
Abstract
Aims: The study aimed to confirm the efficacy and safety of WATCHMAN LAA closure in atrial fibrillation patients unsuitable for oral anticoagulation.
Methods and results: The EWOLUTION registry prospectively collects all clinical data on 1,005 European patients implanted with a WATCHMAN device. Following the procedure, 605 patients (60.2%) received dual antiplatelet therapy according to the local standard; DAPT was discontinued in 85% of patients within one year. CHA2DS2-VASc and HAS-BLED scores were 4.61.6 and 2.41.2, respectively. The periprocedural SAE rate was 3.3% (2.0% major adverse cardiac events), mostly resolving without sequelae. Device embolisation or pericardial effusion occurred in one (0.2%) and two (0.3%) patients, respectively. TEE (median 62 days post implant, IQR: 47-97) confirmed effective sealing (no leak >5 mm) in 99.2% of patients. Device thrombus was present in 22 patients (4.0%), one patient developed a stroke. One-year mortality in the DAPT group was 9.6% (N=58) reflecting the advanced age and comorbidities in this population. The ischaemic stroke rate at one year was 1.4% (expected based on CHA2DS2-VASc: 7.5%), none fatal. The major bleeding rate was 2.5%, or 2.1% excluding periprocedural events (expected rate on VKA based on HAS-BLED: 5.1%).
Conclusions: LAA closure with the WATCHMAN device followed by DAPT therapy in a high-risk patient population is safe. At one year, the intervention is associated with a substantial risk reduction regarding ischaemic stroke and major bleeding compared to the expected rate based on CHA2DS2-VASc and HAS-BLED scores.
Abbreviations
AF: atrial fibrillation
DAPT: dual antiplatelet therapy
EWOLUTION: Registry on WATCHMAN outcomes in Real-Life Utilization
LAA: left atrial appendage
NOAC: non-vitamin K antagonist oral anticoagulants
Introduction
Recent guidelines have incorporated interventional stroke prevention, namely left atrial appendage (LAA) occluder therapy, as part of the routine treatment algorithm in patients with reasons to seek an alternative to long-term oral anticoagulation1. Randomised trials have shown non-inferiority of the therapy to warfarin regarding stroke risk reduction2; indeed, strokes following LAA occluder therapy were clinically less impairing compared to such events on oral anticoagulation in the PREVAIL trial on WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) implantation3. Yet these WATCHMAN trials, initiated prior to non-vitamin K antagonist oral anticoagulants (NOAC) becoming available, were performed in patients eligible for warfarin therapy. The studies employed a post-procedural drug regimen of 45 days of warfarin followed by dual antiplatelet therapy (DAPT). Patients were switched to aspirin monotherapy after six months. In order to place this therapeutic option correctly in the current treatment algorithm, large, prospective registries are helpful to evaluate safety and efficacy in patients unsuitable for oral anticoagulation.
The EWOLUTION registry provides data on LAA closure employing the WATCHMAN device in a real-world population in Europe. The study design and data up to the three-month time point have been published previously4,5. According to current European standards, most patients received DAPT therapy directly post procedure; the current manuscript summarises the one-year outcome data in this particular subgroup.
Methods
As reported previously, EWOLUTION is an all-comers, multicentre, prospective, non-randomised cohort study regarding the safety and efficacy of LAA occlusion employing the WATCHMAN device4-6. Indications for LAA closure as well as post-procedural drug therapy were at the discretion of the investigators; centres were encouraged to recruit consecutive patients to represent real-life practice and avoid selection bias. At least 39 sites out of the 47 centres confirmed that they enrolled consecutive patients. Signed informed consent was obtained prior to attempted WATCHMAN implantation in all subjects. The study adhered to international rules for scientific studies, the Helsinki principles, with local ethics committee approval in all participating centres. The electronic clinical report form provides fields for anticipated serious adverse events (SAE) such as procedure-specific events, e.g., perforation, tamponade, device embolisation, neurological events, device thrombosis and bleeding according to the BARC criteria6. The definition of major bleeding (which includes fatal and life-threatening) aligns with the left atrial appendage occlusion (LAAO)-specific modifications and refinements described in a consensus document on definitions, endpoints and data collection requirements7.
