
Abstract
Aims: Interventional left atrial appendage closure (LAAC) is an emerging alternative to oral anticoagulation (OAC) for stroke prevention in atrial fibrillation (AF) in concomitance with a contraindication for standard OAC. This sub-analysis of the LAARGE registry aimed to investigate differences between different LAA morphologies in a real-world setting.
Methods and results: This prospective, multicentre, observational registry included 562 patients from 37 centres with ineligibility for long-term OAC between April 2014 and January 2016. Baseline characteristics, indications, procedural data and complications were registered according to each LAA morphology (i.e., chicken wing, cauliflower, windsock, cactus and atypical morphologies). Implantation success was high across the four typical anatomies (≥97.5%, p=n.s.); only atypical anatomies exhibited a lower success rate (94%). The cactus-shaped LAA was linked to a trend indicating a shorter fluoroscopy time, while the atypical LAA was linked to a significantly prolonged fluoroscopy time (p=0.089 and p=0.025 versus the overall mean, respectively). Periprocedural and intra-hospital complications were generally rare, with no differences among the different morphologies (p=n.s.).
Conclusions: Procedural success as well as the complication rates of LAAC were not different among the four typical LAA morphologies. A lower implantation success rate was only obvious in patients with atypical LAA morphologies. ClinicalTrials.gov Identifier: NCT02230748
Abbreviations
AF: atrial fibrillation
BARC: Bleeding Academic Research Consortium
CT: computed tomography
DAPT: dual antiplatelet therapy
ICE: intracardiac echocardiography
LAA: left atrial appendage
LAAC: left atrial appendage closure
LAARGE: Left-Atrium-Appendage Occluder Register - GErmany
MRI: magnetic resonance imaging
OAC: oral anticoagulation
TOE: transoesophageal echocardiography
VKA: vitamin K antagonist
Introduction
Atrial fibrillation (AF) is the most common arrhythmia with an estimated age-dependent prevalence of 1-2% and is associated with a fivefold increased risk of ischaemic stroke1-3. Oral anticoagulation (OAC) with either vitamin K antagonists (VKA) or the more recently introduced factor II/Xa inhibitors is the gold standard for reducing the risk of stroke in patients with AF and a CHA2DS2-VASc score of ≥24. However, this treatment is underutilised in such patients due to poor patient compliance, contraindications and potential bleeding complications5.
Left atrial appendage (LAA) closure (LAAC) is a guideline-conforming alternative to OAC either in patients with a high bleeding risk or in those unwilling to be treated lifelong with such medication4. LAAC is non-inferior to VKA for the prevention of stroke in AF patients with a moderate stroke risk, with an improved rate of bleeding6. In patients ineligible for OAC, dual antiplatelet therapy (DAPT) can be safely prescribed until the LAA occlusion device is endothelialised7,8.
At present, the interdependence between this interventional procedure and the underlying LAA morphology remains unclear. LAA anatomy is known to be highly heterogeneous but, typically, four different morphologies are described (Figure 1): cauliflower, cactus, windsock and chicken wing9,10. This sub-analysis of the Left-Atrium-Appendage Occluder Register - GErmany (LAARGE) (ClinicalTrials.gov Identifier: NCT02230748) aimed to investigate how indication, procedural characteristics, complications and post-procedural measures differ among the different LAA morphologies.
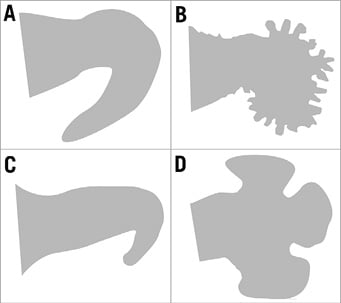
Figure 1. The four typical LAA morphologies. A) Chicken wing. B) Cauliflower. C) Windsock. D) Cactus-shaped.
Methods
THE REGISTRY
LAARGE is a prospective, non-randomised, multicentre real-world registry that encompassed consecutive patients from 37 voluntary participating centres. Recruitment into the registry started in April 2014 and ended in January 2016. In total, 562 patients with documented LAA morphology were included in this sub-analysis. Patients should have been treated according to current recommendations11.
