
Abstract
Aims: The aim of this study was to investigate the feasibility, safety and efficacy of percutaneous closure for prevention of thromboembolic events in patients with atrial fibrillation (AF) and left atrial appendage (LAA) thrombus.
Methods and results: The study included consecutive patients with AF and LAA thrombus who underwent transcatheter occlusion in eight high-volume centres. Clinical and transoesophageal echocardiography (TEE) follow-up was carried out as per each centre’s protocol. Twenty-eight patients were included. The location of the LAA thrombus was distal in 100% of cases. Technical and procedural success was achieved in all patients. A cerebral protection device was used in six cases. There were no periprocedural adverse events. Follow-up was complete in all patients (total 32 patient-years). No death or thromboembolic events were reported. There was one major bleeding during follow-up. Among the 23 patients undergoing TEE, device thrombosis was present in one patient. No significant peri-device leaks were observed.
Conclusions: In this multicentre study, percutaneous closure in selected patients with distal LAA thrombus appears to be feasible and safe, and is associated with high procedural success and a favourable outcome for the prevention of AF-related thromboembolism. Special implant techniques avoiding mechanical mobilisation of the thrombotic mass and the liberal use of cerebral embolic protection devices are recommended.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia and is associated with a significant risk of stroke1. The major cardioembolic source in non-valvular AF is the left atrial appendage (LAA)2. Therefore, appropriate anticoagulation is the established strategy for stroke prevention with mortality benefits in patients with AF regardless of rhythm control3. Percutaneous closure of the LAA provides a potential alternative therapy to oral anticoagulation (OAC) therapy for stroke prophylaxis in patients with AF, especially in those who cannot tolerate anticoagulants or who are at high risk of major bleeding4,5. The presence of persistent LAA thrombus despite appropriate antithrombotic therapy or in patients with a contraindication to systemic OAC is a major concern, as these patients are at an even higher risk of embolic complications. Thus far, there is no valid alternative other than antithrombotic therapy intensification with the subsequent haemorrhagic risk or open heart surgery in patients generally with several additional comorbidities. In this sense, percutaneous LAA device occlusion might represent a valid alternative in selected patients with LAA thrombus. Thus, the aim of this study was to report the feasibility, safety and efficacy of percutaneous LAA occlusion in patients with AF and LAA thrombus.
Methods
STUDY POPULATION
The study included consecutive patients with AF and LAA thrombus who underwent transcatheter occlusion in high-volume centres. Data collected from each centre were transferred to a dedicated database and analysed retrospectively. The following variables were collected: demographics, baseline characteristics, indications for LAA closure, CHA2DS2-VASc score, HAS-BLED score, baseline antithrombotic medication, procedure, periprocedural adverse events, clinical follow-up, and transoesophageal echocardiography (TEE) follow-up, when available. All patients provided written informed consent before the procedure.
PROCEDURAL DESCRIPTION AND CLINICAL ENDPOINTS
Details regarding the procedure have been published elsewhere6. TEE and computed tomography (CT) were the most frequently applied imaging modalities for LAA thrombus evaluation and to assist proper sizing of the device7,8. The device implantation and the result of the procedure were mainly based on fluoroscopy and TEE or, alternatively, intracardiac echocardiography (ICE)9. The selection of the LAA occlusion device and the use of cerebral protection systems were left to the individual operator’s judgement. Definitions and clinical endpoints were defined according to the Munich consensus document10. Technical success was defined as successful deployment of the device, while procedural success was defined as technical success without procedure-related complications10. Pericardial effusion/tamponade was considered significant if drainage was required. Bleeding was defined according to Bleeding Academic Research Consortium (BARC) criteria11. Events were labelled as periprocedural if they occurred within seven days of the LAA closure procedure or before hospital discharge. The choice and duration of antithrombotic therapy were individualised depending on patient history, indication for LAA occlusion, and physician preference. Clinical follow-up for subjects was not pre-specified but carried out as per each institution’s standard practice. All outcomes were adjudicated locally without an independent events committee. TEE to confirm appropriate device implantation and to exclude residual device-related leak or thrombosis was performed according to the protocol of each centre or if clinically indicated.