Study enrolment occurred from October 2013 to May 2015. At a total of 47 centres in 13 countries, 1,025 patients were enrolled, with 1,005 successful LAA WATCHMAN implants employing current techniques. Follow-up is according to the local institutions’ standard of care and will continue for two years post enrolment. Post-implant data collection includes LAA imaging data via transoesophageal echocardiography (TEE), oral anticoagulant (OAC) or antiplatelet medications and adverse events. At the time of this one-year analysis, 90% of active patients have completed at least 11 months of follow-up, and 871 patients (87%) have had at least one TEE exam during follow-up (Figure 1).
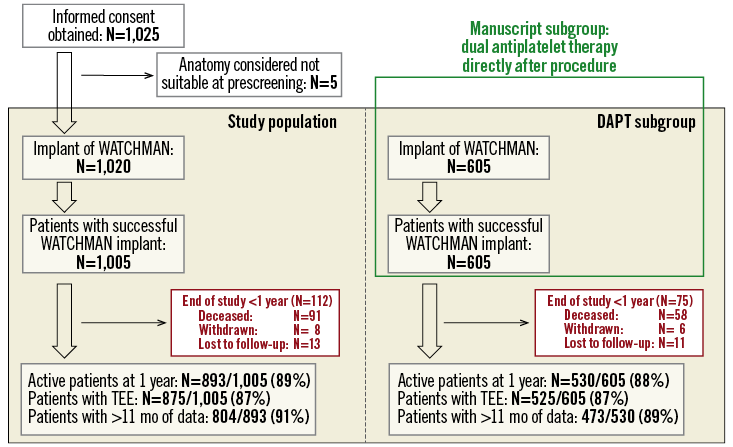
Figure 1. Study flow chart of the prospective, monitored, multicentre EWOLUTION registry. This manuscript reports on the efficacy of LAA closure employing the WATCHMAN device in patients without post-procedural treatment with oral anticoagulation. Six hundred and five patients received dual antiplatelet therapy (DAPT) directly after the procedure; 9.6% of patients died during the first year.
STATISTICAL ANALYSIS
Continuous variables are summarised using the mean, standard deviation, median and range. Categorical variables are summarised using counts and percentages. Rates of stroke/bleeding events are calculated as number of events per 100 patient-years (PY). The individual patient annual risk for stroke and bleeding was recorded based on each subject’s CHA2DS2-VASc and HAS-BLED scores and then the average risk score for the study population was calculated. Annual risks of stroke and bleeding were then extrapolated from published risk score literature in similar cohorts of patients8,9 in order to determine relative risk reductions. The mortality rate is calculated via the Kaplan-Meier method to account for censoring. The rate of device-associated thrombus is calculated via the Kaplan-Meier method, taking into account only patients with TEE performed. P-values are based on log-rank tests for time-to-event analysis and Fisher’s exact test for binomial proportions. Identification of heart failure decompensations and other SAE was at the discretion of the centre. Events and relevant source documents were additionally reviewed by the sponsor Medical Safety Group (MSG)4. For all patient deaths, source documents were requested at the sites and reviewed by the MSG. All centres were monitored by an outside contract research organisation (CRO) and every centre was visited at least once.
While previous analysis of the database focused on periprocedural safety and post-procedural drug therapy4,5, this analysis aims to focus on efficacy regarding stroke prevention at one year following LAA occluder therapy. As oral anticoagulation itself confers stroke prevention and randomised trials have already studied patients eligible for oral anticoagulation, we focused on the group of patients ineligible for OAC and transiently treated with dual antiplatelet therapy.
