
Abstract
Aims: While LAA closure has recently been incorporated into both European and US guidelines for stroke prevention, uncertainties regarding post-procedural drug therapy so far limit its adoption. The aim of this analysis is to compare real-world outcome data stratified for the post-procedural drug regimen employed.
Methods and results: One thousand and five patients were implanted with a WATCHMAN device in the prospective EWOLUTION study at 47 centres; 73.5% of the patients were deemed contraindicated for long-term OAC therapy. Here we report on three-month data including the first follow-up TOE exam for 94% of the study population. Following LAA closure, patients received DAPT, VKA, NOAC, single antiplatelet or no therapy (60.3%, 15.4%, 10.9%, 7% and 6.5%, respectively). Device thrombus (2.6%), stroke (0.4%) and major bleeding SAE (2.6%) rates were low overall and did not vary by post-implantation medication strategy. Patients on NOAC had the lowest bleeding rate, without an increase in device thrombus or stroke rates.
Conclusions: LAA closure with the WATCHMAN device is feasible in patients with a relative or absolute contraindication to oral anticoagulation. Neither DAPT nor NOAC therapy leads to a significant increase in device thrombus, stroke or bleeding compared to the standard VKA regimen. Numerically, NOAC therapy had the lowest event rate.
Abbreviations
ASD: atrial septal defect
CAD: coronary artery disease
(D)APT (dual) antiplatelet therapy
HEENT: head, ears, eyes, nose and throat
LAA: left atrial appendage
(N)OAC (non-vitamin K) oral anticoagulants
SAE: serious adverse event(s)
TIA: transient ischaemic attack
TOE: transoesophageal echocardiography
VKA: vitamin K antagonists
Introduction
Left atrial appendage (LAA) closure is a non-pharmacologic alternative for stroke prevention in patients with non-valvular atrial fibrillation. The device is intended to close off the LAA, considered to be a major source of thromboembolism1, and thus offer protection against stroke as an alternative to long-term oral anticoagulation therapy (OAC)2. Important outstanding questions concerning LAA closure in Europe and the USA revolve around the different post-procedural medication strategies and their impact on stroke, thrombus and bleeding risk3,4.
EWOLUTION (Registry on Watchman Outcomes in Real-Life Utilization) is a prospective, multicentre, observational study, collecting real-world clinical data on procedural success, leakage and complications, as well as long-term patient outcomes. Recruitment finished in May 2015 and follow-up data collection will continue for up to two years on all participants. In this study, no restrictions are made regarding post-procedural drug regimens as the device moves outside of the randomised clinical trial environment into broader clinical use. Discretion is left to the treating physician, guided by the evolving international guidelines, approved device indications and medication practices5,6.
Previous publications have summarised the design, patient baseline characteristics and acute implant results of the EWOLUTION patient cohort7,8. Key characteristics of the study participants include presentation with advanced age, a high prevalence of cardiovascular disease, and the presence of risk factors for thromboembolic stroke and bleeding. The current manuscript focuses on bleeding, strokes and device-associated thrombus during the first three months of follow-up, including a post-procedural transoesophageal echocardiography (TOE), stratified for post-procedural drug regimen.
Methods
As reported previously, EWOLUTION is a multicentre, prospective, non-randomised cohort study regarding safety and efficacy of LAA occlusion employing the WATCHMAN® device (Boston Scientifc Corp., Marlborough, MA, USA)7,8. Signed informed consent was obtained prior to attempted WATCHMAN implantation. Participating centres were encouraged to include consecutive patients from their local routine practice without any selection criteria; eligibility was solely determined by local, national and international guidelines on LAA occlusion during the recruitment period. The study adhered to international rules for scientific studies, and the principles of the Declaration of Helsinki, with local ethics committee approval at all participating centres. The electronic clinical report form provides fields for anticipated serious adverse events (SAE) such as procedure-specific events (perforation, tamponade, device embolisation, neurological events, device thrombosis and bleeding according to the BARC criteria)7. All centres and events are monitored by an outside contract research organisation. Rates of events are calculated via the Kaplan-Meier method to account for censoring. P-values are based on log-rank tests for time-to-event analysis.
