Abstract
The increasing interest in left atrial appendage occlusion (LAAO) for ischaemic stroke prevention in atrial fibrillation (AF) fuels the need for more clinical data on the safety and effectiveness of this therapy. Besides an assessment of the effectiveness of the therapy in specific patient groups, comparisons with pharmacological stroke prophylaxis, surgical approaches and other device-based therapies are warranted. This paper documents the consensus reached among clinical experts in relevant disciplines from Europe and North America, European cardiology professional societies and representatives from the medical device industry regarding definitions for parameters and endpoints to be assessed in clinical studies. Adherence to these definitions is proposed in order to achieve a consistent approach across clinical studies on LAAO among the involved stakeholders and various clinical disciplines and thereby facilitate continued evaluation of therapeutic strategies available.
Introduction
Left atrial appendage occlusion (LAAO) is a device-based therapy for stroke prevention in patients with non-valvular atrial fibrillation (AF), which continues to evolve. Important issues remain to be clarified including the outcome and safety of this local site-specific therapy versus systemic anticoagulant therapy, comparison of the multiple approaches being studied, the specific patient population and risk benefit ratio in these populations as well as the long-term follow-up. These clinical initiatives will benefit from standardisation of definitions that will enhance the ability to make meaningful comparisons of the safety and efficacy of the diverse approaches available.
The present document is the output of a two-day consensus conference that was organised on 28-29 August 2014 in Munich, Germany. It is complementary to the EHRA/EAPCI consensus document1 by providing definitions for the parameters and characteristics assessed for LAAO and other stroke prevention therapies compared with LAAO. Within the field of interventional cardiology, the consensus documents published by the Valve Academic Research Consortium (VARC)2,3 contributed significantly to the use of consistent definitions for research purposes. Where meaningful, these definitions have been adopted within this document, with modifications relevant to specific aspects of LAAO, such as venous access and transseptal puncture.
Atrial fibrillation, stroke and left atrial appendage occlusion
In a typical cohort of non-treated non-valvular AF patients, the annual rate of ischaemic stroke is approximately 5%, although much higher risk populations for thromboembolism and for bleeding can be identified using risk scores such as CHA2DS2-VASc (or CHADS2) and HAS-BLED4. Oral anticoagulation (OAC) with vitamin K antagonists (VKA) or non-VKA oral anticoagulants (NOAC) has been demonstrated to reduce significantly this risk of stroke or systemic embolism by more than 60%5,6. However, VKA therapy is associated with clinically relevant bleeding4,5. NOACs less frequently result in OAC-associated life-threatening bleeding6, but major bleeding may not be less than with VKA therapy, and gastrointestinal bleeding has often been more pronounced with NOACs, which therefore may not be the preferred therapy for AF patients with a high bleeding risk. The overall bleeding risk as a drug class may be lower with NOACs compared to warfarin, but it is not zero. Moreover, other AF patients have absolute contraindications to pharmacological stroke prophylaxis or may suffer a systemic thromboembolisation event despite adequate OAC accounting to “failed therapy”. The finding that 91% of thrombi in this setting originate in the left atrial appendage (LAA)7 constitutes the rationale for stroke prevention by exclusion of the LAA as applied using several therapeutic approaches. Surgical approaches include the total excision of the LAA or exclusion by ligation or stapling8,9 as well as epicardial clips applied to close the LAA after obtaining access by sternotomy or less invasive thoracoscopic approaches10,11. While these surgical approaches are applied with variable success, they are highly invasive techniques, and particularly surgical excision or exclusion is done concomitantly along with surgical AF ablation, valve repair/replacement or coronary artery bypass grafting.
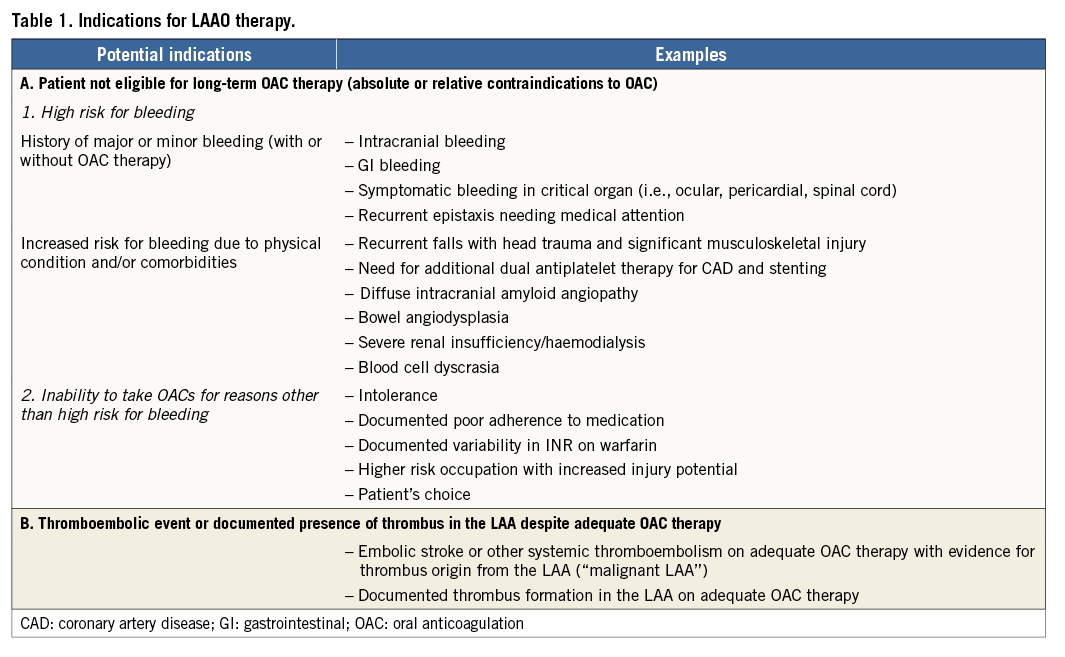