Results
Here we report the one-year outcome data of patients receiving DAPT following WATCHMAN implantation as part of the EWOLUTION all-comers registry. In total, EWOLUTION recruited 1,025 patients; 1,005 were implanted with a WATCHMAN device and 605 received dual antiplatelet therapy directly after the procedure (Figure 1). At the time of this analysis, 473 patients had had an 11-month data entry; median time of follow-up for the purpose of this analysis was 372 days. Final study visit and database entries will be completed two years after LAA occluder therapy. Patient characteristics in the EWOLUTION DAPT subgroup reflect the all-comer design of the study: patients were old (31.1% >80 years) and had a high CHA2DS2-VASc score of 4.61.6 (Table 1). Not surprisingly, 83.8% of the DAPT patients were deemed contraindicated to oral anticoagulation.
Safety data regarding WATCHMAN LAA occlusion are displayed in Table 2 and Table 3 as well as in Figure 2. The periprocedural (seven-day) major adverse cardiac events rate observed in this EWOLUTION cohort was 2%; two patients died during the first week. One patient died due to right heart failure following a MitraClip (Abbott Vascular, Santa Clara, CA, USA) procedure with three clips, possibly leading to an increased transmitral gradient; a second patient died due to unsuccessful resuscitation following an asystole after cardioversion four days after the procedure. One additional patient died >7 days post procedure secondary to air embolism during implantation leading to multi-organ failure and sepsis. The seven-day device/procedure-related SAE rate was 3.3% (n=19) (Table 3). Between seven and 30 days two fatal gastrointestinal bleeding events occurred on the same drug regimen that the patient received prior to WATCHMAN LAA occluder therapy. A third patient died due to clostridium difficile enteritis following a colonoscopy due to chronic inflammatory bowel disease with perforation.
