinion, percutaneous LAAC compared to OAC is:
The majority of respondents (59.4%) stated that LAAC is “as effective as OAC in preventing stroke and safer due to lower bleeding risks”. The other participants were almost equally divided among those who thought that LAAC is “less effective in preventing stroke but safer due to bleeding reduction” (19.5%), and those who considered LAAC “as effective as OAC in preventing stroke but with high periprocedural events rate” (Figure 10, Figure 11, Online Table 1).
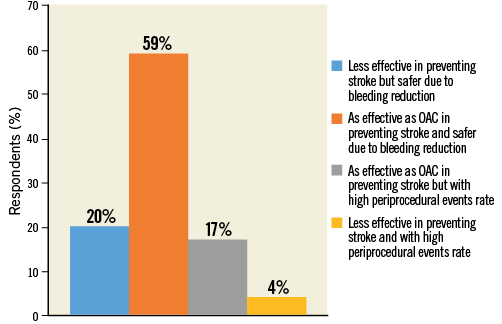
Figure 10. Question 7: In your opinion, percutaneous LAAC compared to OAC is:
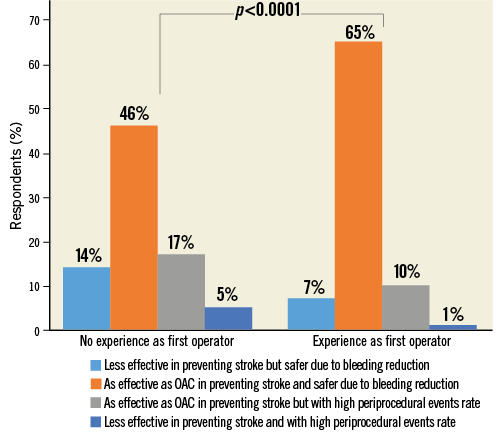
Figure 11. Question 7 stratified by experience as operator.
Q8. What worries you most about LAAC devices?
Respondents were most concerned about procedural complications of LAAC (40.3%), followed by device cost (28.8%) (Figure 12, Online Table 1). Those who considered available evidence around LAAC “Scarce” in Q4 were more prone to believe that LAAC has limited efficacy in stroke prevention (“Limited efficacy in preventing stroke” vs. no “Limited efficacy in preventing stroke” in Q4: 24.7% vs. 8.7%, p<0.0001). Operators were more frequently worried about procedural complications than non-operators (47.4% vs. 36.6%, p=0.009). Finally, a greater number of non-European respondents indicated “Cost” of devices as a major issue compared with European respondents (40.2% vs. 19.2%, p<0.0001).
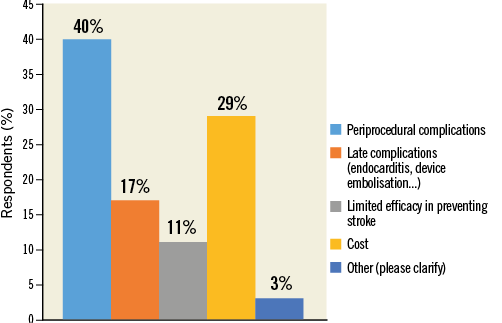
Figure 12. Question 8: What worries you more about left atrial appendage closure devices?
Q9. Do you think a short course of OAC is needed early after LAAC?
The majority of participants (55.4%) believed that there is a rationale for OAC to prevent thrombosis after the procedure prior to endothelialisation of the implanted device (Figure 13, Online Table 1). However, operators who declared direct experience with LAAC procedure (any device) more frequently responded that OAC early after LAAC was not needed. This remained consistent when stratified according to the device used (operators vs. non-operators: any device 31.5% vs. 10.2%, p<0.0001; WATCHMAN: 22.6% vs. 9%, p=0.044; Amulet: 34% vs. 25%, p=0.279; both devices: 37.7% vs. 4%, p<0.0001). Moreover, in the subgroup of first-operator respondents, those with prior selective experience with WATCHMAN answered more frequently “Yes, to ensure proper endothelialisation of the implanted device” compared to physicians selectively experienced with Amulet (56.6% vs. 17.6%, p<0.0001).