ENROLMENT
Patients ≥18 years planned for LAAC with all three types of non-valvular AF, a CHA2DS2-VASc score ≥2 and ineligibility for long-term OAC were included11. The sole exclusion criterion was absence of written informed consent from the individual patient. Participating centres were encouraged to include all patients consecutively to avoid recruitment bias. The study was carried out according to the principles of the Declaration of Helsinki and was approved by the Landesärztekammer Rheinland-Pfalz. Written informed consent was obtained from all study patients.
PROCEDURE AND INTRA-HOSPITAL FOLLOW-UP
Preprocedural screening and indication, the conduct of the implantation procedure as well as post-procedural management, including the antithrombotic treatment, were at the discretion of the operating physician. The operators’ levels of experience in LAAC were diverse (<10 to >100 prior implantations). After confirming the indication and the technical feasibility of the LAA, the procedure was performed under conscious sedation or general anaesthesia. Transoesophageal echocardiography (TOE) guidance or intracardiac echocardiography (ICE) was used to rule out an intracardiac thrombus intraprocedurally, to facilitate transseptal puncture and to screen for peri-device leaks before complete device deployment12. Peri-device leaks >5 mm were considered relevant13. Intraprocedural and post-procedural clinical events as well as imaging procedures were assessed by each centre and transferred via an electronic case report form. The verification of these events was conducted by the steering committee. Relevant documents were requested from the treating site in such cases. Distinctive scores, e.g., the Bleeding Academic Research Consortium (BARC) score14, were used to grade event severity.
LAA ANATOMY
LA and LAA diameters and surfaces were measured by TOE. The particular LAA anatomy was determined based on TOE, computed tomography (CT) and/or magnetic resonance imaging (MRI) by the treating physician12. LAAs were allocated to one of four predefined morphologies. The chicken wing-shaped LAA is defined by a dominant lobe with an early and sharp bend. The cauliflower-shaped LAA consists of a short major lobe branching into innumerable small ramifications in its distal part with a high trabecularisation. The windsock-shaped LAA’s dominant lobe is tapered at its distal end. There might also be a sharp bend, but, if so, it is clearly localised in the distal end. The cactus-shaped LAA is characterised by a short major lobe subdivided into several secondary lobes. Only anatomies that did not match these typical morphologies were termed atypical.
OUTCOME MEASURES
The primary efficacy outcome measure of this sub-analysis was the rate of successful device implantation for each LAA morphology. Secondary outcome measures were the differences in the indications and the procedural characteristics among the five groups. The safety outcome measure was the difference in the number of intra-hospital complications.
STATISTICAL ANALYSIS
Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC, USA). Continuous data are presented as medians with interquartile ranges (25th and 75th percentiles), or as means with standard deviation, and categorical data as frequencies with group-related percentages. Categorical variables were compared between the patient groups using the Pearson’s chi-squared test, or by the Freeman-Halton test for rare events, as indicated in the Tables. Metric variables were compared using the Kruskal-Wallis test. The statistics were based on the available cases. In case of significant differences among the groups, outlying categories were identified by comparing each category to the overall mean, in the vein of the so-called analysis of means. Otherwise, no adjustment for multiple testing was made. P-values <0.05 (two-tailed) were considered significant.
Results
BASELINE CHARACTERISTICS
The baseline characteristics of the intervened patients were mostly evenly distributed among the five groups (Table 1). Antithrombotic pre-treatment did not differ significantly among the groups (p=n.s.). Table 2 shows the results of the pre-interventional cardiac imaging procedures. The number of lobes was significantly different among the groups (p<0.001).
INDICATIONS FOR LAAC
Participating centres could document more than one indication in the same patient (Table 3). The main indication was a prior bleeding event which in 71.9% of cases was of moderate or severe intensity (p=n.s.). Patient choice for the interventional procedure instead of chronic OAC was documented in about one fourth of procedures (p=n.s.).