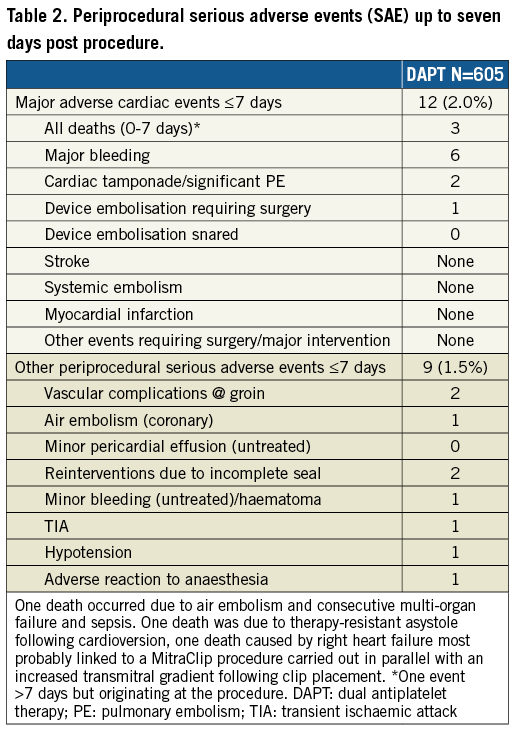
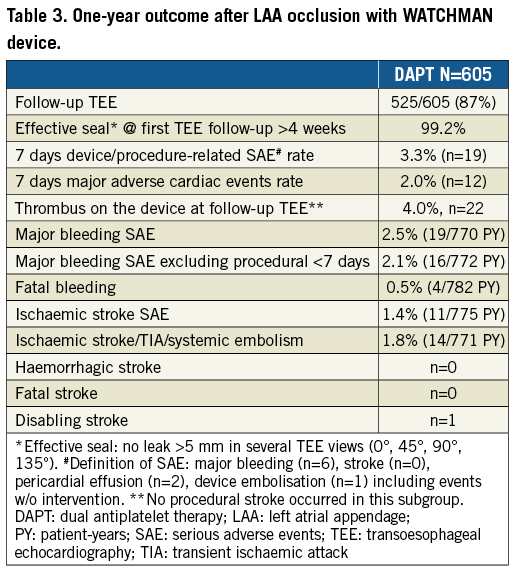
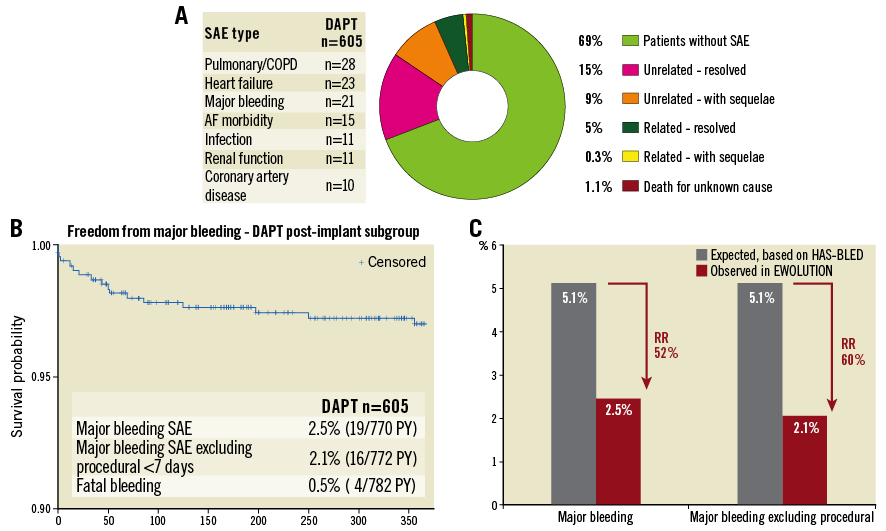
Figure 2. Presence or absence of serious adverse events at one year. A) Overview of all serious adverse events at one year. Major bleeding is the most relevant SAE related to the underlying disease and possibly linked to the dual antiplatelet therapy. B) Kaplan-Meier analysis of major bleeding events; most events occurred in the first three months while on dual antiplatelet therapy. C) Comparison of expected bleeding rate if patients were on oral anticoagulation based on average HAS-BLED score in the EWOLUTION DAPT subgroup9.
Up to the one-year time point, two more patients had a fatal bleeding, reflecting the high-risk patients included in the EWOLUTION study, particularly in this DAPT subgroup. Major bleeding was the most common SAE (n=19) (Table 3, Figure 2B); other events including device embolisation (one observed) and pericardial effusion (n=2) occurred rarely. The expected rate of major bleeding in EWOLUTION would have been 5.1% if vitamin K antagonists (VKA) had been used (Table 4); the observed rate of major bleeding was 2.5%, and 2.1% if periprocedural bleedings were not included (Figure 2C, Table 4). By one year, DAPT was discontinued in 85% of patients in EWOLUTION (Figure 3A, Figure 3B). The registry did not capture the reason for DAPT treatment but it is suspected to be linked to an underlying coronary artery disease with possibly repetitive acute coronary syndromes. Bleeding occurred mostly within the first 180 days, probably linked to the bleeding risk that comes with DAPT; after switching to aspirin monotherapy, the number of events was very low (Figure 2B).