Study enrolment occurred from October 2013 to May 2015. In a total of 47 centres in 13 countries, there were 1,025 patients enrolled with 1,005 successful LAA WATCHMAN implants employing current techniques9. Centres were encouraged to recruit consecutive patients to represent real-life practice and avoid selection bias. At least 39 sites have enrolled consecutive patients. A comparison of SAE in those 39 centres versus those with non-consecutive enrolments (n=3) or unknown (n=5) did not show any difference regarding the occurrence of SAE (p=0253).
Follow-up is according to the local institution’s standard of care and will continue for two years post enrolment. Post-implant data collection includes LAA imaging data via TOE, OAC or antiplatelet medications and adverse events. At the time of this analysis, 94% of patients have completed at least three months of follow-up and 871 patients (87%) have had at least one LAA imaging examination (Figure 1).
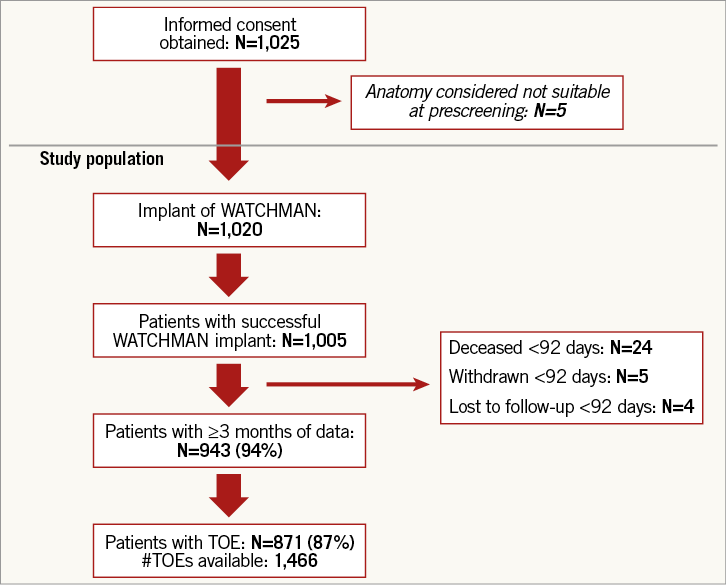
Figure 1. Patient flow chart in the EWOLUTION study. EWOLUTION: Registry on Watchman Outcomes in Real-Life Utilization; TOE: transoesophageal echocardiography
STATISTICAL ANALYSIS
Continuous variables are summarised using the mean, standard deviation, median, range, and interquartile range and are compared using ANOVA tests. Categorical variables are summarised using counts and percent and compared using the Fisher’s exact test. Kaplan-Meier event rates were calculated for adverse events up to 92 days (three months) post implant, with the corresponding log-rank p-value for between-group comparisons where appropriate. Identification of heart failure decompensations and SAE was at the discretion of the centre. Events and relevant source documents were additionally reviewed by the sponsor Medical Safety Group (MSG)8. For those subjects not experiencing an event, time to last contact was used as the censoring time. All centres were monitored by an outside contract research organisation (CRO) and every centre was visited at least once.
Multivariate Cox regression models were used to estimate potential predictors of events of interest. These potential predictors were chosen based on their clinical relevance and potential relationship to the outcome of interest although the event rate was low. Univariate models were also run for those potential predictors that were included in the multivariate analysis, but were used for descriptive purposes of the individual impact of a variable on the outcome of interest and were not used to guide the decision as to which variables to include in the multivariate model based on the significance level in the univariate model. For categorical variables in the multivariate model, the reference group was specified for each model separately. In the model predicting device-associated thrombi, vitamin K antagonists (VKA) was set as the reference group, while in the model predicting bleeding SAE non-vitamin K oral anticoagulants (NOAC) was set as the reference group. Eligibility for oral anticoagulation therapy (OAT) was also included as a predictor in both the model predicting device-associated thrombi as well as the model predicting bleeding SAE. Other predictors that were included in the models were CHA2DS2-VASc (all SAE and device-associated thrombi models) and HAS-BLED (all SAE and bleeding SAE models) scores at baseline. For categorical variables in the univariate model, binary indicators were created for each level of the categorical variable and included in the model as a single predictor.