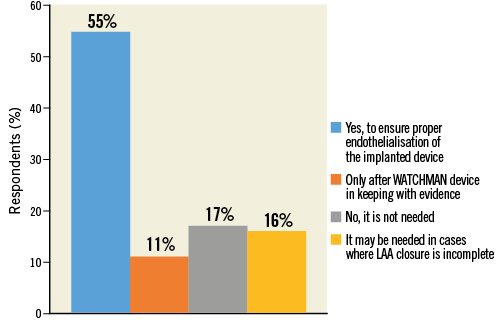
Figure 13. Question 9: Do you think a short course of oral anticoagulants is needed early on after LAAC?
Interpretation of the survey results
This survey confirms confidence in percutaneous LAAC, with more than seven out of ten respondents belonging to the interventional cardiology community thinking that the availability of NOACs should not limit the use of LAAC devices, despite a lack of randomised data comparing these two strategies of stroke prevention. Moreover, the majority of respondents declared that LAAC is as effective as OAC in preventing stroke and safer due to reduced bleeding risk (59.4%). Only a very small proportion of practitioners (4.3%) evaluated the safety/efficacy profile of these devices negatively (“less effective - compared to OAC - and with higher periprocedural events rate”).
Along the same lines, a large proportion of participants (86%) considered the scientific evidence for LAAC to be at least “average”, of whom 46% considered it “Good”. Although two randomised trials have confirmed the safety and efficacy of the WATCHMAN device9,10, only observational data exist for the Amulet device at present. While many respondents expressed uncertainty regarding the comparative effectiveness of Amulet versus WATCHMAN, one in three believed that the data supporting the use of WATCHMAN may be safely applied to the Amulet device.
The surveyed community expressed concerns regarding the occurrence of periprocedural complications (40% of respondents). The cost of the device was also raised as a potential limitation to wider use of this treatment modality, particularly outside European countries, with four out of ten in this group underlining this issue.
Finally, the community appeared uncertain regarding the need for a short course of OAC after LAAC.
It may be considered surprising that the large majority of respondents believed the availability of NOAC not to be a restriction to the future growth of LAAC procedures, despite established evidence on the improved safety/efficacy profile of NOAC compared to VKA. Likewise, Amulet utilisation was found to be high, in particular in Europe, where it was the most used device, although Amulet itself has not yet been validated in randomised clinical trials. Conversely, the concerns of physicians on periprocedural complications were expected.
Limitations
This survey has important limitations, which should be carefully weighed when interpreting the results. First, only a small percentage of invited practitioners took part in it. Therefore, the results are not necessarily representative of the opinion of the broader community. However, a low participation rate is a common limitation of surveys in general, in particular when the population targeted is that of professionals at an advanced career stage. Second, there is probably a selection bias towards respondents positively predisposed to the use of LAAC devices. Third, the respondents from European countries were more often first operators than those from non-European countries. Thus, the two sets of participants were somewhat different.
Conclusions
Despite concerns regarding the potential for periprocedural complications and increased device cost, the surveyed interventional cardiology community was predominantly in favour of LAAC as an effective and safer alternative to OAC for stroke prevention in patients with atrial fibrillation at high bleeding risk, regardless of a prior bleeding history. There was no consensus as to whether a short course of OAC is necessary after LAAC implantation nor regarding the comparative effectiveness of the two currently most used LAA occluders in practice.
| Impact on daily practice The balance between bleeding and ischaemic risk, especially in an aged and frail population, remains an unmet clinical need. Ischaemic stroke is a serious and disabling disease and it has a major impact on survival as well as on public health and costs. Similarly, bleeding events have a negative prognostic impact on mortality, morbidity and quality of life. LAAC has emerged as a viable and potentially growing treatment option in patients with atrial fibrillation at high bleeding risk. The need for risk mitigation processes regarding procedural complications and costs were the most frequently raised concerns. Lack of randomised data in support of the Amulet device or lack of comparative effectiveness data versus NOAC does not seem to undermine the value of LAAC in practice in the opinion of the respondents. |
Acknowledgements
The authors would like to express their gratitude to the staff of Europa Organisation/EuroPCR for the support given during the survey development.