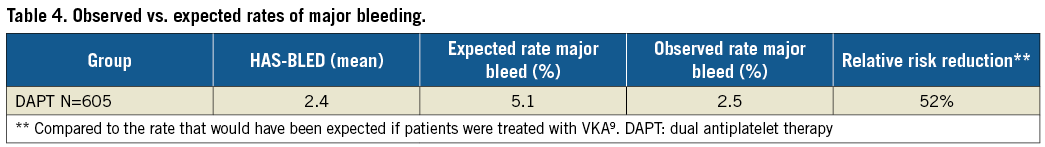
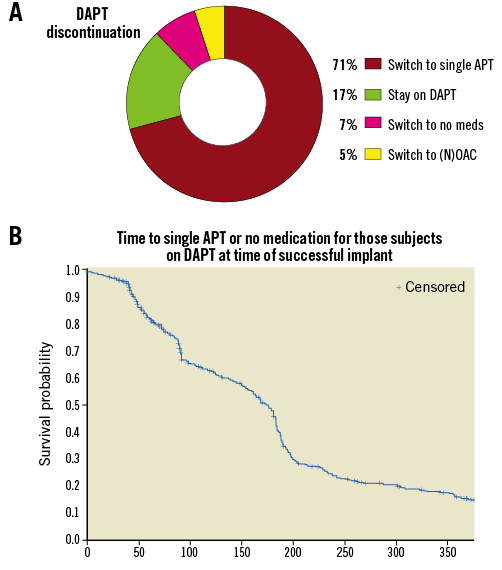
Figure 3. Periprocedural drug treatment following WATCHMAN LAA closure, including discontinuation of DAPT and switch to aspirin monotherapy and no treatment. A) Total percentage of patients switched from DAPT. B) Kaplan-Meier analysis of change from DAPT to aspirin monotherapy or nothing over the first year.
Regarding the complete list of SAE at one year, 0.3% of patients experienced a serious adverse event that did not completely resolve and was related to the device (Figure 2A). In total, 31% of patients in the EWOLUTION DAPT subgroup experienced an SAE during the first year, mostly related to their underlying disease including chronic obstructive pulmonary disease (COPD), heart failure, morbidity related to atrial fibrillation, infection and impaired renal function (Figure 2A). In total, there were 288 events in 71 different categories. After one year, 90.4% of patients were alive. The cause of death was known in 51/58 patients, mostly related to the underlying disease and not caused by ischaemic cerebrovascular or peripheral disease: 2.6% cardiovascular death and 5.8% non-cardiovascular death.
There was a high rate of TEE follow-up in EWOLUTION: 87% of the patients had TEE data available, allowing the assessment of an effective seal in 99.2% of the patients (Table 3). Device thrombus was present in 22 patients (4.0%). Medications were adjusted in 20 patients, while no action was taken in the remaining two. More specifically, 12 patients were transiently switched to OAC (two warfarin, 10 NOAC), four patients stayed on DAPT and one patient stayed on single antiplatelet therapy (APT). Two patients discontinued anticoagulation despite the presence of a thrombus (one patient switched from DAPT to single APT, one patient switched from DAPT to nothing). At the next follow-up (FU), 19 patients had completely resolved thrombus including the two patients where no action was taken. The remaining three cases are unknown, as the patients had no TEE performed after the thrombus was discovered. In patients with a thrombus detected at routine TEE, there were no subsequent reports of adverse events such as stroke, transient ischaemic attack (TIA) or embolism. In the patients with a stroke where TEE or computed tomography (CT) imaging was performed, one instance of device thrombus was observed.
The one-year data of EWOLUTION regarding efficacy in the DAPT subgroup are summarised in Table 5 and Figure 4. Based on their CHA2DS2-VASc score, a rate of 7.5% ischaemic strokes would have been expected in the DAPT subgroup in the absence of stroke preventive therapy. DAPT treatment was continued beyond one year in 9.8% of patients (n=59), probably due to the comorbidity of an acute coronary syndrome. EWOLUTION revealed a stroke rate of 1.4/100 PY (11/775 PY; 11 events in the cohort). The observed rate of ischaemic stroke/TIA/systemic embolism was 1.8/100 PY (14/771 PY; 14 events in the cohort) corresponding to an 83% risk reduction as compared to the expected rate of 10.5 based on CHA2DS2-VASc in the absence of stroke prevention therapy. There was no report of haemorrhagic stroke. No fatal stroke was observed at one-year follow-up. There was one disabling stroke reported. The expected rate of ischaemic stroke was 5.3-fold higher. The Kaplan-Meier curve shows a slow, but steady increase in event rates (Figure 2A) very similar to the stroke events with oral anticoagulation; there is no increase in strokes during the first few months when the device endothelialises. The rate of stroke in the subgroup of patients with a CHA2DS2-VASc ≥3 (n=540 patients) was 1.61 per 100 PY.