While percutaneous LAAO was initially developed to replace OAC, in Europe and most recently in North America it is currently considered for non-pharmacological stroke prevention in AF patients in whom long-term OAC is not considered a first-choice therapy12-15. The ESC guidelines for the management of AF16 recommend that interventional, percutaneous LAA closure may be considered in patients with a high stroke risk and contraindications to long-term oral anticoagulation (class IIb, level B). Surgical excision of the LAA may be considered concomitantly in AF patients undergoing open heart surgery (class IIb, level C). The same recommendations are included in the ESC/EACTS guidelines on myocardial revascularisation with respect to patients with AF undergoing percutaneous coronary intervention or coronary artery bypass grafting17. The US guidelines do not supply any recommendation because until very recently none of the LAAO devices had been approved in the USA. In March 2015, the Food and Drug Administration (FDA) announced the approval of the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA)18. The FDA stated that the WATCHMAN device is indicated to reduce the risk of thromboembolism from the LAA in patients with non-valvular AF who: (1) are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy, (2) are deemed by their physicians to be suitable for warfarin, and (3) have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin. Noteworthy, while all randomised studies so far have included patients eligible for warfarin therapy, European registries and common sense in the panels have led to considering this option mainly for patients with absolute or relative contraindications to warfarin. Nevertheless, at the moment, there is no scientific consensus on the definitions of absolute or relative contraindications to OAC therapy for patients with AF, so the exact indications for LAAO have yet to be clarified19. Acknowledging this fact, potential indications for LAAO therapy and some common examples are provided in Table 1.
Percutaneous LAAO encompasses occluding the LAA with a mechanical device through a catheter-based, transseptal approach or ligating the LAA through a combined strategy requiring transvenous, transseptal and transpericardial access. Patient cohorts, treated with this therapy, have stroke rates lower than expected based on their risk factors15,20, confirming the role of the LAA as the predominant origin of atrial thrombi. The randomised controlled PROTECT AF trial21 demonstrated the non-inferiority of LAAO with the WATCHMAN device compared to dose-adjusted warfarin therapy in the prevention of ischaemic stroke, systemic embolism and cardiovascular death. At a longer-term follow-up (3.8 years) of the study cohort, there was evidence of superiority in cardiovascular and all-cause mortality in comparison to warfarin22. Patients in this study received warfarin until appropriate LAA occlusion was confirmed and device-related thrombus excluded by transoesophageal echocardiography (TEE) at 45 days after implantation. The randomised controlled PREVAIL study23 failed to show the non-inferiority of LAAO with the WATCHMAN device for overall efficacy. However, event rates in the control group were lower than expected, and LAAO was non-inferior to warfarin for ischaemic stroke or systemic embolism prevention >7 days after device implantation. Moreover, the study showed that the WATCHMAN device could be safely implanted by new operators.
Most common complications related to LAAO therapy are cardiac perforation, pericardial effusion, tamponade, device embolisation, systemic thromboembolism and injury related to vascular access24. Despite higher initial procedural complications, operators showed a positive learning curve in the implantation of the LAAO device25-27, with a significant reduction of complication rates to 2-3%26.
Recently, a hybrid approach for epicardial LAA ligation has been introduced, combining transcatheter endocardial techniques and epicardial access by minimal invasive surgery28,29. While initial results showed the feasibility and safety of this technique, limited early experience similar to the other LAAO devices is going through a similar learning curve with a slightly higher rate of bleeding and cardiac tamponade in small series of patients reported in retrospective studies30. The efficacy and safety of this technique has yet to be fully established in larger multicentre randomised controlled studies or registries. This is particularly important for devices that have not yet been tested in randomised control trials.
Mortality
A meaningful assessment of mortality associated with LAAO should address the timing relative to the index procedure as well as the underlying causes. Mortality definitions provided in Table 2 are based on the definitions included in the VARC-2 consensus3. For consistency and comparability with other studies, the traditional definition of procedural mortality should refer to the periods between implantation and hospital discharge or between implantation and 30-day follow-up.