STATISTICAL ANALYSIS
All data were analysed with SPSS, Version 24 (IBM Corp., Armonk, NY, USA). The normality of the distribution for continuous variables was tested using the Shapiro-Wilk test. Continuous variables were presented as means±standard deviation and, in case of non-normal distribution, as median and interquartile range (IQR) or minimum-maximum range, as appropriate. Categorical variables were presented as frequencies and percentages. Technical and procedural success rates were calculated as percentages of the total number of patients. Patient-years were calculated as the product of patient number and the mean years of follow-up. Rates of stroke/bleeding events were calculated as number of events per 100 patient-years. The individual patient annual risk for stroke and bleeding was recorded based on each subject’s CHA2DS2-VASc and HAS-BLED score and then the median risk score for the study population was calculated. The annual risk of stroke and bleeding was then extrapolated from published risk score literature in order to determine relative risk reductions12,13.
Results
POPULATION
A total of 28 patients from eight high-volume centres (≥20 procedures/year) were included. All thrombi were confined to the distal part of the LAA except in two cases, in which they were located in the mid-to-distal portion of the LAA (Figure 1). Baseline patient characteristics are summarised in Table 1. The median age was 72 years, and 10 patients (35.7%) were >75 years old. Permanent AF was present in 21 patients (75%), whereas nine (32%) had a history of stroke/TIA. The median CHA2DS2-VASc score was 4. Based on the CHA2DS2-VASc score, the expected annual risk of thromboembolism was 7.8%. The median HAS-BLED score was 3. A score of ≥3 was present in 20 patients (71.4%). The predicted annual risk of major bleeding was 5.8%. The main indication for LAA closure (Table 2) was a history of previous major bleeding (53.6%), followed by thromboembolism on OAC associated with persistent LAA thrombus (“malignant LAA”) despite adequate pharmacological therapy (28.6%).
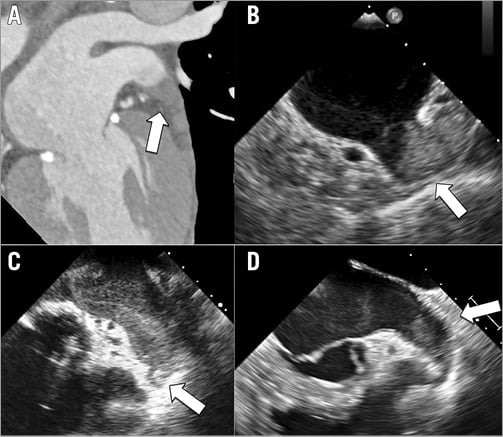
Figure 1. Case examples of patients with left atrial appendage thrombus. Preprocedural cardiac computed tomography (A) and transoesophageal echocardiography (B to D) showing distal appendage thrombus (arrow).
PROCEDURAL AND IN-HOSPITAL OUTCOMES
As shown in Table 3, general anaesthesia was used in the majority of cases. In order to prevent thrombus dislodgement during the procedure, any contrast injection, guidewire or catheter manipulation in the LAA was avoided or reduced to a minimum (“no-touch technique”) (Figure 2). In fact, left atrium contrast injection was reported in only 57% of cases. The most commonly used device was the AMPLATZER™ Amulet™ (54% of cases), followed by the AMPLATZER™ Cardiac Plug (both St. Jude Medical, St. Paul, MN, USA) and LAmbre™ (Lifetech Scientific [Shenzhen] Co., Ltd. Shenzhen, China). The device was deployed at the first attempt in 26/28 cases (93%). A cerebral protection device was used in six cases (21.4%), including four cases with the TriGUARD™ (Keystone Heart, Tampa, FL, USA), one case with the Sentinel™ (Claret Medical, Santa Rosa, CA, USA), and one case with the FilterWire EZ™ (Boston Scientific, Marlborough, MA, USA). In one case, on visual examination, small debris were captured in the cerebral protection devices at the end of the procedure. Both technical and procedural success was achieved in all patients. No periprocedural adverse events were reported. In-hospital median length of stay was 3.5 (IQR 2-6.75) days.
