Abstract
Aims: The WATCHMAN® device was proven non-inferior to oral anticoagulation (OAT). However, periprocedural risks and uncertainty regarding patients with absolute contraindication for OAT limit the overall utilisation of this approach. We investigated the periprocedural safety of dual platelet inhibition and primary utilisation of larger device diameters in order to minimise peri-device leakage and repositioning of the device during the implantation process.
Methods and results: Since 2010, 59 consecutive patients have been treated with the WATCHMAN® device and followed for six months. In patients with contraindications to warfarin, dual antiplatelet therapy (DAPT) was used during the first 45 days after implantation instead of warfarin. Device size was chosen 15-30% greater than the LAA diameter in order to minimise device repositioning and leakage, and to prevent device embolisation. Small, non-trabecular recesses at the superior ridge towards the inferior, left pulmonary vein were regularly observed and followed by transoesophageal echo regarding thrombus formation. We observed a 3.3% rate of pericardial effusions and a 5% rate of thrombi at the device during the healing period of 45 days and no device embolisations. One thromboembolic event without clinical sequelae was observed during the six-month follow-up period. All patients stopped DAPT at six months as no primary or secondary leakage >5 mm around the device perimeter was observed.
Conclusions: Our data suggest that DAPT can be used safely during the first 45 days in patients with contraindications to warfarin. An algorithm employing larger devices in relation to the LAA ostium with consecutively larger compression improved procedural safety compared to the current standard regarding leakage and device repositioning.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1-2% of the European population, and its prevalence is expected to increase significantly in the next 40 years1,2. It is known that in non-valvular AF patients the primary location of thrombus formation is the left atrial appendage (LAA), with over 90% of thrombi originating from the LAA3. However, traditionally only a small number of patients, undergoing cardiac surgery for other reasons, could be treated by surgical closure of the left atrial appendage3. Therefore, until recently long-term anticoagulation was the primary way to reduce the risk of cerebrovascular events in AF. However, oral anticoagulation therapy (OAT), usually performed with warfarin, carries a significant risk of bleeding complications due to a very narrow therapeutic window. Studies have shown that proper management of warfarin intake is difficult in a significant number of patients and is often discontinued due to labile INR levels or bleeding complications4,5. This, however, leaves these patients with a markedly elevated risk of cardioembolic events.
In order to avoid this therapeutic dilemma and to provide an alternative way of managing the stroke risk in AF, the WATCHMAN® device (Boston Scientific, Natick, MA, USA) for interventional closure of the left atrial appendage was introduced commercially in 2009. In the phase III trial PROTECT-AF, the WATCHMAN® device met the criteria defined for non-inferiority compared with oral anticoagulation using warfarin6.
Percutaneous LAA closure, however, carries certain risks as well. Besides periprocedural pericardial effusion, the thrombogenic potential of the device itself during the healing phase of approximately 45 days is of concern. In the PROTECT-AF trial, 4.2% of the patients who received a WATCHMAN® device developed thrombi on the device during the first 12 months (resulting in a stroke risk of 0.3 per 100 patient years)7. Similar data were obtained in the CAP registry8.
However, the optimal strategy for the first six weeks in which warfarin was used in PROTECT-AF remains uncertain, especially in patients with relative or absolute contraindications to warfarin.
Recently, new oral anticoagulants such as dabigatran (Pradaxa®) raised hopes for a new era in anticoagulation by avoiding several negative aspects of warfarin, but early experiences with these substances outside of clinical trials suggest a significant risk of bleeding with these substances9. Major bleeding occurred in 3.1%/3.6%/2.1% of patients per year with dabigatran, rivaroxaban and apixaban, respectively10-12. The bleeding risk for oral anticoagulation therapy can be calculated by the HAS-BLED score introduced in the 2010 ESC AF guidelines2. If the periprocedural risk of LAA closure can be minimised, patients with a HAS-BLED score >3 and/or recent bleeding events may be treated most effectively with LAA closure.
AIM OF THE REGISTRY
In this single-centre registry we addressed two issues regarding periprocedural safety of the implantation: we investigated whether dual antiplatelet therapy (DAPT) during the first six weeks after WATCHMAN® implantation is as safe as warfarin therapy and whether a device diameter 15-30% greater than the LAA diameter is a safe alternative to the 8-20% compression currently recommended.