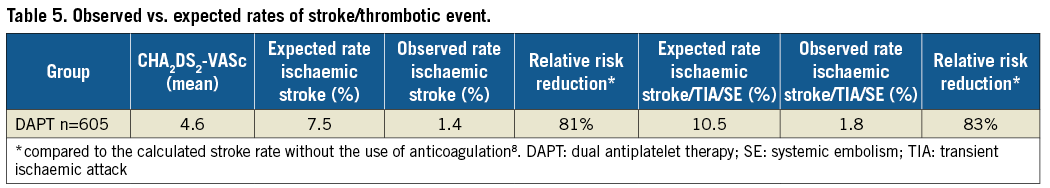
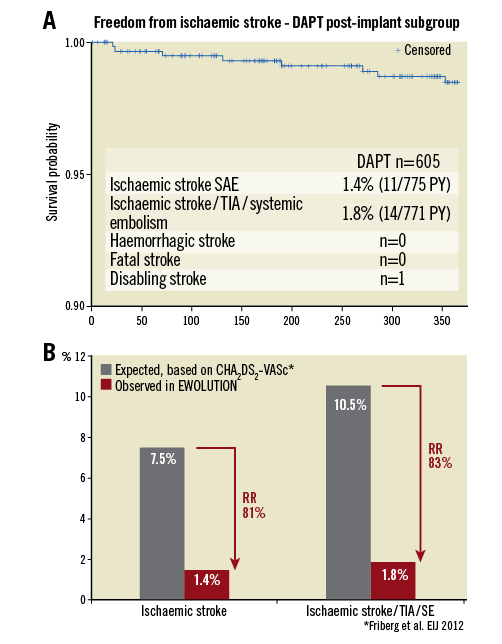
Figure 4. Efficacy of LAA occlusion. A) Kaplan-Meier analysis of ischaemic stroke rate during the first year after LAA closure. B) Annual stroke rate and stroke/TIA/systemic embolism observed in EWOLUTION compared to expected rate based on the average CHA2DS2-VASc score of patients in EWOLUTION8.
Discussion
This EWOLUTION data analysis provides one-year efficacy data on a high-risk, OAC-ineligible patient population receiving LAA closure followed by DAPT. The study shows a rate of ischaemic stroke of 1.4% in the 605 patients with WATCHMAN LAA occluder therapy; compared to the rate expected from the CHA2DS2-VASc score this represents an 81% risk reduction. This result is very similar to the overall EWOLUTION patient cohort where ischaemic strokes were observed at a rate of 1.1%, albeit also with a lower CHA2DS2-VASc score (84% risk reduction)10. Major bleeding rates in the DAPT patient cohort were not negligible, yet reduced by around 50% as compared to the expected rates on oral anticoagulation based on the HAS-BLED score. The bleeding rate became very low once periprocedural DAPT therapy was switched to monotherapy (71%) or no therapy (7%).
The European atrial fibrillation (AF) guidelines from 2012 were the first to discuss LAA closure therapy, mainly for patients ineligible for long-term oral anticoagulation11. Accordingly, patients receiving WATCHMAN LAA closure in Europe are usually high-risk, warfarin- and NOAC-ineligible patients. DAPT is mostly employed as post-procedural drug regimen based on the results of the ASAP registry12. Our previous results from the EWOLUTION registry have led to a change in the WATCHMAN instructions for use (IFU); these now specifically allow both DAPT as well as a three-month course of NOAC5. This very flexible scheme allows tailoring post-procedural anticoagulation to the individual patient. The one-year outcome data of a cohort of 605 patients treated with DAPT following WATCHMAN implantation in the EWOLUTION registry now add further scientific information to this European perspective on LAA closure therapy.