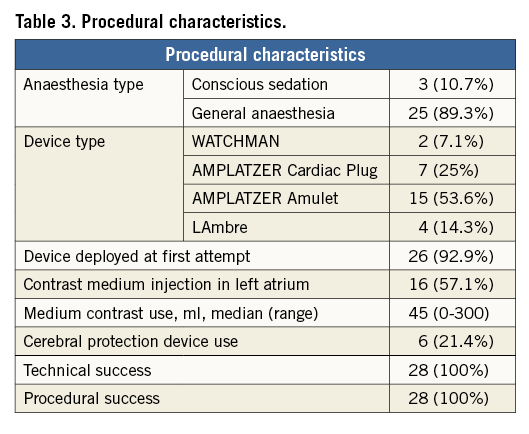
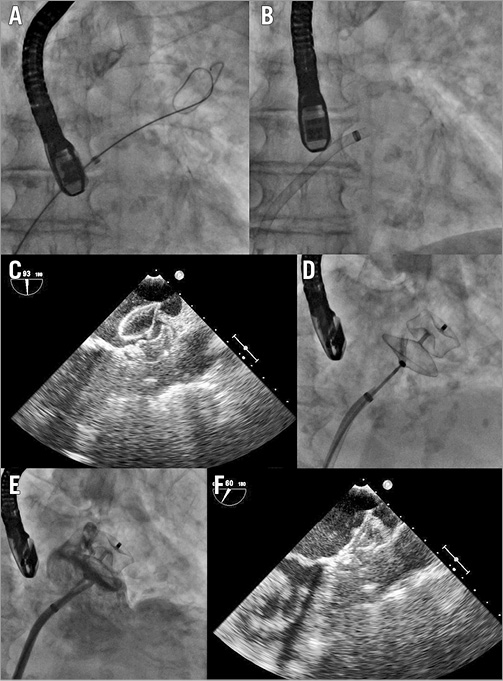
Figure 2. Step-by-step left atrial appendage (LAA) closure strategy. A) A stiff guidewire was positioned into the left upper pulmonary vein (LUPV). B) The transseptal sheath was advanced over the guidewire into the LUPV and then it was exchanged for the delivery sheath, which was then pulled back gently from the vein and moved forward towards the LAA ostium. The AMPLATZER Amulet device was deployed without the delivery sheath entering deeply into the left atrial appendage, and its stability was confirmed by a sustained “tug test” at transoesophageal echocardiography (TEE) (C) and fluoroscopy (D). Well-deployed LAA occlusion device was confirmed by final angiography (E) and TEE (F).
CLINICAL OUTCOMES
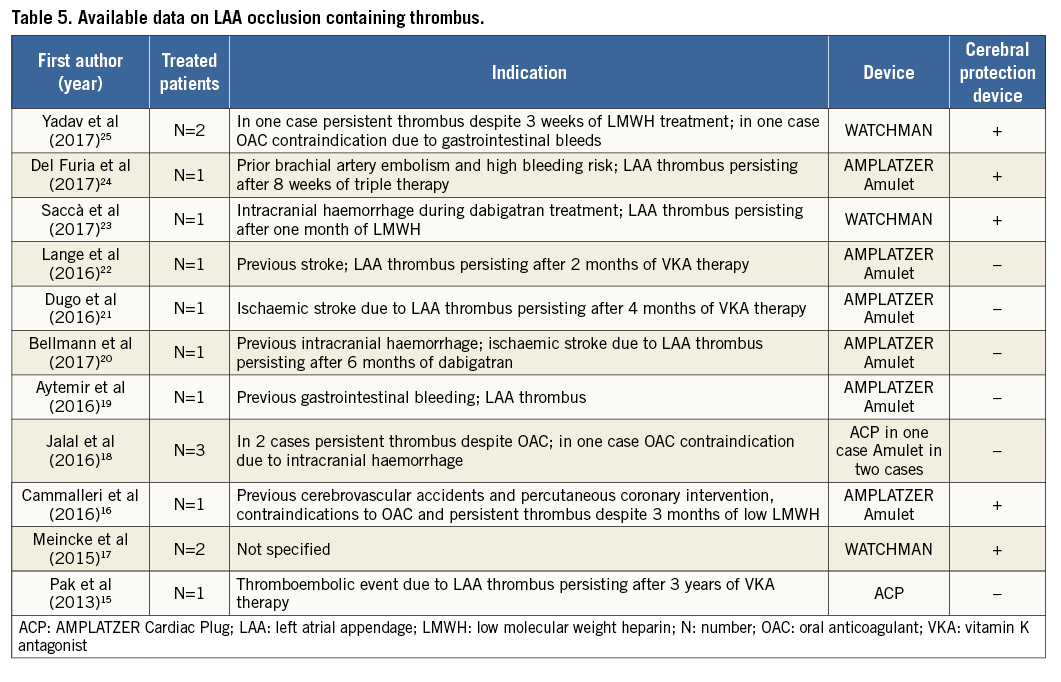
Clinical follow-up was complete in all patients. Median follow-up was 255 days (IQR 106-646 days), resulting in a total of 32 patient-years. Table 4 summarises the changes in antithrombotic medication between baseline and last follow-up. No death or systemic embolic events were reported. There was one major bleeding (BARC criteria 3a) at follow-up.