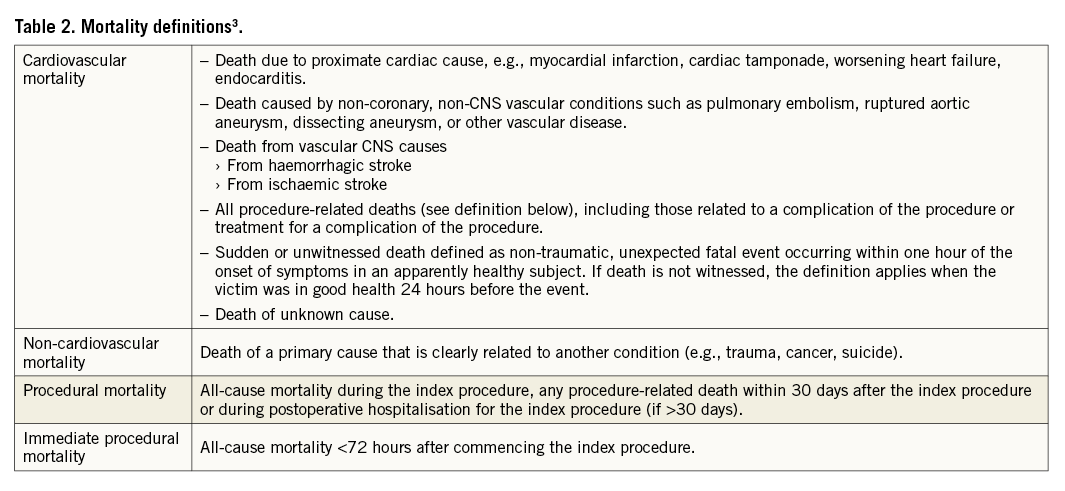
With respect to the cause of death, all-cause mortality is subdivided into cardiovascular and non-cardiovascular mortality. By conservative approach, sudden or unwitnessed death and any death of unknown cause are classified as cardiovascular death. LAAO studies should report on all three categories of mortality, defined in Table 2.
Stroke and transient ischaemic attack and peripheral embolism
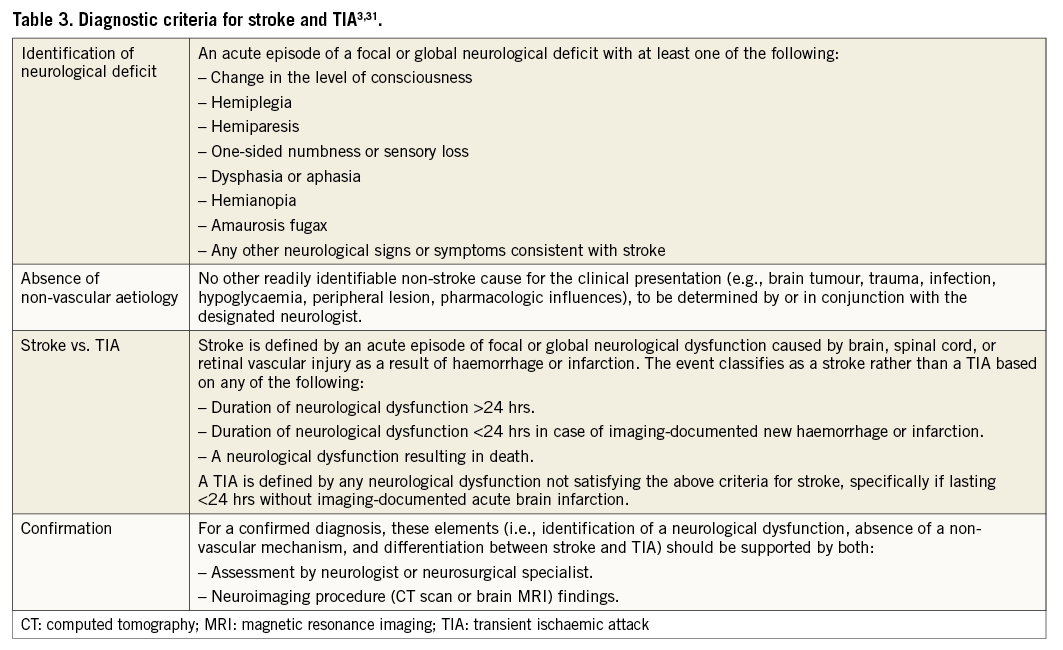
Stroke is defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of haemorrhage or infarction. A transient ischaemic attack (TIA) should be clearly distinguished from ischaemic stroke, based on focal neurological symptoms lasting <24 hours and imaging-confirmed absence of acute brain infarction. Therefore, it is mandatory to recommend imaging confirmation as part of the diagnosis of TIA. Stroke assessment requires a neuroimaging and neurological examination, preferably by a neurologist. Although in registry studies such extensive diagnostics may not be feasible, strokes should minimally be adjudicated by a neurologist based on written information.
An overview of diagnostic criteria for stroke and TIA is provided in Table 3.
Infarction of the central nervous system (CNS) is defined as cerebral, spinal cord or retinal cell death attributable to ischaemia, based on:
– Pathological, imaging, or other objective evidence of cerebral, spinal cord or retinal focal ischaemic injury in a defined vascular distribution, or;
– Neuroimaging (CT or MRI) evidence of cerebral, spinal cord, or retinal focal ischaemic injury, or;
– Clinical evidence of cerebral, spinal cord, or retinal focal ischaemic injury based on acute onset symptoms persisting ≥24 hours, imaging excluding brain haemorrhage, and other aetiologies excluded.