Procedural safety of LAA closure for stroke risk reduction in patients with AF is important for the overall net benefit of the procedure. Bleeding is numerically the highest SAE, occurring at a rate of 2.5/100 PY; fatal bleeding was observed in four patients (0.5%). Similar data at three months were recently observed in the AMPLATZER Amulet European registry13. The Kaplan-Meier curve suggests that bleeding is linked to DAPT therapy. The absolute number of bleeding events, however, is still lower than observed in a similar high-risk population on NOAC therapy14. Reducing the duration of DAPT therapy or replacing DAPT with transient NOAC therapy on a reduced dosing scheme may be an alternative to DAPT and should be explored further.
After one year, close to 10% of the patients receiving WATCHMAN LAA closure followed by DAPT had died. Generally, octogenarians have a massively increased stroke and bleeding risk; these patients in particular will benefit from a procedure with reduced bleeding risk compared to NOAC therapy. In our subgroup analysis, >30% of patients were older than 80 years - there was no increase in the periprocedural event rate in this group. Therefore, the decision on LAA closure should be based on an individual balance of stroke and bleeding risk as well as life expectancy.
In patients with a CHA2DS2-VASc score ≥3 the rate of ischaemic stroke was as low as 1.61/100 PY. These numbers compare well with data from patients with a CHA2DS2-VASc score ≥3 on apixaban or warfarin in the ARISTOTLE trial (event rate of 1.48 and 2.03 per 100 PY, respectively)15. Large registries from the USA on patients with a CHA2DS2-VASc score ≥3 have found dabigatran, apixaban and rivaroxaban to be associated with a stroke or peripheral embolism rate of 1.35, 1.67 and 1.69 per 100 PY, respectively16. To confirm non-inferior efficacy of LAA closure compared to NOAC therapy, a randomised trial is necessary; such trials are in preparation. In addition, a study design comparing low-dose NOAC therapy plus LAA closure vs. full dose NOAC therapy might be attractive since recently published data describe a rate of thrombus in the LAA of around 1% despite efficient oral anticoagulation, particularly in patients with a CHA2DS2-VASc score ≥517.
Limitations
EWOLUTION is a prospective registry. As such, one limitation of the study is the lack of a randomised control. The clinical observations at one year described here are associated with, but not proven to be causally related to, the intervention of LAA closure. In addition, comparing event rates regarding ischaemic stroke and bleeding based on risk factor scores may either overestimate or underestimate the true event rate in this population. However, both the HAS-BLED and the CHA2DS2-VASc scores have been validated in large patient cohorts. This study includes only one device for LAA closure, namely the WATCHMAN, and results would not necessarily apply to other devices.
Future directions
Several new trials on other devices aiming for FDA approval randomise different devices vs. WATCHMAN implantation but not drug therapy. Further insights into the efficacy of LAA closure are therefore expected only from the randomised ASAP-TOO study18. This study started in February 2017, comparing WATCHMAN LAA closure to antiplatelet therapy or nothing for stroke prevention in patients unsuitable for oral anticoagulation.
Conclusions
The one-year outcome data of patients on dual antiplatelet therapy after WATCHMAN LAA closure in the EWOLUTION registry provide important insights regarding the safety and efficacy of interventional stroke prevention. Patients with atrial fibrillation at high risk of ischaemic events and unsuitable for oral anticoagulation were studied. The safety of the procedure has greatly improved and no device- or procedure-related late events such as pericardial effusion/tamponade were observed. There was a high rate of complete occlusion (>99% on TEE), resulting in a >80% risk reduction for ischaemic events compared to the expected rate based on CHA2DS2-VASc score in the absence of stroke prevention therapy in this population. Major bleeding rates were halved compared to the expected rates based on HAS-BLED score under VKA therapy. While EWOLUTION was not a randomised trial, a highly positive net benefit of WATCHMAN LAA closure is strongly supported by these data, given that 90% of serious adverse events resolved completely.