PROCEDURAL DATA
The implantation success rate was high in all LAA morphologies and statistically evenly distributed among the four classic LAA anatomies (p=n.s.) (Table 4). A significantly lower success rate was registered (p<0.001) only for the group with not clearly attributable, i.e. atypical, anatomies. In this group, the number of implantation attempts was lower compared to the total average (p=0.022). For these atrial appendices, the WATCHMAN® occluder (Boston Scientific, Marlborough, MA, USA) was predominantly chosen (p<0.001), whereas its use was below average for the chicken wing morphology (p<0.001) (Figure 2). No peri-device leak >5 mm was observed (p=n.s.). Compared to the mean LAA ostial diameter of 20.1±3.8 mm measured at 45°, the mean device size was 25.4±3.8 mm, and thus 26% larger (5.2±3.5 mm; each p=n.s.). There was a clear positive correlation between ostial diameter and device size (Pearson correlation coefficient 0.57). Though the total duration of the intervention was not statistically different among the five groups (p=n.s.), there was a trend towards a shorter fluoroscopy time in the patients with a cactus-shaped LAA (p=0.089) and a prolonged time in those with atypical morphologies (p=0.025) compared to the overall mean.
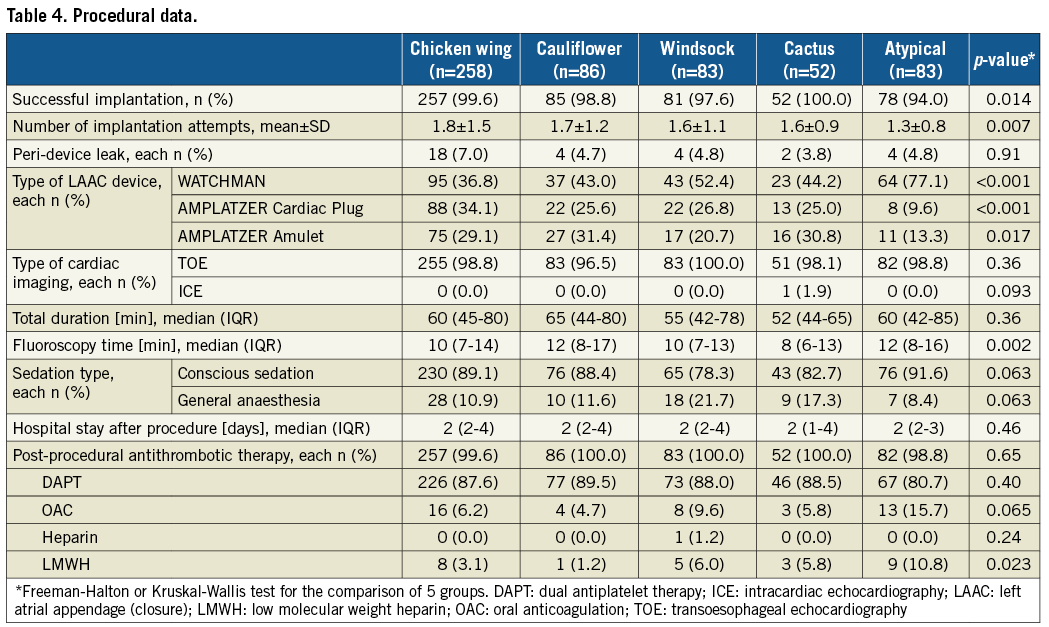
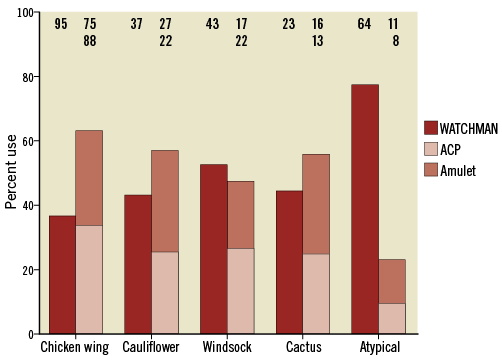
Figure 2. Percentage and number of device types used. ACP: AMPLATZER Cardiac Plug
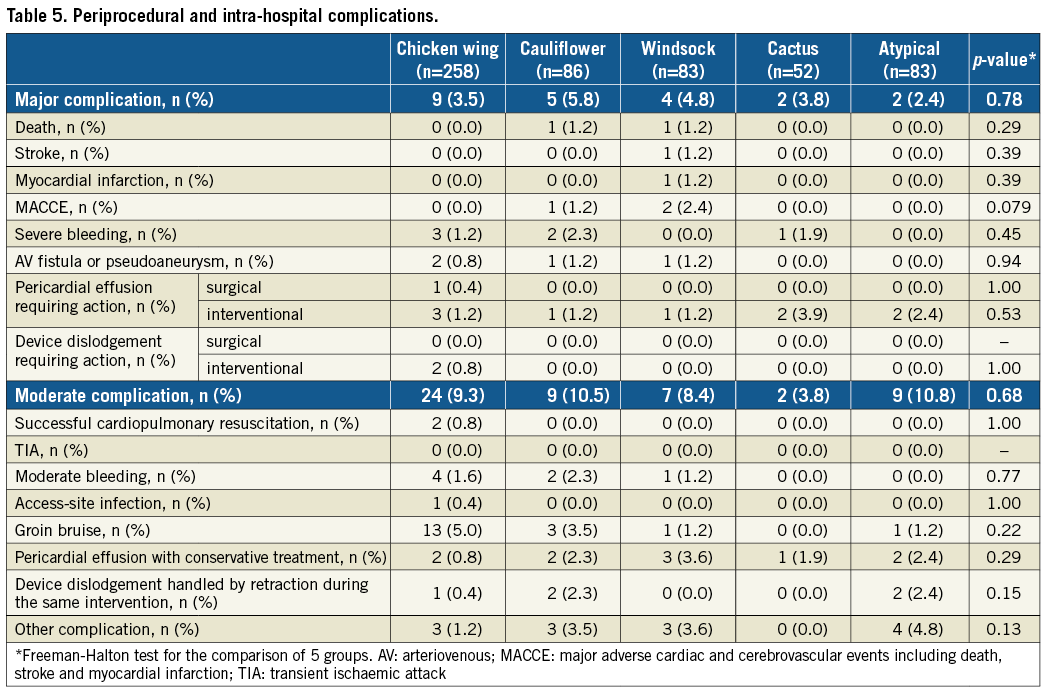
PERIPROCEDURAL AND INTRA-HOSPITAL SAFETY EVENTS