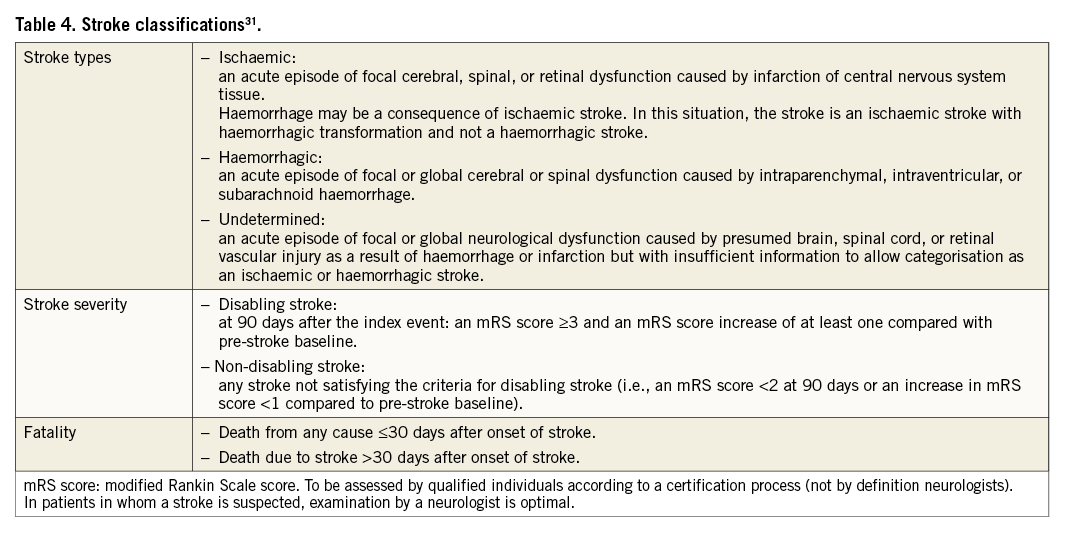
Strokes should be classified according to the definitions provided by the Clinical Data Interchange Standards Consortium (CDISC)31, as listed in Table 4.
Cognitive function assessment
Assessment of cognitive function should be considered before, shortly after and during long-term follow-up of patients undergoing LAAO procedures.
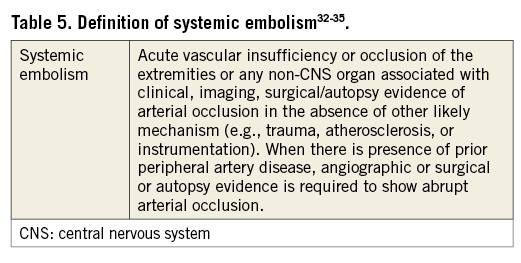
Systemic embolism
Although trials on VKA and NOAC therapies32-35 as well as on LAAO14,19,21,23,36 have applied systemic embolism as a primary endpoint for effectiveness, definitions have been variable and inconsistent.
The definition provided in Table 5 is composed from definitions applied by several trials on VKA and NOAC therapies and is proposed for all patients enrolled in device or drug arms of LAAO studies.
Additional details with regard to thromboembolic events
To understand better the aetiology of stroke and systemic embolism, studies on LAAO should document and report on all relevant procedural conditions, such as antithrombotic therapy, timing, extent and target ACT of heparinisation, the occurrence of air embolism, catheter and/or device exchanges during the procedure, and the duration of the procedure.
In case of stroke or systemic embolism, all studies of any type should require the following to be performed as soon as possible after the event:
– full neurological examination;
– imaging (CT or MRI of the brain);
– TEE to identify potential embolic sources.
In studies comparing a device therapy with pharmacological treatment, the above examinations should be performed in both study arms.
Device-related aspects to be assessed by TEE following an ischaemic event include thrombus on the device and peri-device leaks. Besides event-triggered TEE, regular TEE is recommended in all patients, with and without events, to monitor the device status and presence of thrombus or leaks and evaluate their clinical significance. Studies should obtain an appropriate baseline neurological assessment to allow comparison with post-event neurological evaluation.
Pericardial effusion/tamponade
Pericardial effusion with or without tamponade is a potentially severe complication of endocavitary cardiac catheterisation; classification of their severity within the context of LAAO would benefit from a more detailed and consistently applied definition. Therefore, a definition based on the actual treatment is proposed. Acknowledging the fact that, in current clinical practice, pericardiocentesis is not considered a critical, high-risk intervention per se, the definitions listed in Table 6 arise.
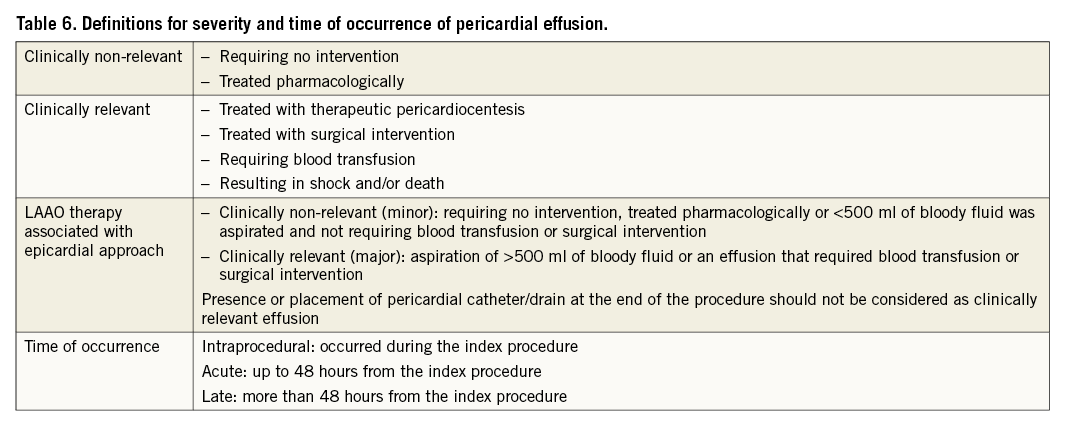
All patients should have a baseline echocardiogram. LAAO studies should report on all pericardial effusions with severity classified according to the definitions in Table 6, and specify effusions with tamponade as a subgroup. Of note, the qualification of the event as a major complication does not depend on the presence of tamponade.