Conflict of interest statement
R. Colleran reports support from the Irish Board for Training in Cardiovascular Medicine sponsored by Merck Sharp & Dohme (MSD). R. Byrne reports receiving institutional research grants from Boston Scientific and HeartFlow and lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
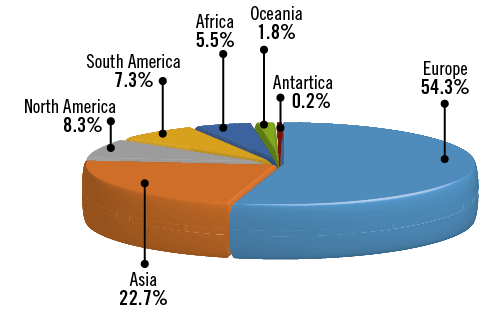
Online Figure 1. Region of work.
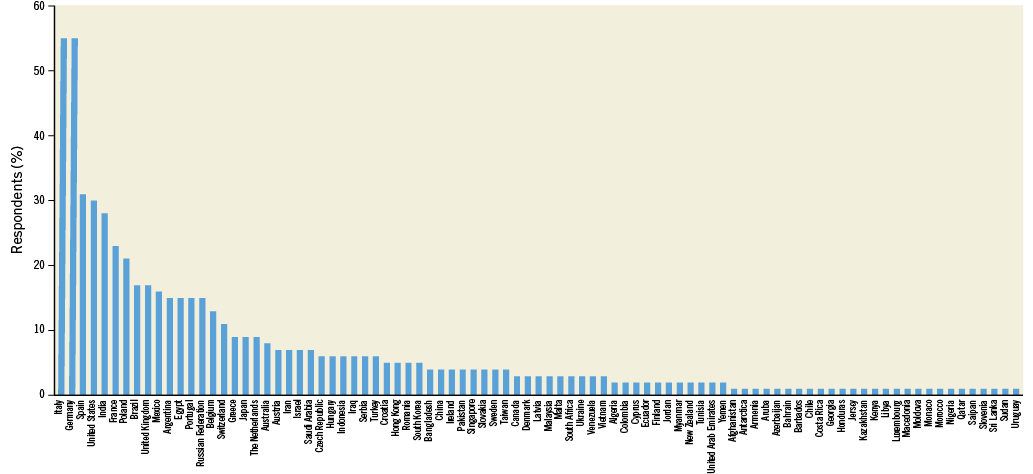
Online Figure 2. Country of work.
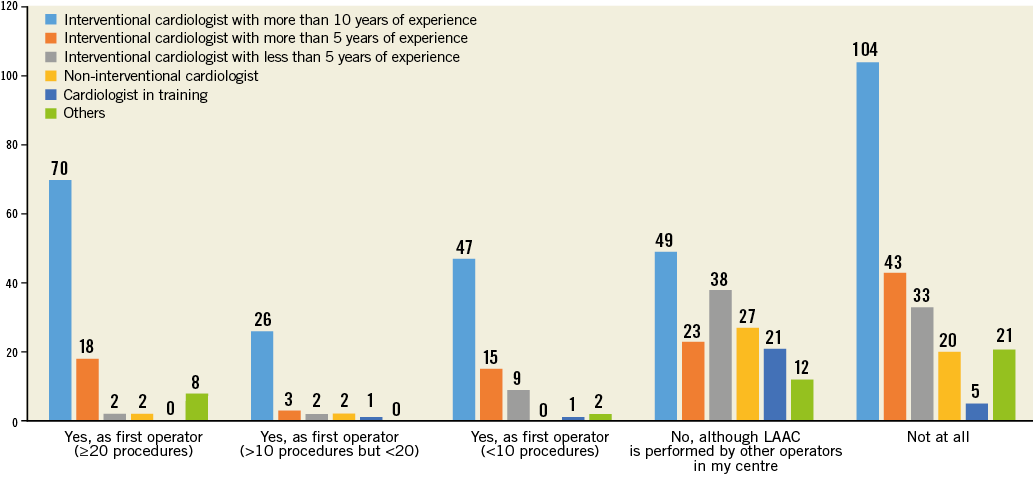
Online Figure 3. Question 1 stratified by professional figure.