Methods
PATIENT SELECTION
All patients at our institution with AF at high risk for thromboembolic complications and one of the following criteria were screened for LAA closure with the WATCHMAN® device:
– Prior bleeding complication while using warfarin
– Major bleeding prior to using warfarin leading to markedly elevated risk of recurrence
– HAS-BLED score >3
– Walking instability with recurrent falls
– Inability to maintain INR levels within the therapeutic range with warfarin
PROCEDURAL DETAILS
Since March 2010, 59 consecutive patients with relative or absolute contraindications for warfarin have been treated with the WATCHMAN® device at our institution. During the procedure heparin was administered intravenously with activated clotting time (ACT) guidance (ACT >250 seconds). No protamine was given after the procedure; the puncture site was closed using manual compression.
OVERSIZING
In order to avoid device embolisation, leakage and the need for repositioning or device change during the procedure, the device size was chosen 15-30% greater than the LAA diameter instead of 8-20% as currently suggested by the manufacturer (Table 1, Figure 1).
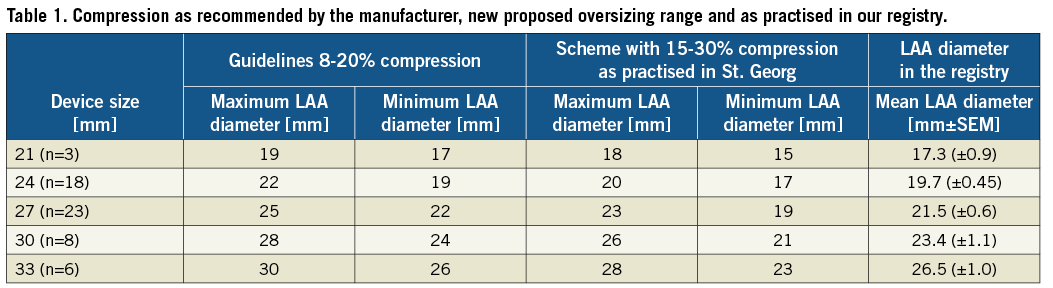
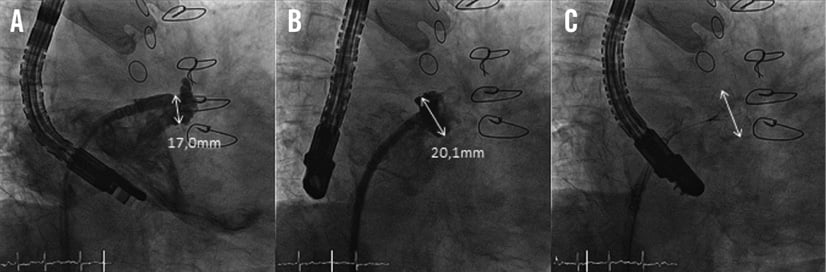
Figure 1. Fluoroscopic view of the LAA during WATCHMAN® implantation. A) Measurement of the LAA diameter prior to implantation. B and C) After implantation of a 24 mm WATCHMAN® device with and without contrast dye injection.
RECESSES
In some patients, small recesses were discovered at the junction from the left atrium to the LAA (Figure 2). Due to a lack of trabeculation we regarded them as a part of the left atrium and left them uncovered by the device. We hypothesised that this anatomical structure does not carry a significant thrombogenic potential and re-analysed the recessi at the follow-up TEE.
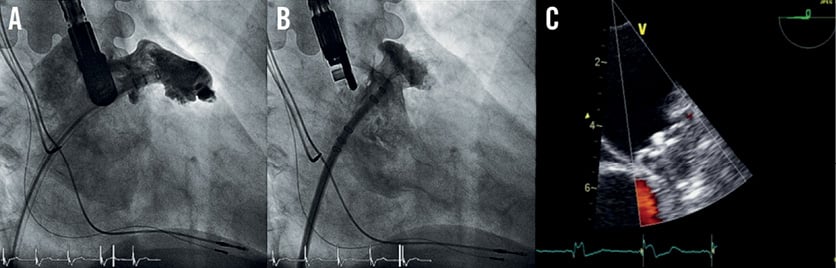
Figure 2. A) Contrast-enhanced fluoroscopic view of the LAA prior to implantation with a recessus at the LAA/left atrial junction. B) After implantation of the WATCHMAN® device without covering the recessus. C) TEE view after implantation proving no residual flow.