The post-procedural antithrombotic regimen was based on DAPT in the vast majority of cases (87.0%; p=n.s.). More than one complication in the same patient could be documented. Complications were generally rare and dominated by bleeding events (Table 5). No LAA morphology was predominantly associated with pericardial effusion (each p=n.s.). All intraprocedurally dislodged devices could be retrieved by a catheter intervention.
Discussion
To our knowledge, LAARGE is the first multicentre registry which analyses precisely the differences in indication, details of the implantation procedure, periprocedural complications and postprocedural measures related to the different LAA morphologies.
It is of particular interest to note that no differences in the procedural success, i.e., completed device implantation and peri-device leak ≤5 mm, were observed among the four typical LAA morphologies (p=n.s.). This is despite the fact that some LAA morphologies, especially the chicken wing-shaped LAAs, had been regarded as interventionally challenging15. A significantly lower number of implantation attempts in the atypical group (p=0.022 compared to the total average) should have been related to a higher percentage of successful implantations but, in fact, the implantation success in the atypical morphologies was significantly lower (94%; p<0.001 compared to the total average). This could signify a subgroup of LAA morphologies amongst these atypical ones that is a priori unsuitable for interventional closure and, thus, the interventions might have been interrupted after one attempt. According to the definitions of the different LAA morphologies, the number of lobes was significantly different among the groups (p<0.001)9,10, but this was not accompanied by differing numbers of implantation attempts among the four typical LAA morphologies (p=n.s.).