Results
Patient enrolment, demographic data, and acute safety results have been reported previously8. Briefly, of the 1,005 successfully implanted subjects with baseline data available, the average age was 73.4 years and 25.6% were ≥80 years of age. The majority of subjects were male (59.8%) and had a history of hypertension (86.9%), with approximately one third having congestive heart failure, 13.3% with left ventricular dysfunction, 28.7% having type 2 diabetes, 10.8% history of transient ischaemic attack (TIA), 19.3% history of ischaemic stroke, 14.7% history of haemorrhagic stroke, 31.6% history of major bleeding, 42.1% vascular disease, 16.1% abnormal renal function, 4.4% abnormal liver function. Subjects were at a high risk of both stroke and bleeding, with 72.9% of patients having a CHA2DS2-VASc score ≥4 and 40.3% having a HAS-BLED score ≥3. The final analysis identified 73.4% of patients as being contraindicated for OAC.
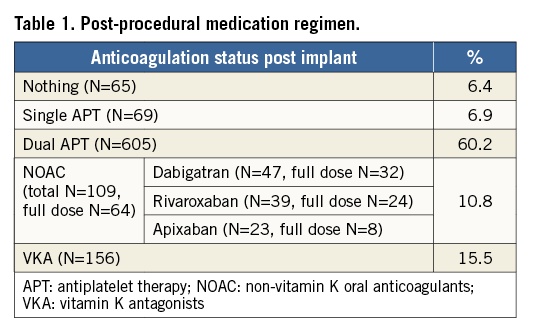
POST-IMPLANT MEDICATION USAGE PATTERNS
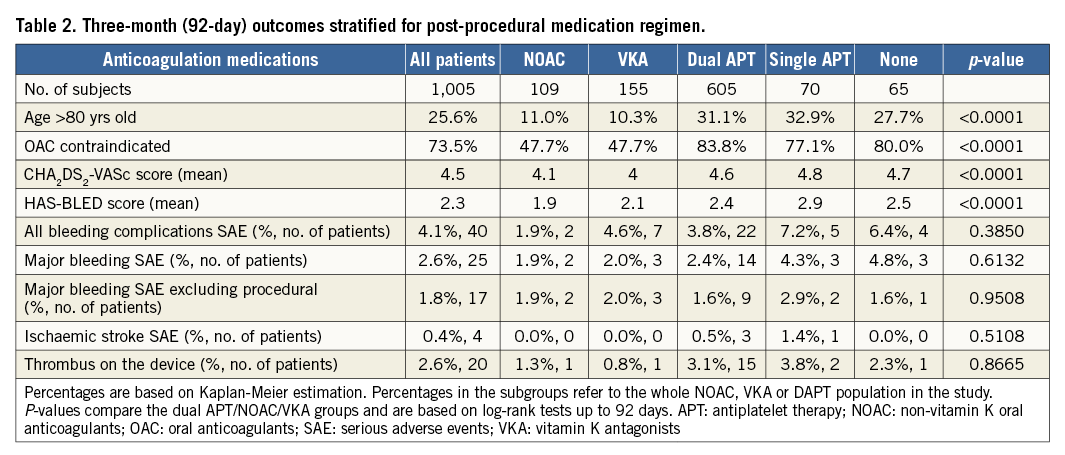
Post-implant medication strategies included either dual or single antiplatelet therapy (60% and 7% of subjects, respectively), or oral anticoagulation (16% VKA and 11% NOAC), while 6% of subjects received no anticoagulation or antithrombotic therapy (n=65). For the patients taking NOAC (n=109), the majority were prescribed dabigatran (n=47), then rivaroxaban (n=39) and apixaban (n=23), with 59% of these patients taking full dose (Table 1). In general, post-implant drug therapy was at the discretion of the operator. There was a significant difference in the mean CHA2DS2-VASc score at baseline for groups defined by their post-procedural medications (p<0.001); HAS-BLED similarly varied between these groups (p<0.001) (Table 2).
THREE-MONTH CLINICAL OUTCOMES AND TOE FOLLOW-UP DATA
During the first three months, 24 patients died and five patients withdrew. One patient had a periprocedural air embolism and a prolonged intensive care unit (ICU) stay; the patient died three weeks later. For three patients the cause of death is unknown. Two patients had a fatal gastrointestinal (GI) bleed while on dual antiplatelet therapy (DAPT) medication. One respiratory failure was possibly aggravated by the TOE procedure. The other 16 patients died from causes independent of procedural or device risks, thus reflecting the high-risk population included in this registry: heart failure (five patients), multiorgan failure and/or sepsis (six), renal failure (two), cancer (one), colitis (one), cardiac arrest (one). As already reported for the one-month time point after LAA closure8, no significant difference regarding the SAE rate between patients contraindicated to oral anticoagulation (73.5%) and patients eligible for OAC was observed.