MEDICAL TREATMENT AND FOLLOW-UP
When OAT was feasible, the postprocedural protocol of the PROTECT-AF study was followed with warfarin for 45 days, dual antiplatelet therapy (DAPT) with aspirin 100 mg/day and clopidogrel 75 mg/day until six months after implantation, followed by aspirin monotherapy (Figure 3).
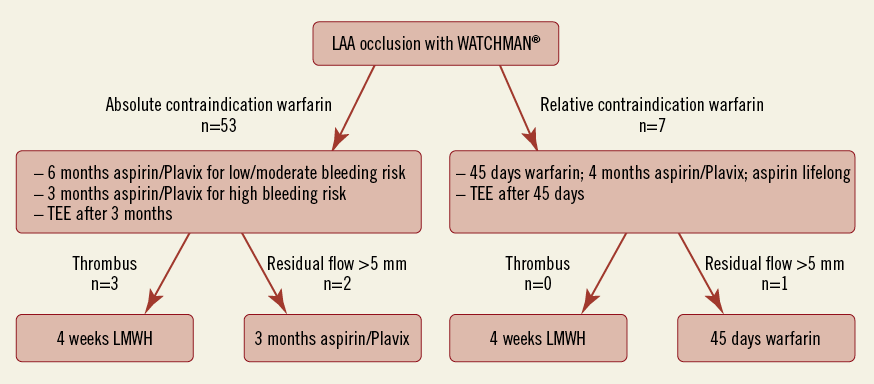
Figure 3. Flow chart of the postprocedural anticoagulation regime.
In the presence of relative or absolute contraindications to warfarin a dual antiplatelet therapy was initiated using aspirin (100 mg/day) and clopidogrel (75 mg/day) and maintained for at least three months. At three-month and six-month follow-up the device was evaluated using echocardiography. With proper position on echocardiography and in the absence of thrombi, clopidogrel was discontinued after three months in patients at very high risk for bleeding complications and at six months in patients with low or moderate bleeding risk.
In case of a peri-device flow >5 mm on follow-up echocardiography, the initial anticoagulation regime (either warfarin or aspirin/clopidogrel) was prolonged for another six weeks (warfarin) or three months (aspirin/clopidogrel) followed by TEE.
Results
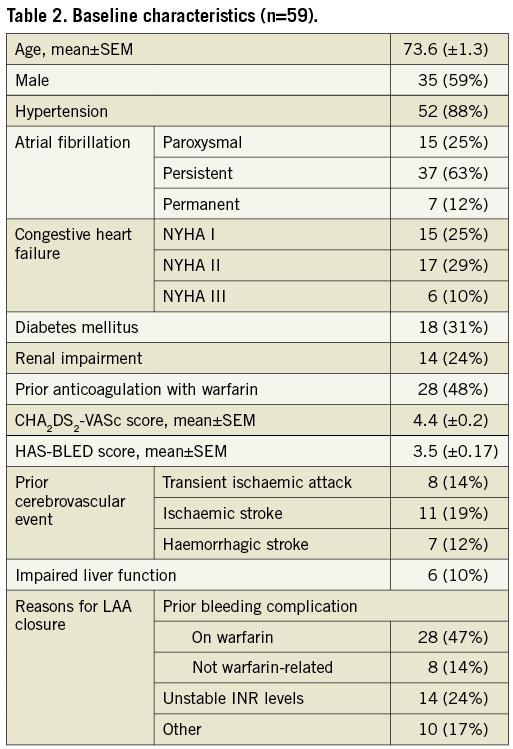
BASELINE CHARACTERISTICS
The demographic and procedure-related characteristics are shown in Table 2. The mean age was 73.6 (±1.3), and 59% of the patients were male. Mean CHA2DS2-VASc score and HAS-BLED score were 4.4 (±0.2) and 3.5 (±0.17), respectively. Eighty-eight percent had absolute or relative contraindications to warfarin: in 36 patients (61%) this was due to bleeding complications.