Bleeding
In the currently most comprehensive definitions of bleeding associated with cardiovascular interventions, the Bleeding Academic Research Consortium (BARC)37 includes six severity categories (Type 0 to 5). In an update of their endpoint definitions for transcatheter aortic valve implantation3, the VARC decided to maintain the original severity categories of life-threatening, major and minor bleeding2. The definitions for bleeding in an LAAO context, provided in Table 7, primarily follow the VARC-2 definitions3, with some LAAO-specific modifications and refinements, and cross-reference to the types of bleeding defined by the BARC (i.e., in contrast to VARC-2, BARC 3a is never considered minor bleeding).
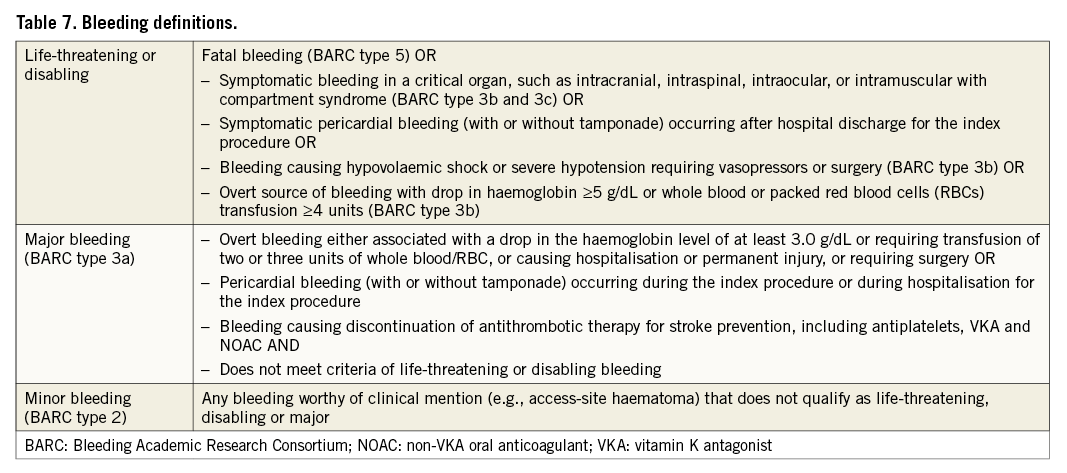
Pericardial bleeding is the most common complication of LAAO. When pericardial bleeding occurs during the index procedure or before hospital discharge for the index procedure and is treated with therapeutic pericardiocentesis without sequelae, it is not considered life-threatening or disabling bleeding but only major bleeding. However, symptomatic pericardial bleeding after hospital discharge (with or without clinical tamponade) is considered life-threatening. Pericardial effusion, including haemorrhagic effusion, should be classified as a device-specific complication according to Table 6. Consistent with the consensus published by the International Society on Thrombosis and Haemostasis38, asymptomatic bleeding is not considered life-threatening, even if it occurs in a critical organ. As a result, asymptomatic pericardial bleeding as an incidental finding from imaging is not classified as life-threatening. By its impact on stroke prevention in high-risk patients, bleeding that leads to a physician’s decision to discontinue pharmacological stroke prophylaxis should be considered a major event. The definitions in Table 7 are adequate for all types of occlusion devices (endocardial and epicardial) and can also be applied to subgroups receiving pharmacological therapy.
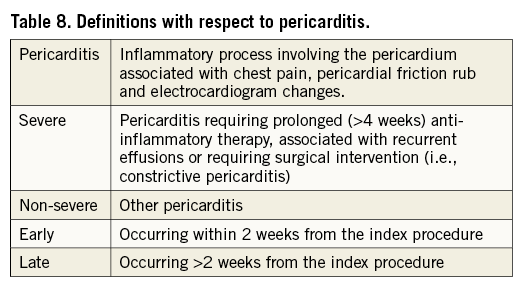
Pericarditis
Pericarditis may occur as a result of a cardiac intervention, particularly when using an epicardial approach. Table 8 provides definitions with respect to pericarditis that should be applied in comparative studies on LAAO and other LAA-targeted therapies.
Myocardial infarction
Endoluminal occlusion of the LAA does not usually cause tissue necrosis of the LAA. In contrast, epicardial closure, either device-based or surgical, may result in myocardial necrosis. This should be differentiated from necrosis due to a myocardial infarction. Epicardial closure-related necrosis may cause enzyme elevation, but does not result in ischaemia, typical ECG changes and regional wall motion abnormalities. Elevated cardiac enzymes and abnormal ECG related to the necrosis of the LAA after the epicardial technique should not be considered as MI in the absence of an acute coronary cause. Overall, the standard definitions of MI3,39 should be used for cohort studies on LAAO as well as trials comparing LAAO with other options for stroke prevention.