While previously predominant procedure-related SAE, such as pericardial effusion/cardiac tamponade (n=7) and stroke (n=4), could be minimised, other events related to post-procedural drug regimen and patient selection, such as bleeding complications (n=47), heart failure (n=21) and other cardiovascular events (n=39), became more abundant. Most SAE were transient in nature, with only 0.5% of patients who had an SAE possibly related to the device or procedure (or unknown) with residual clinical effects at three months.
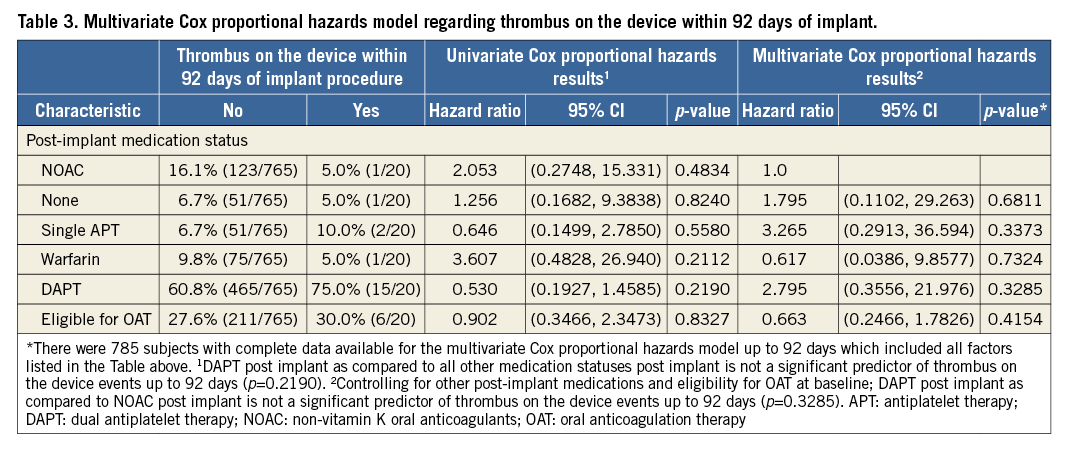
The overall rate of device-associated thrombus was 2.6% (n=20); all cases of device thrombus were detected by routine TOE not linked to clinical events. Looking at individual medication regimens, no statistical difference could be detected (p=0.8665). However, numerically the highest event rate was 15 patients with device thrombus in the DAPT group (Table 2). While we consider the patient numbers to be too low to draw any definite conclusions, it is noteworthy that even the patient groups with only single antiplatelet therapy or no therapy at all did not show increased risk of stroke or device thrombus. The overall three-month ischaemic stroke SAE rate was 0.4%; none of the strokes occurred in a patient where TOE had detected a thrombus. The rates did not vary significantly between medication groups (p=0.5043), though three strokes occurred in subjects on DAPT (0.5%) and there were zero events in subjects on NOAC or VKA (Table 2). Multivariate Cox proportional hazard analysis did not find any statistical link between device thrombus and post-procedural drug regimen or patient eligibility for oral anticoagulation (Table 3).
In the EWOLUTION study, the most prevalent serious adverse event in the 92-day post-implantation period was bleeding complications, with 40 subjects experiencing an event (4.1%), 25 of them being classified as major bleeding (2.6%). The rate of bleeding SAE did not vary significantly based on the subject’s post-implantation drug regimen but was numerically lowest for subjects on NOAC (1.9% – both cases were on rivaroxaban) compared to the other regimens (p=0.3850) (Table 2). Multivariate Cox proportional hazard analysis did not find a statistical link between bleeding and post-procedural drug regimen or patient eligibility for oral anticoagulation (Table 4).