TRANSOESOPHAGEAL ECHOCARDIOGRAPHIC FOLLOW-UP
During follow-up, TEE was performed in 23 patients (82.1% of cases). Device thrombus was identified in one case (4.3% of evaluated patients), observed 23 months after LAA occlusion. The thrombosis was asymptomatic. The patient, discharged on dual antiplatelet therapy, started warfarin, and device thrombus resolution was confirmed at control TEE. No significant peri-device leaks (jet width >5 mm) were observed, while minor leaks were found in two patients (8.7%).
Discussion
The main finding of this multicentre, international study is that percutaneous LAA closure appears feasible and safe, and is associated with favourable outcomes for the prevention of thromboembolic events in selected patients with distal LAA thrombus that persists despite appropriate antithrombotic therapy, or who cannot take OAC.
It has been reported that the incidence of LAA thrombus on TEE among patients scheduled for AF ablation who have been adequately anticoagulated is up to 11% in patients with a CHADS2 score of 4-614. Therefore, the prevalence of LAA thrombus may be higher in patients referred for LAA closure indication, as they have a high CHA2DS2-VASc score and often cannot be adequately treated with OAC. The presence of a thrombus in the LAA is considered a contraindication to percutaneous LAA occlusion, as manipulation of catheters, guidewires, sheaths or devices in the LAA may lead to systemic embolisation. Consequently, patients with known LAA thrombus have been excluded from percutaneous LAA closure studies. Our study population was at high bleeding risk (HAS-BLED 3), presented contraindications to anticoagulation, or LAA thrombus persisted despite adequate pharmacological treatment (notably anticoagulant and/or antiplatelet therapy was present at baseline in 27/28 patients). Intensification of antithrombotic therapy or surgical excision of the LAA would have been the only alternatives for reducing the risk of cardioembolic events in these cases, but was often not feasible due to the high haemorrhagic or surgical risk, respectively.
Available data about LAA closure in patients with thrombus are sparse and limited to case reports and very small case series (Table 5)15-25. These have all reported procedural success using a minimal manipulation implantation technique or inconstant use of a cerebral embolic protection device. To the best of our knowledge, our study is the largest reported series of percutaneous LAA closure in patients with known LAA thrombus.
In the present cohort, device implantation was successful in all cases, comparable to success rates recently reported from recent large all-comers multicentre studies (≈99%)26,27. Of note, the use of cerebral protection systems was not constant and was based on operator preference more than thrombus location/characteristics. The use of such devices, in fact, needs arterial vascular access and device manipulation in the aortic arch, and prolongs the duration of the procedure, which may increase the risk of periprocedural complications in such fragile patients, especially when used by low-volume operators. Moreover, embolic events can occur despite the use of these devices, as these filters do not prevent embolisation events in coronary, renal, mesenteric and femoral arteries, which may be easier to manage percutaneously or surgically28. However, these devices are successful in preventing the most devastating complication that could occur in this cohort of patients, i.e., embolic stroke. Considering the added protection given by cerebral embolic devices, we do not see a valid reason not to use them routinely in this high-risk group of patients, if they are available. The costs of the device are the main limitation to its routine use. On the other hand, all procedures were carried out without any or with only minimal catheter or guidewire manipulation or direct contrast medium injection in the LAA. The exchange between the transseptal sheath and the delivery sheath was performed in the left upper pulmonary vein using a stiff guidewire. The wire and sheath were advanced carefully using fluoroscopy and, above all, TEE or ICE guidance, to avoid inadvertently entering the LAA. This, along with the skill and experience of operators, may be associated with the high technical and procedural success rates and the fact that no periprocedural embolic events occurred. This last observation is in line with what was initially observed with LAA occlusion experience in the PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) trial29, and in the Continued Access Protocol registry30, where operator-related experience was associated with a decrease in the rate of complications over time. However, the authors of the present study believe that this type of intervention should only be reserved to very experienced operators, as limited device manipulation in the LAA is mandatory to avoid thrombus dislodgement.