| Impact on daily practice The introduction of NOAC as the standard drug regimen for stroke prevention in patients with atrial fibrillation has greatly improved treatment options in this setting. However, unresolved issues remain, particularly in the elderly: an increasing stroke risk is combined with a high bleeding risk, limiting the options regarding long-term oral anticoagulation. Misdosing NOAC in the fear of bleeding events ignoring the increased stroke risk has become a common issue. The one-year EWOLUTION data, together with the randomised data from the PROTECT-AF and PREVAIL studies, strengthen the recommendation to offer LAA closure to patients not able to tolerate full dose oral anticoagulation. Given the wealth of data that has become available over the last 12 months regarding this intervention, current guidelines may need to be updated. |
Acknowledgements
The following investigators and institutions participated in the EWOLUTION study. Investigators are listed after centres in alphabetical order. Al Qassimi Hospital: Arif Al Nooryani; Asklepios Klinik Saint Georg: Felix Meincke; Asklepios Klinik Weissenfels: Thomas Fiedler; Ospedale di Cirie: Gaetano Senatore; Beaumont Hospital: David Foley; Cardioangiologisches Centrum Bethanien: Boris Schmidt; CHRU de Lille: François Brigadeau; CHU Grenoble Hopital Michallon: Pascal Defaye; CHU Henri Mondor: Emmanuel Teiger; CHU La Timone Hospital: Jean-Louis Bonnet; Dominikus-Krankenhaus: Christof Wald; Elisabeth Krankenhaus Essen: Thomas Schmitz; Erasmus MC - University Medical Center Rotterdam: Tamas Szili-Torok; Evangelisches Krankenhaus Bielefeld: Wladimir Tschishow; Fondazione Centro San Raffaele: Patrizio Mazzone; Freeman Hospital: David Crossland; Herzkatheter Asklepios Wandsbek: Martin W. Bergmann; Hôpital Bichat: Alec Vahanian; Hospital Clinico Salamanca: Ignacio Cruz-Gonzalez; Hôpitaux du Haut-Leveque: Jean-Benoit Thambo; Johannes Gutenberg Universitaet Mainz: Tommaso Gori; John Radcliffe Infirmary Oxford II: Timothy Betts; King Fahed Medical City Prince Salman Cardiac Center: Faisal Al Smadi; Klinikum Neuperlach: Harald Mudra; Krankenhaus Barmherzige Bruder: Robin Molitoris; Medisch Centrum Leeuwarden: Richard Folkeringa; Medisch Spectrum Twente: Yorick Stevenhagen; NCN Nouvelles Cliniques Nantaises: Daniel Gras; Onze Lieve Vrouw Ziekenhuis: Tom De Potter; Ospedale Ferrarotto: Corrado Tamburino; Ospedale Sacro Cuore Don Calabria: Giulio Molon; Regional Vascular Center: Vladimir Protopopov; Royal Victoria Hospital: Mark Spence; Department of Cardiology, University of Medical Sciences, Poznan: Marek Grygier; Santa Maria, Lisbon: Eduardo Infante Oliveira; St. Antonius Ziekenhuis: Lucas Boersma; St. Katharinen Krankenhaus: Horst Sievert; State Cardiology Research Center: Evgeny Merkulov; State Research Institute of Circulation Pathology: Evgeny Pokushalov; Szpital Uniwersytecki: Adam Sukiennik; The Brompton Hospital: Tom Wong; Universitatsmedizin Greifswald: Mathias Busch; University Berlin, Charite Virchow Standort: Leif-Hendrik Boldt; University KH Bonn: Georg Nickenig; University Leipzig: Martin Neef; Vivantes Klinikum Am Urban: Hüseyin Ince; Vivantes Klinikum im Friedrichshain: Stephan Kische.
Funding
Boston Scientific Corporation provided funding for the study.
Conflict of interest statement
M. Bergmann, H. Ince, B. Schmidt, T. Betts and L. Boersma have received lecture and consultancy fees from Boston Scientific outside the current work. The other authors have no conflicts of interest to declare.