Access-related complications
Complications associated with obtaining vascular access are an important category of procedural complications of LAAO device implantation. A definition of these complications should include all adverse events that are directly or indirectly related to any of the vascular access sites (venous and arterial) used during the procedure. The events listed in Table 9 are considered vascular access-related complications. Of note, some of these events also qualify as bleeding and should be reported in both categories. Although for some of the events in Table 9 other causes cannot be excluded, their occurrence within seven days after the procedure most likely qualifies them as access-related. Additional definitions for access-related complications associated with epicardial and/or minimally invasive surgical approaches are provided in Table 10.
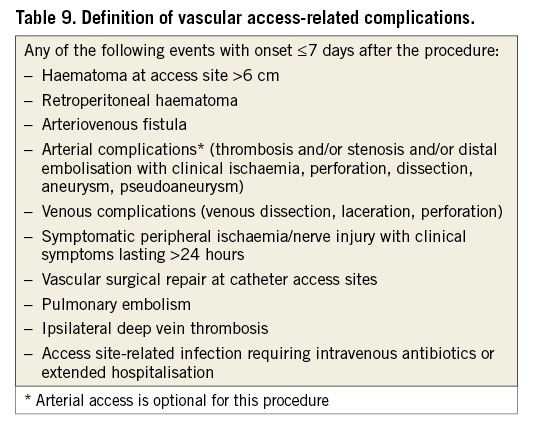
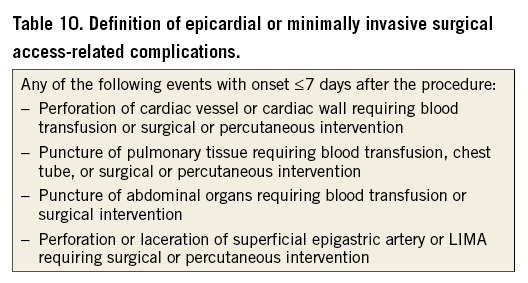
Any of the events listed in Table 9 and Table 10 that occur >7 days post procedure are not considered access-related. Consistent with the VARC-2 consensus3, vascular complications that are not related to the access site should be reported separately as non-access-related vascular complications. These may include events within and outside of the seven-day procedural window.
Renal and hepatic injury
The use of contrast medium for angiography and/or cardiac CT prior to or during an interventional procedure may constitute a renal or hepatic burden. In this context, it should be emphasised that severe renal or hepatic insufficiency is a contraindication to VKA or NOAC, and consequently may be a reason to consider device-based LAAO. For classification of acute kidney injury, the definitions of the Acute Kidney Injury Network (AKIN)40 that are included in the VARC-2 consensus3 are adopted (Table 11).
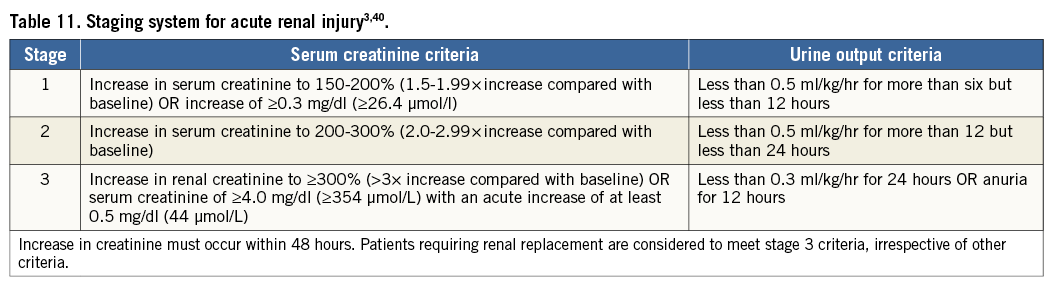
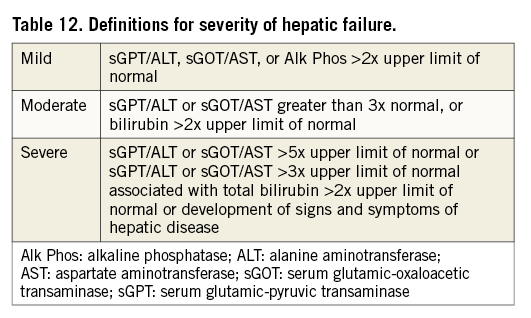
For classification of hepatic failure, the alert levels defined for the RE-LY trial, comparing dabigatran with warfarin for stroke prevention in AF patients41, are considered appropriate (Table 12).
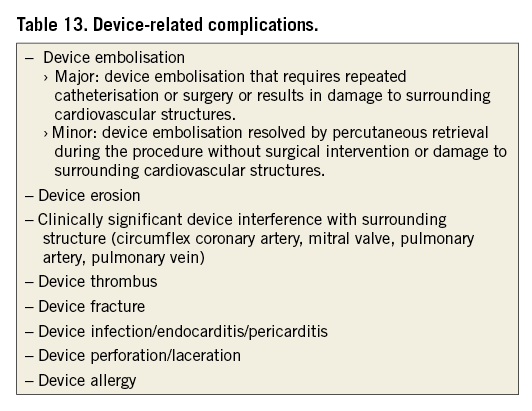
Device-related complications
Essentially, all complications that are a result of the presence of the device should be considered device-related complications. Table 13 specifies the device-related complications relevant to LAAO by endocardial or epicardial devices. Regarding device embolisation, surrounding cardiovascular structures include those in the vicinity of the implant location (circumflex coronary artery, mitral valve, pulmonary artery, pulmonary vein) and any cardiovascular structures at the location to which the device migrated. Of note, a residual leak is considered an efficacy issue, rather than a device-related complication.