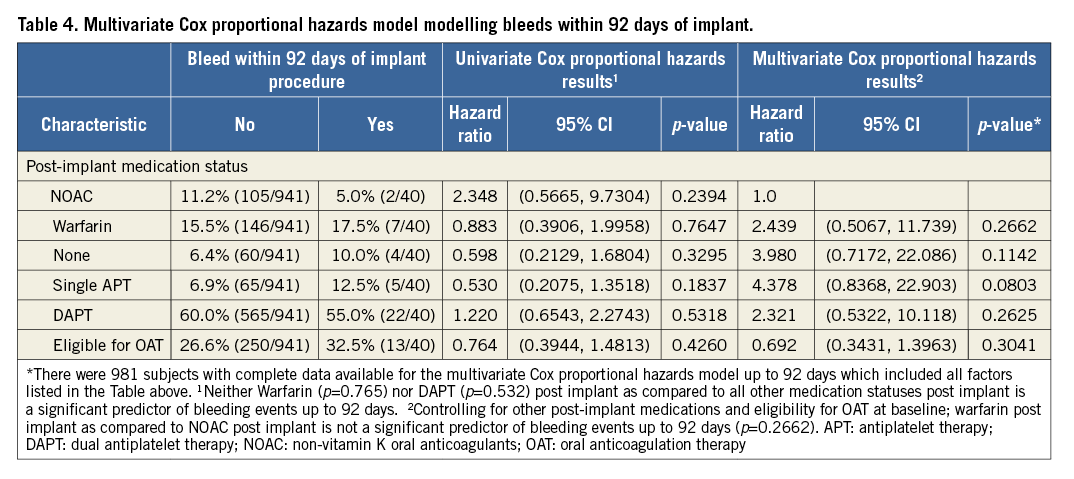
Although not statistically significant and certainly influenced by patient selection, the rates of bleeding and ischaemic stroke were lowest with periprocedural NOAC therapy (Table 2-Table 4). Mean CHA2DS2-VASc and HAS-BLED scores were significantly lower in the NOAC group compared to the VKA and DAPT patient groups (Table 2). A multivariate analysis controlling for CHA2DS2-VASc score at baseline failed to identify post-implant medication strategy as a significant predictor of device-associated thrombi; yet, dual antiplatelet therapy exhibited the highest risk in this study population (HR 4.3, 95% CI: 0.6, 32.5) as compared to VKA (Table 4). The multivariate analysis controlling for HAS-BLED score could not detect differences in bleeding risk (HR 1.5, 95% CI: 0.3, 6.4 and HR 2.2, 95% CI: 0.5, 10.7, respectively, for DAPT and VKA as compared to NOAC) (Table 4).
The hazard ratios calculated both in the univariate as well as in the multivariate Cox proportional analysis regarding thrombus on the device and bleeding found all options of drug treatment to be viable alternatives as there was no significant difference between the groups. Post-procedural three-month NOAC therapy appears to come with a good balance between bleeding risk and thrombus on the device in the high-risk patient cohort included in the EWOLUTION registry as, e.g., DAPT therapy came with a 2.8-fold higher rate of thrombus on the device (Table 3, multivariate analysis) and a 2.32-fold increase in bleeding events (Table 4, multivariate analysis).
Discussion
While LAA closure has been incorporated in both European and US guidelines for stroke prevention in recent years, some uncertainties have so far limited the adoption of the technique to a broader extent. These include the post-procedural drug regimen during the first three months while the device endothelialises and an anticoagulation therapy is deemed to be necessary prior to switching the patient to single antiplatelet therapy. EWOLUTION provides data on almost all possible regimens during this time frame. While periprocedural events such as pericardial effusion, air embolism and stroke could be minimised by current implantation techniques, bleeding complications emerged as the most frequent SAE within the first three months, closely linked to the drug regimen employed during this time frame. The next step to minimise risks conferred by LAA closure employing the WATCHMAN device is therefore to identify the optimal post-procedural drug regimen, minimising risks for device-related thrombus, stroke and bleeding.
In line with current practice in most centres in Europe, the majority of patients in EWOLUTION received dual antiplatelet therapy. One hundred and nine patients (10.9%) received NOAC. Some participating centres have apparently adopted DAPT as their default post-procedural drug regimen since 17% of patients receiving DAPT were eligible for OAC. Numerically, those prescribed DAPT after WATCHMAN implantation had the highest rate of thrombus on the device (3.1% vs. 0.8% with VKA and 1.3% with NOAC), though this event rate was statistically not different to the other drug regimens. Patients receiving DAPT had higher CHA2DS2-VASc and HAS-BLED scores compared to patients receiving OAC. The absolute rate of 3.1% device-associated thrombus is in the range of past randomised trials on WATCHMAN with a 45-day VKA course. Comparing EWOLUTION with the ASAP prospective registry that also employed dual antiplatelet therapy following LAA closure in a patient cohort with a mean CHA2DS2-VASc score of 4.4±1.7, the event rates are quite similar: out of 150 patients enrolled, six patients (4%) in ASAP developed a thrombus on the device10. In summary, DAPT therapy following WATCHMAN LAA closure is the routine option for patients ineligible for OAT. Interestingly, we have found that, even in patients with single antiplatelet therapy or no drug therapy aimed at reducing thrombus formation, LAA closure with the WATCHMAN device appears to be safe. We conclude that all three main drug regimens (VKA, DAPT, NOAC) appear to be safe and effective. The low rate of thrombus on the device and bleeding events with post-procedural NOAC compared to post-procedural VKA or DAPT appears to be consistent even when considering the differences between the groups regarding CHA2DS2-VASc, HAS-BLED scores or focusing on the OAT non-eligible patients.