PROCEDURAL RESULTS AND COMPLICATIONS
In 58 of the 59 patients the device could be positioned successfully (98%). In four (6.7%) patients the device had to be changed to another size after the first implantation attempt. During eight (13.5%) procedures the device had to be repositioned due to a negative TUG test. The mean device compression was 18.7% (±1.1). No patient showed a peri-device flow >2 mm immediately after implantation (Table 3).
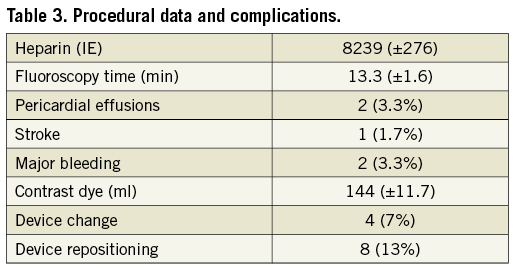
Two cases of periprocedural pericardial effusion occurred, one due to misplacement of the septal puncture site and one postprocedural. Both could be managed by interventional pericardiocentesis; the device was well positioned in both cases. The compression of the device in the second case was 17% and therefore within the recommended range. Both patients could be discharged free of symptoms a few days later. None of the patients with compressions greater than 20% suffered from pericardial effusions or other device-related complications.
One patient (1.7%) suffered from an ischaemic stroke during the procedure. The exact reason remains unclear. However, we consider a thrombus formation at or within the sheath as the most likely mechanism.
CLINICAL OUTCOME
The only cerebral event during the follow-up period (6.3±0.46 months) was a transient ischaemic attack in one patient. The symptoms resolved completely within 24 hours. No thrombus or LAA leakage was detected by TEE, thus we interpreted this event as unrelated to the LAA. One patient developed anaemia of unknown origin while taking aspirin and clopidogrel. Otherwise, there were no major bleedings recorded during dual antiplatelet therapy.
At three-month follow-up a peri-device leakage of 2-5 mm/>5 mm was detected in four (7%) and three (5%) patients, respectively. The differences between initial assessment and follow-up echo may be explained by slightly different planes. Leakages between 2 and 5 mm detected at three months were occluded by six months and were not linked to clinical events in our series.
DEALING WITH THROMBUS FORMATION ON THE DEVICE
In three patients (5%; 90% confidence interval [CI]: 2.2 to 13.3%), routine follow-up echocardiography revealed thrombi attached to the WATCHMAN® device during the six-month follow-up period (Figure 4). One thrombus was detected after six weeks, the other two 12 weeks after implantation. In one of these patients, clopidogrel was discontinued prematurely at six weeks after WATCHMAN® implantation due to suspected minor intestinal bleeding.
These three patients were treated with low-molecular-weight heparin (LMWH) for at least four weeks with regular echocardiography follow-ups. In all cases the thrombus resolved completely under LMWH treatment and no clinical or radiologic thromboembolic event occurred.
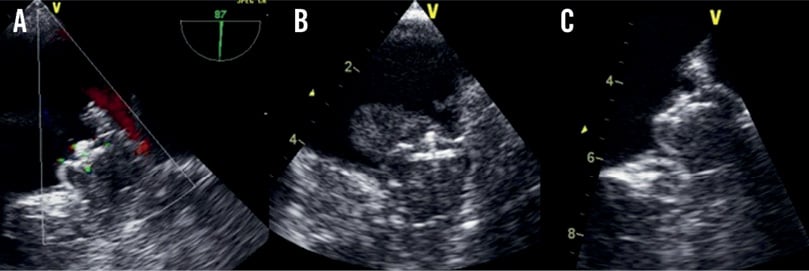
Figure 4. A) Intraprocedural TEE view after implantation. B) Follow-up TEE with a thrombus attached to the device. C) Same patient after low-molecular-weight heparin treatment without thrombus.
Discussion
This registry finds dual antiplatelet therapy during the first six months following LAA closure with the WATCHMAN® device to be a safe alternative for patients with absolute contraindications for OAT regarding thrombus formation on the device and clinical events. In addition, a strategy of oversizing the device by 15-30% is not associated with increased risk of pericardial effusion but reduces peri-device leakage in comparison to recent publications7.