No thromboembolic events were reported at follow-up, resulting in a 100% reduction in the rate of stroke/TIA/systemic thromboembolism, compared with the expected rate of 7.8 events per 100 patient-years, according to a median CHA2DS2-VASc score of 412 (Figure 3). However, the expected rate of thromboembolic events based on the CHA2DS2-VASc score might underestimate the real risk of this population, which was higher, considering the presence of LAA thrombosis. The expected annual rate of major bleeding was 5.8%13. The observed annual rate of major bleeding in our study was 3.1% (1/32 patient-years), which corresponds to a 47% risk reduction compared to the rate that would have been expected during vitamin K antagonist (VKA) therapy based on a comparable HAS-BLED score (Figure 3). This finding was consistent with the risk reduction in the annual rate of major bleeding reported in a large all-comers registry for both devices, the AMPLATZER Cardiac Plug and the WATCHMAN® (Boston Scientific) (61% and 48%, respectively)5,26. Notwithstanding this, the observed relative reduction in our population may be somewhat overestimated, as the expected rate is calculated assuming VKA use, thus limiting the utility in calculating an imputed benefit in patients who cannot take anticoagulant therapy. In this regard, we observed a large variability in antithrombotic regimen immediately after the procedure and during follow-up, which reflects: 1) the lack of consensus about the optimal pharmacological strategy to adopt after percutaneous LAA closure, and 2) the absence of any OAC contraindication in 11 patients, in whom the anticoagulant therapy was maintained after the procedure.
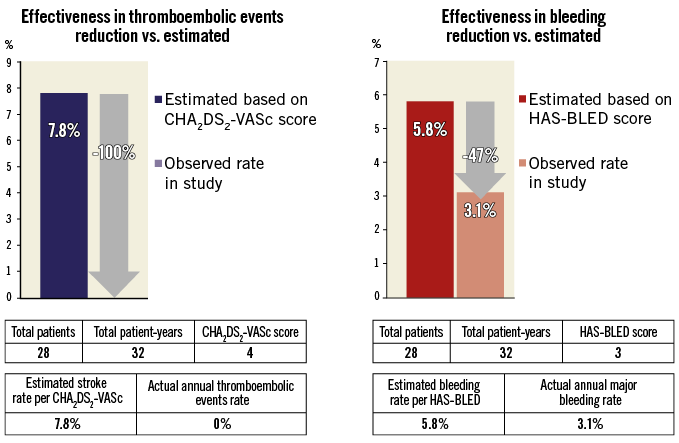
Figure 3. Effectiveness of left atrial appendage occlusion in reduction of thromboembolism and bleeding based on annual rate predicted by CHA2DS2-VASc score and HAS-BLED score, respectively.
Limitations
This is a non-randomised, retrospective, observational study. There was no control group. CT/TEE LAA baseline imaging features were not available. The techniques and tools for device implantation were left to operators’ discretion. TEE follow-up was not available for all patients. The clinical and TEE results were self-reported without independent adjudication. Brain magnetic resonance imaging or dedicated neurologic assessment was not systematically performed, thus subclinical micro-embolisation and silent brain ischaemia could not be ruled out. Because of the relatively small sample size, our results are not generalisable and do not permit identifying the ideal candidate for this procedure, as this was a highly selected group of patients with distal (or mid-to-distal) LAA thrombus treated by experienced operators in high-volume centres31.
Conclusions
The results of this multicentre experience show that, in selected cases of patients with distal LAA thrombosis in whom OAC is ineffective or contraindicated, percutaneous LAA occlusion seems to be feasible, safe and effective to prevent AF-related thromboembolic events and as such it should not be considered contraindicated. Further studies are needed with larger sample sizes and longer follow-up periods to confirm these findings and to assess the overall safety of the procedure.
| Impact on daily practice The presence of left atrial appendage thrombus is generally considered a contraindication to transcatheter closure. However, in selected cases, when various antithrombotic therapies fail to resolve a left atrial appendage thrombus or in patients unable to take oral anticoagulants, percutaneous device occlusion may serve as a bail-out strategy to prevent thromboembolic events. Special implant techniques avoiding mechanical mobilisation of the thrombotic mass and the use of cerebral embolic protection devices are recommended. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

