
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and an independent risk factor for ischaemic stroke. It is estimated that AF is present in approximately 1-2% of the total population, although this number may be as high as 5-10% in those aged more than 70years. In addition, it is foreseen that the prevalence of AF will triple in the western world in the next four decades. As aconsequence, stroke prevention in AF patients is amajor public health priority.
Traditionally, vitamin K antagonists (e.g., warfarin) have been used as oral anticoagulant (OAC) therapy for stroke prevention in AF patients at increased stroke risk. In the last few years, warfarin has increasingly been replaced by direct oral anticoagulants (DOACs), which have been found to be as effective for stroke prophylaxis but cause significantly fewer major bleedings than warfarin. However, these newer DOACs also have their shortcomings, as DOACs are still associated with a2-4% occurrence of major bleedings, and more than 20% of patients are reported to discontinue their treatment even during follow-up in the large randomised DOAC trials. Hence, there is aneed for anon-pharmacological option as stroke prevention for AF patients. As the left atrial appendage (LAA) is aprominent source of cardiac emboli in patients with non-valvular atrial fibrillation (NVAF), percutaneous LAA closure may be an option in these patients. The safety and efficacy of LAA closure as stroke prevention in patients with NVAF has been investigated in afew randomised controlled trials (RCTs) and some large-scale multicentre registries.
Over the past decade, percutaneous LAA closure has slowly but gradually been picked up by the medical community following agrowing body of evidence indicating that LAA closure is asafe and effective strategy of stroke prevention in NVAF patients. However, the current indications for percutaneous LAA closure are different in the USA from those in the EU, as stated in the respective guidelines. Following the US guidelines as well as the Food and Drug Administration (FDA) approval statement, implantation of the WATCHMAN™ LAA closure device (Boston Scientific, Marlborough, MA, USA) may be considered as an alternative treatment for NVAF patients with high stroke risk who are eligible for warfarin but have an appropriate reason to seek anon-pharmacologic alternative to warfarin. This recommendation is based on two pivotal RCTs studying the safety and efficacy of the WATCHMAN LAA closure device - PROTECT AF1 and PREVAIL2. The combined five-year outcomes of both trials demonstrated that LAA closure with the WATCHMAN device provides stroke prevention in NVAF comparable to warfarin, with additional reductions in major bleeding, particularly haemorrhagic stroke, and mortality. However, in both studies, patients who received LAA closure continued warfarin for at least 45days after the procedure, as per protocol. Consequently, current US guidelines do not recommend LAA closure for patients with an absolute contraindication for (D)OAC, but only for those patients with relative contraindications for (D)OAC and those patients interested in being managed off (D)OAC (e.g., poor compliance, lifestyle decision). Applying these recommendations to the US population would categorise 1,723,700patients as being eligible for LAA closure. However, only 40,000 LAA closures have been performed so far, indicating an LAA closure adoption rate of only 2.3% in the USA until today (Figure 1).
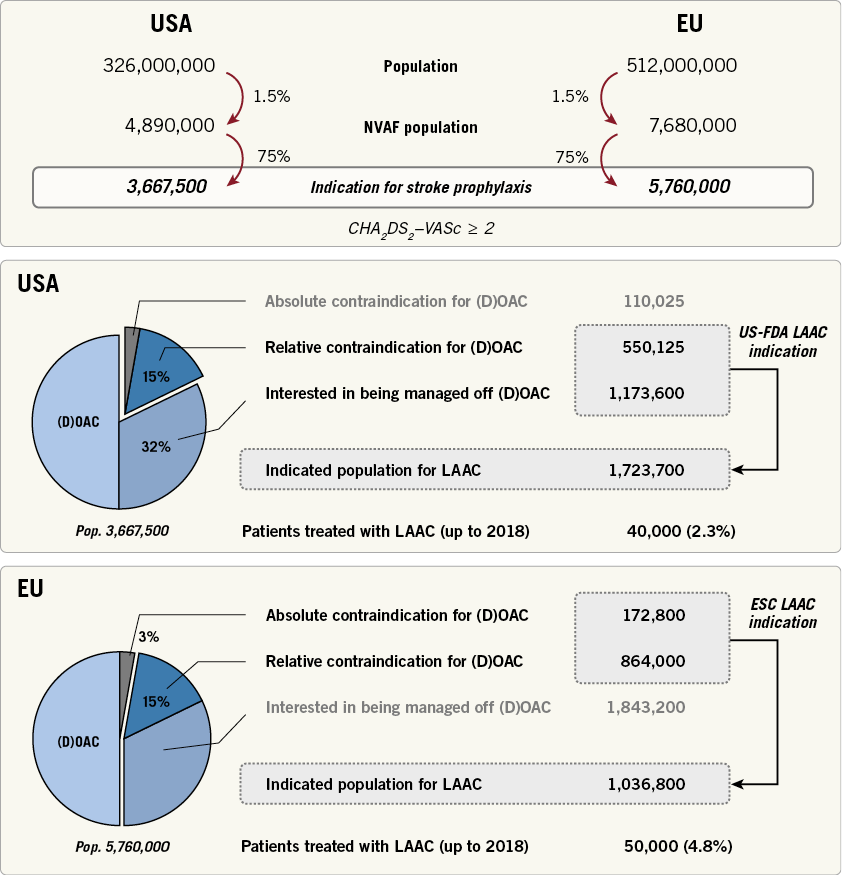
Figure 1. Indications and current use of percutaneous left atrial appendage closure (LAAC) in USA and EU. DOAC: direct oral anticoagulation; ESC: European Society of Cardiology; FDA: Food and Drug Administration; NVAF: non-valvular atrial fibrillation