The study finds patients with periprocedural NOAC therapy to have the numerically lowest rate of thrombus formation on the device, stroke and bleeding up to the three-month time point. NOAC have the advantage of a shorter half-life than both VKA and DAPT, yet should be efficient in preventing thrombus formation in the low-flow area of the left atrium. In addition, NOAC are at least as effective as VKA in preventing strokes from all sources. EWOLUTION is the first prospective, large-scale study to report on outcomes with NOAC as adjuvant therapy post WATCHMAN implant. In line with these results, a retrospective study initiated after the results of our prospective registry were presented as a late breaking trial at EuroPCR 2016 reports on similar good outcomes using NOAC in the periprocedural phase of LAA closure employing the WATCHMAN device11. Our multivariate analysis controlling for CHA2DS2-VASc and HAS-BLED scores as well as post-procedural drug regimen found that the good results of NOAC in this time frame were not explained by a lower-risk group as captured by these two scores. A randomised controlled trial with a NOAC arm would be needed to appreciate fully the difference that may exist when comparing to VKA or DAPT.
The recent GARFIELD-AF European all-comers registry on patients with atrial fibrillation found a mortality rate of 1.25% per 100 person-years12. The mortality rate of 23.5% at three months driven by the comorbidities of EWOLUTION patients does reflect the high-risk patient characteristics included in this study; however, in retrospect these patients should not have received an LAA closure procedure. The local centres did not regard them as critically ill at the time of the procedure. In fact, these patients had a very high-risk score and therefore implanters thought that they would benefit rapidly from the improved stroke protection in comparison to no oral anticoagulation. Assessing frailty and sarcopaenia as well as other measures of life expectancy will probably need to be part of the work-up prior to LAA occlusion.
Limitations
While EWOLUTION is a prospectively designed study, one limitation of the study is the lack of a randomised control. Only conclusions regarding associations of predictors with outcomes can be made; however, the broad entry criteria and large study size increase the generalisability of the results. Follow-up is limited to three months post procedure in this report, but the trial is ongoing, and the study is expected to provide continued insights with additional data collection and analysis. The final endpoint is two-year follow-up. This study includes only one device for LAA closure and results will not necessarily apply to other devices. New alternative devices have been developed, including second-generation versions that attempt to improve upon the performance of early versions, though initial clinical results have not yet demonstrated an improvement.
Conclusions
The data from the first three months of follow-up in the EWOLUTION study indicate that LAA closure with the WATCHMAN device can be successfully performed, with low rates of acute adverse events, for both OAC-indicated and contraindicated subjects, in the face of varied use of post-procedural medications.
Within the first three months after LAA closure, bleeding unrelated to the procedure or device, but related to post-procedural drug regimen, emerged as the most relevant SAE within this report. Both DAPT and NOAC appear safe as post-implant medication alternatives to VKA. In a real-world experience outside of the randomised clinical trial setting, the three-month results of EWOLUTION suggest that WATCHMAN can be safely implanted in patients with non-valvular atrial fibrillation, both those eligible for and those contraindicated to OAC, regardless of whether DAPT, VKA or NOAC is employed for periprocedural anticoagulation.
| Impact on daily practice Effective stroke protection in patients with atrial fibrillation is mandatory for both quality of life as well as mortality if one additional risk factor is present. In particular, extracranial bleeding as well as issues around drug compliance and comorbidities such as coronary heart disease with a need for effective, long-term platelet inhibition limits utilisation of the only effective drug therapy, namely oral anticoagulation. LAA closure has emerged as an effective interventional strategy for stroke prevention in patients with atrial fibrillation, eliminating the need for oral anticoagulation. The three-month data from the 1,000-patient, prospective EWOLUTION registry indicate a low rate of periprocedural serious adverse events with current implantation techniques employing the WATCHMAN device. In addition, post-procedural therapy with dual antiplatelet therapy as well as transient NOAC therapy was effective regarding inhibition of device thrombus formation. Imaging data at three months confirmed a high rate of effective LAA sealing. These data support the routine utilisation of LAA closure with the WATCHMAN device for stroke prevention in patients with atrial fibrillation where oral anticoagulation is not the optimal long-term treatment option. |
Acknowledgements
The following investigators and institutions participated in the EWOLUTION study. Investigators are listed after centres in alphabetical order.