LAA occlusion and residual leaks
Effective LAA occlusion, i.e., elimination of the LAA as a thromboembolic source, is the primary technical objective of an LAAO procedure, irrespective of whether the occlusion is achieved from the endocardium or epicardium. Residual leaks have been observed after surgical LAA exclusion, endocardial LAAO and epicardial LAA closure. Although incomplete surgical LAA ligation is a common observation, its clinical significance is unclear42. It has been hypothesised that the creation of a small communication between the LAA and the LA causes local stagnation of blood flow42. This would result in a thrombogenic source with similar risk compared with the initial situation. A similar mechanism would apply to incomplete epicardial LAA closure by minimally invasive techniques.
In the PROTECT AF study21, LAA occlusion was evaluated by TEE at 45 days after implantation, and complete closure or a leak represented by a jet <5 mm in diameter was a condition for warfarin discontinuation. The criterion of 5 mm was based on results reported from surgical LAA exclusion, being the only relevant data available when the study was designed. Similarly, the PREVAIL trial23 considered adequate LAA sealing characterised by a jet <5 mm, while other studies36,43 defined a jet <3 mm as a mild or small leak. A study on the clinical impact of residual leaks44 did not find a significant effect of either the existence of a leak or its size on the composite endpoint of all-cause stroke, systemic embolism and cardiovascular or unexplained death. However, authors emphasised that the low event rate requires a larger sample to draw definite conclusions. Despite the existence of residual leaks in the PROTECT AF cohort, LAAO was demonstrated to be non-inferior to warfarin21 and resulted in a statistically significant improved clinical outcome compared to warfarin at long-term follow-up25. Residual flow is not an uncommon finding after LAA exclusion, irrespective of the applied approach. As its clinical significance is still poorly understood, any criterion to classify the size of the residual leak appears to be highly arbitrary. Therefore, the current consensus is to assess this parameter in studies on any type of LAA exclusion following a consistent methodology, outlined in Table 14.
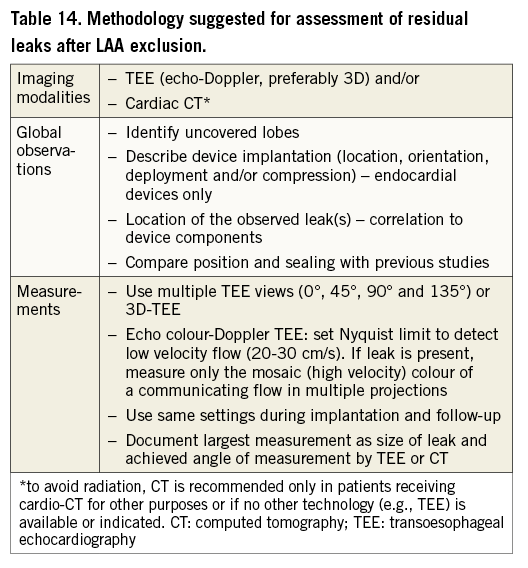
Studies should report on the distribution of the size of residual leaks. In addition, relevant clinical endpoints, such as ischaemic and all-cause stroke, systemic embolism and cardiovascular or unexplained death should be stratified with respect to the presence and size of leaks. Until the clinical significance of residual leaks has been clearly revealed, use of the term “complete closure” seems only justified in case of complete absence of residual flow.
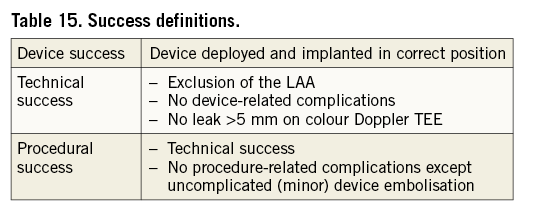
Device, technical and procedural success
Table 15 provides definitions of device, technical and procedural success, consistent with most LAAO studies reported so far. Correct device position, as an aspect of device success, is to be assessed as soon as possible after release of the device from its delivery system and accounting for the manufacturer’s recommendations for implantation. This assessment should also address the device stability, for instance verified by applying gentle traction to the device before release45.
Antithrombotic therapy post procedure
Antithrombotic therapy after LAAO varies and may include OACs (VKA or NOAC), antiplatelet drugs (aspirin, clopidogrel, etc.) single or combination, for the short term or for life, or no treatment. This depends on the device instructions for use, the patient history, the indication for LAAO, the presence of significant residual leaks, etc. For example, based on the results of the PROTECT AF trial, warfarin is prescribed for 45 days after LAAO with the WATCHMAN device (and until a TEE confirms the absence of significant leak)21, whereas based on solely empirical data LAAO with Amplatzer devices is followed by dual antiplatelet therapy for one to three months14. Studies should report data on antithrombotic therapy post procedure in detail, including the duration of therapy, the doses and any potential changes at follow-up.