On the other hand, the latest EU guidelines recommend percutaneous LAA closure as stroke prevention in NVAF patients with contraindication(s) for long-term anticoagulant therapy (Class IIb, level of evidence B)3. These EU guidelines are partially supported by the ASAP study, which evaluated the safety and efficacy of the WATCHMAN device in patients with NVAF who were ineligible for warfarin therapy4. Recently, further evidence for the WATCHMAN and ACP/Amulet™ device (St.Jude Medical, St.Paul, MN, USA) was provided in the large-scale, prospective EWOLUTION and AMPLATZER Amulet observational studies, reporting satisfactory real-world safety and efficacy outcomes with LAA closure in NVAF patients with (mainly) contraindications for OAC5,6. Applying these EU guideline recommendations to the current EU population would categorise 1,036,800patients as being eligible for LAA closure. However, only 50,000 LAA closures have been performed so far, indicating an LAA closure adoption rate of 4.8% to date (Figure 1).
Taken together, this indicates that percutaneous LAA closure as astroke prevention therapy is still largely underused worldwide and that only asmall percentage of NVAF patients with indication(s) for LAA closure currently benefit from this therapy. Clearly, many challenges are still ahead in order to increase the adoption rate of LAA closure in the coming years. First of all, it will be essential to have abetter dissemination of the current data and evidence to both referring physicians and eligible patients, and not merely to the operators who perform percutaneous LAA closure. Furthermore, the introduction and implementation of new imaging and device technologies will be needed in order to improve further the procedural safety and efficacy as well as clinical outcomes. As percutaneous LAA closure is astroke prevention therapy, it must be an absolute condition that this procedure has ahigh safety profile. Therefore, an optimised preprocedural planning, (e.g., by the use of computed tomography and computational modelling), and better designed LAA occluders are warranted. Also, studies comparing the economic impact of aone-time LAA closure procedure vs. lifelong (D)OAC treatment are essential in these times where health economics are increasingly important. Finally, the body of evidence for LAA closure will need to be strengthened by conducting new, large prospective RCTs. The ongoing ASAP-TOO trial will be the first well-designed study comparing LAA closure using the WATCHMAN device with current optimal medical therapy in patients deemed not eligible for OAC therapy7; completion of this trial is expected in 2020. Moreover, large RCTs comparing LAA closure with the newer DOACs in NVAF patients at increased stroke risk will be essential for further acceptance of this non-pharmacological approach. In the case that these trials demonstrate non-inferiority, or even superiority, of LAA closure as compared to DOAC, apatient population of more than three million could benefit from LAA closure in the western world alone. All this will be needed to convince possible patient candidates, referring physicians, authorities and payers of the merits of this procedure and could finally result in aparadigm shift in which percutaneous LAA closure is no longer a“last resort” option for NVAF patients not suitable for long-term OAC treatment, but could become a“first-line” option for NVAF patients at increased stroke risk.
Conflict of interet statement
O. De Backer has been a consultant for Abbott Vascular. The other authors have no conflicts of interest to declare.