Al Qasimi Hospital: Arif Al Nooryani; Asklepios Klinik Saint Georg: Felix Meincke; Asklepios Klinik Weissenfels: Thomas Fiedler; Ospedale di Cirie: Gaetano Senatore; Beaumont Hospital: David Foley; Cardioangiologisches Centrum Bethanien: Boris Schmidt; CHRU de Lille: François Brigadeau; CHU Grenoble Hopital Michallon: Pascal Defaye; CHU Henri Mondor: Emmanuel Teiger; CHU La Timone Hospital: Jean-Louis Bonnet; Dominikus-Krankenhaus: Christof Wald; Elisabeth Krankenhaus Essen: Thomas Schmitz; Erasmus MC - University Medical Center Rotterdam: Tamas Szili-Torok; Evangelisches Krankenhaus Bielefeld: Wladimir Tschishow; Fondazione Centro San Raffaele: Patrizio Mazzone; Freeman Hospital: David Crossland; Herzkatheter Asklepios Wandsbek: Martin W. Bergmann; Hôpital Bichat: Alec Vahanian; Hospital Clinico Salamanca: Ignacio Cruz-Gonzalez; Hospitaux du Haut Leveque: Jean-Benoit Thambo; Johannes Gutenberg Universitaet Mainz: Tommaso Gori; John Radcliffe Infirmary Oxford II: Timothy Betts; King Fahed Medical City Prince Salman Cardiac Center: Faisal Al Smadi; Klinikum Neuperlach: Harald Mudra; Krankenhaus Barmherzige Bruder: Robin Molitoris; Medisch Centrum Leeuwarden: Richard Folkeringa; Medisch Spectrum Twente: Yorick Stevenhagen; NCN Nouvelles Cliniques Nantaises: Daniel Gras; Onze Lieve Vrouw Ziekenhuis: Tom De Potter; Ospedale Ferrarotto: Corrado Tamburino; Ospedale Sacro Cuore Don Calabria: Giulio Molon; Regional Vascular Center: Vladimir Protopopov; Royal Victoria Hospital: Mark Spence; Samodzielny Publiczny Szpital: Marek Grygier; Santa Maria: Eduardo Infante Oliveira; St. Antonius Ziekenhuis: Lucas Boersma; St. Katharinen Krankenhaus: Horst Sievert; State Cardiology Research Center: Evgeny Merkulov; State Research Institute of Circulation Pathology: Evgeny Pokushalov; Szpital Uniwersytecki: Adam Sukiennik; The Brompton Hospital: Tom Wong; Universitatsmedizin Greifswald: Mathias Busch; University Berlin, Charite Virchow Standort: Leif-Hendrik Boldt; University KH Bonn: Georg Nickenig; University Leipzig: Maika Klein; Vivantes Klinikum Am Urban: Hüseyin Ince; Vivantes Klinikum im Friedrichshain: Stephan Kische.
Funding
Boston Scientific Corporation provided funding for the study.
Conflict of interest statement
The following authors have received lecture and consultancy fees outside of the submitted work broadly related to LAA closure technology: M. Bergmann has received lecture fees and consultancy fees from Boston Scientific; T. Betts has received lecture fees and consultancy fees from Boston Scientific; H. Sievert has received lecture fees and consultancy fees from Boston Scientific, Biosense Webster, Coherex, Gore, Lifetech and Occlutech; B. Schmidt has received lecture fees and consultancy fees from Boston Scientific and St. Jude Medical; K. Stein is an employee at BSC as Chief Medical Officer. L. Boersma has received lecture fees and consultancy fees from Boston Scientific and Medtronic; H. Ince has received lecture fees and consultancy fees from Boston Scientific. The other authors have no conflicts of interest to declare.