More precisely, although a greater compression (device diameter/LAA diameter ratio) was used, there were only two pericardial effusions (3.4%). This is a rate similar to the amount observed in the PROTECT-AF trial6. One of these effusions occurred during the transseptal puncture and was therefore definitely unrelated to the device itself. Both incidences occurred in the initial phase of our programme. In the second case following the first 19 implantations, the device compression was 17% and thereby within the range recommended by the manufacturer. In addition, previously published data suggest that an incomplete LAA occlusion is an issue in a significant number of patients, though its clinical significance remains controversial7,13. In our follow-up echocardiographies, we observed peri-device leaks >2 mm in seven patients (12%). In comparison, 37% of the patients in PROTECT-AF showed a leakage >1 mm. Thus, oversizing of the device could help to avoid peri-device leakage and might thereby help to improve long-term safety. However, due to the relatively small number of patients in our registry compared to PROTECT-AF, there is a certain likelihood of an incorrect low number of peri-device leakages.
During the PROTECT-AF trial, 4.2% of the patients developed thrombi attached to the device. In three out of twenty of these patients this led to thromboembolic events; this equals 0.3 events per 100 patient years6. Our experience demonstrated that a change in the anticoagulant management during the first 45 days as performed in our registry did not lead to higher rates of thrombus formation.
However, thrombus formation does occur with the current generation of devices. One section of interest is the so-called threaded insert, i.e., the part with which the device is attached to the catheter before release. This part is believed to carry a particular thrombogenic potential. Therefore, in future devices this problem could be addressed by improved device designs and the use of less thrombogenic materials.
We discovered small recesses proximal to the entry of the LAA in a significant number of our patients. Before the era of transcatheter LAA closure these structures were of little interest. Now, they raise the question whether they have to be covered by the device. As they are anatomically part of the left atrium, the WATCHMAN® device does not cover these structures. The WATCHMAN® device is the only LAA closure system with a randomised comparative trial to oral anticoagulation6. Thus, the design with closure of the trabecular LAA but leaving the ridge towards the inferior pulmonary vein (often carrying the aforementioned recessus) uncovered is currently the only strategy that results in stroke protection similar to oral anticoagulation. During our follow-up period we detected no thrombi related to this structure, nor did we find higher levels of thromboembolic events in our patients compared to previously published data6,8.
A second discussion is ongoing regarding the triangle between the device and the upper ridge towards the pulmonary vein. This area was hypothesised to be the root of device-attached thrombi. In the three cases of thrombus formation on the device in our registry, the thrombus appeared at the centre part near the threaded insert of the device rather than at the upper ridge. Therefore, it appears unnecessary to cover this area in order to prevent ischaemic events in patients with atrial fibrillation.
With the availability of the new oral anticoagulants (dabigatran, rivaroxaban, apixaban), using one of these drugs for the first 45 days during endothelialisation of the device may prove to be an alternative strategy. To date this has not been tested but it will need to be validated.
Limitations
The limited number of patients and the lack of randomisation with a direct control group prevent definite conclusions being drawn from these data. In addition, the short follow-up period of six months limits the ability to conclude that LAA occlusion with the WATCHMAN® device can be effective in reducing ischaemic events. Regarding the likelihood of thrombus formation, we have a 90% confidence interval of 2.2-13.3%.
Conclusions
Dual antiplatelet therapy during the first 12 weeks after WATCHMAN® implantation demonstrates a similar rate of device-associated thrombus formation compared to warfarin therapy (PROTECT-AF) during this time. Given the high CHA2DS2-VASc and HAS-BLED scores in our population, the therapy appears safe and effective even though the registry included only 59 patients. In case of thrombus formation on the device, treatment with LMWH for at least four weeks with regular echocardiographic follow-up is recommended.
Oversizing of the device can reduce the need for repositioning and device change without an increased risk of pericardial effusions.
Leaving small recesses proximal to the entry of the LAA uncovered by the device does not lead to an elevated risk of thromboembolic events.
Conflict of interest statement
The authors have no conflicts of interest to declare.