Summary/conclusions
Several studies have shown the safety and efficacy of LAAO for stroke prevention in AF patients who are contraindicated or less suited to long-term oral anticoagulation. In order to explore further and demonstrate the potential of this therapy, additional clinical evidence is required. This document proposes a consistent approach in the assessment and reporting of clinical results by providing definitions for parameters relevant to studies on LAAO, including comparisons with other devices and with surgical or pharmacological therapies.
It is acknowledged that several definitions included in this consensus document may present physicians and their staff with challenges as to the assessment of associated clinical endpoints, particularly for stroke and TIA. However, adherence to these definitions is strongly encouraged in order to create a consistent base of evidence for development of further recommendations with regard to LAAO within the context of all therapeutic options for the prevention of stroke and embolism in AF patients and to facilitate accurate and concordant scientific studies comparing different approaches to LAAO.
Acknowledgements
The authors wish to thank D. Dorra, Y. Greipl, S. Masset (all from Boston Scientific, Marlborough, MA, USA), K. Hodgson, W. Stegink, C. Williams (all from St. Jude Medical, Plymouth, MN, USA), D. Claus (Bonn, Germany) and B.A. Albers (Warnsveld, The Netherlands) for their participation in the consensus meeting and/or the preparation and review of this consensus document.
Funding
Funding for transportation, overnight stay and venue was provided by Boston Scientific and St. Jude Medical.
Online Appendix. Participants
The following individuals participated in the consensus meeting that agreed the definitions and were involved in the review of this document.
Physicians. J. Brachman, Coburg, Germany; A.J. Camm, London, United Kingdom; H.C. Diener, Essen, Germany; S. Gafoor, Frankfurt, Germany; D.R. Holmes, Rochester, MN, USA; R. Ibrahim, Montreal, Quebec, Canada; S. Kar, Los Angeles, CA, USA; D. Lakireddy, Kansas City, KS, USA; R.J. Lee, San Francisco, CA, USA; T. Lewalter, Munich, Germany; C.E. Ruiz, New York, NY, USA; J.L. Saver, Los Angeles, CA, USA; H. Sievert, Frankfurt, Germany; K. Tiemann, Munich, Germany; A. Tzikas, Thessaloniki, Greece
Cardiology society representatives. C. Blomström-Lundqvist, Uppsala, Sweden (EHRA); R. Byrne, Munich, Germany (EAPCI); R. Cappato, Milan, Italy (ECAS); M. Näbauer, Munich, Germany (AFNET); S. Schneider, Ludwigshafen, Germany (IHF)
Clinical research organisations. O.I. Soliman, Rotterdam, The Netherlands (Cardialysis)
Industry representatives. Boston Scientific, Marlborough, MA, USA: D. Dorra, Y. Greipl , S. Masset; St. Jude Medical, Plymouth, MN, USA: K. Hodgson, W. Stegink, C. Williams
Others. D. Claus, Bonn, Germany (organisation); B.A. Albers, The Netherlands (manuscript preparation)
Conflict of interest statement
A. Tzikas: consultant, research grant, St. Jude Medical. D.R. Holmes: Mayo Clinic and D. Holmes have licensed technology for LAAO to Boston Scientific. C.E. Ruiz: consultant, research grant, Philips and St. Jude Medical. C. Blomström-Lundqvist: consultant, research grant, Medtronic, Cardiome, Boston Scientific. H-C. Diener: received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from Abbott, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, MSD, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth and Yamanouchi. Financial support for research projects was provided by AstraZeneca, GSK, Boehringer Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi-Aventis, Syngis and Talecris. The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation and Heinz-Nixdorf Foundation. H-C. Diener has no ownership interest in and does not own stocks of any pharmaceutical company. R. Cappato: consultant, research grant, Boston Scientific, Biosense Webster, St. Jude Medical, Medtronic. S. Kar: served as an advisor or consultant for Abbott Vascular and Boston Scientific, received grants for clinical research from Abbott Vascular, Boston Scientific and St. Jude Medical, and owns stock, stock options, or bonds from Coherex Medical, Inc. R.J. Lee: consultant and equity holder in SentreHEART, Inc. R.A. Byrne: reports speaker’s fees from B. Braun Melsungen, Biotronik and Boston Scientific. R. Ibrahim: consultant and proctor, St. Jude Medical. D. Lakireddy: consultant to SJM/Biosense Webster, advisory board for SentreHEART/AtriCure, speaker for Biotronik/Janssen/Boehringer Ingelheim/Pfizer. M. Näbauer: speaker honoraria, St. Jude Medical. J. Brachman: research funding, speaker’s bureau, advisory board, Boston Scientific, St. Jude Medical, Lifetech, Occlutech. J.L. Saver: is an employee of the University of California. The Regents of the University of California receive funding for J.L. Saver’s services as a scientific consultant regarding trial design and conduct to St. Jude Medical. K. Tiemann: equipment grant, Philips. S. Gafoor and H. Sievert’s institution has ownership interest in or has received consulting fees, travel expenses or study honoraria from the following companies: Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm medical, Rox Medical, Sadra, St. Jude Medical, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan. J. Camm: advisor, St. Jude Medical, Boston Scientific, Medtronic, Sorin, Bayer, Boehringer Ingelheim, Pfizer/BMS, and Daiichi Sankyo. T. Lewalter: speaker’s honorarium, St. Jude Medical, Bayer, Boehringer Ingelheim, Pfizer, Daiichi Sankyo. The other authors have no conflicts of interest to declare.