The distribution of the different LAA anatomies is heterogeneous in the existing literature9,10,16. However, in this registry, the LAA morphology was assessed by the operators using TOE, CT and MRI data and based on the same predefined definitions and templates for all centres. Notwithstanding the observed differences in stroke risk among the different LAA morphologies in other studies17,18, the proportion of prior strokes was not significantly unequal in this registry (p=n.s.).
There was a trend towards a shorter fluoroscopy time in the patients with a cactus-shaped LAA (p=0.089) but a significantly prolonged one in those with atypical anatomies (p=0.025). The clear structure of the cactus-shaped LAA might explain a shorter fluoroscopy time as it can be accessed easily and the device anchors well in the cylindrical main lobe. It had been assumed that the LAA anatomy should influence the selection of a certain LAAC device12. In this registry, predominant use of the WATCHMAN occluder was only observed for the atypical LAA morphologies (p<0.001 compared to the total average). It might be speculated that operators considered the WATCHMAN’s nitinol frame construction as being more suitable for the often wide and flat atypical LAA anatomies than the AMPLATZER™’s (St. Jude Medical, St. Paul, MN, USA) pacifier principle12.
The queried indication data did not differ significantly among the groups (each, p=n.s.).
Peri-interventional and intra-hospital complications were generally rare, which is in line with recently published safety data on LAAC19. The documented dominance of bleeding complications is neither untypical for a cardiac intervention with transvascular access nor surprising in a patient collective prone to a pre-existing high bleeding risk. While for the AMPLATZER™ Cardiac Plug and the AMPLATZER™ Amulet™ (both St. Jude Medical) a prospective observational registry revealed no statistical differences in the proportion of major adverse events comparing the different LAA morphologies20, the LAARGE registry confirmed this finding for different devices.
Study limitations
These analyses were based on observational registry data with the inherent limitations of this study type. Respecting the observational character of this registry, the conduct of the interventions was not influenced by the study investigators and was based on the operators’ discretion. This individualised decision algorithm might not have insignificantly influenced the outcome measures but surely reflects clinical practice. We cannot report on cases which were found ineligible for the intervention by a preprocedural assessment. Additionally, not all centres used all types of device. The LAA morphology was allocated by the operators and not by an independent commission, which, indeed, might have caused an inherent interobserver variability. However, all centres were provided with the same templates and written definitions to avoid an unequal assignment. Moreover, follow-up time was limited to the end of hospitalisation in this sub-analysis. Despite all the limitations of this observational registry, it serves as a data source for this little studied topic.
Conclusions
In general, the procedural success and complication rates of LAAC were not different among the four typical LAA morphologies. Only atypical LAA morphologies were linked to a lower success rate.
| Impact on daily practice Left atrial appendage (LAA) closure is an established, effective and safe alternative to oral anticoagulation for stroke prevention in non-valvular atrial fibrillation patients who are ineligible for drug treatment. However, the LAA varies anatomically but differentiating data do not exist. This sub-analysis of the LAARGE registry revealed no significant differences in procedural success and complication rates among the four typical LAA morphologies, while atypical anatomies, not conforming to the classic LAA configurations, were associated with lower implantation success. |
Funding
The study was financed and conducted by the Stiftung Institut für Herzinfarktforschung (IHF), Ludwigshafen am Rhein, Germany.
Conflict of interest statement
J.W. Park is a proctor for St. Jude Medical, Occlutech, Cardia and Lifetech. J. Brachmann has received study support from Abbott. H. Sievert has received study honoraria, travel expenses and consulting fees <25,000€ from Abbott, Ablative Solutions, Ancona Heart, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, CeloNova, Cibiem, Comed B.V., Contego, Hemoteq, Kona Medical, Lifetech, Maquet Getinge Group, Medtronic, Occlutech, pfm medical, St. Jude Medical, Terumo, Trivascular, Valtech, and Vascular Dynamics. V. Geist is a proctor for St. Jude Medical. M. Käunicke is a proctor for Boston Scientific. The other authors have no conflicts of interest to declare.

