
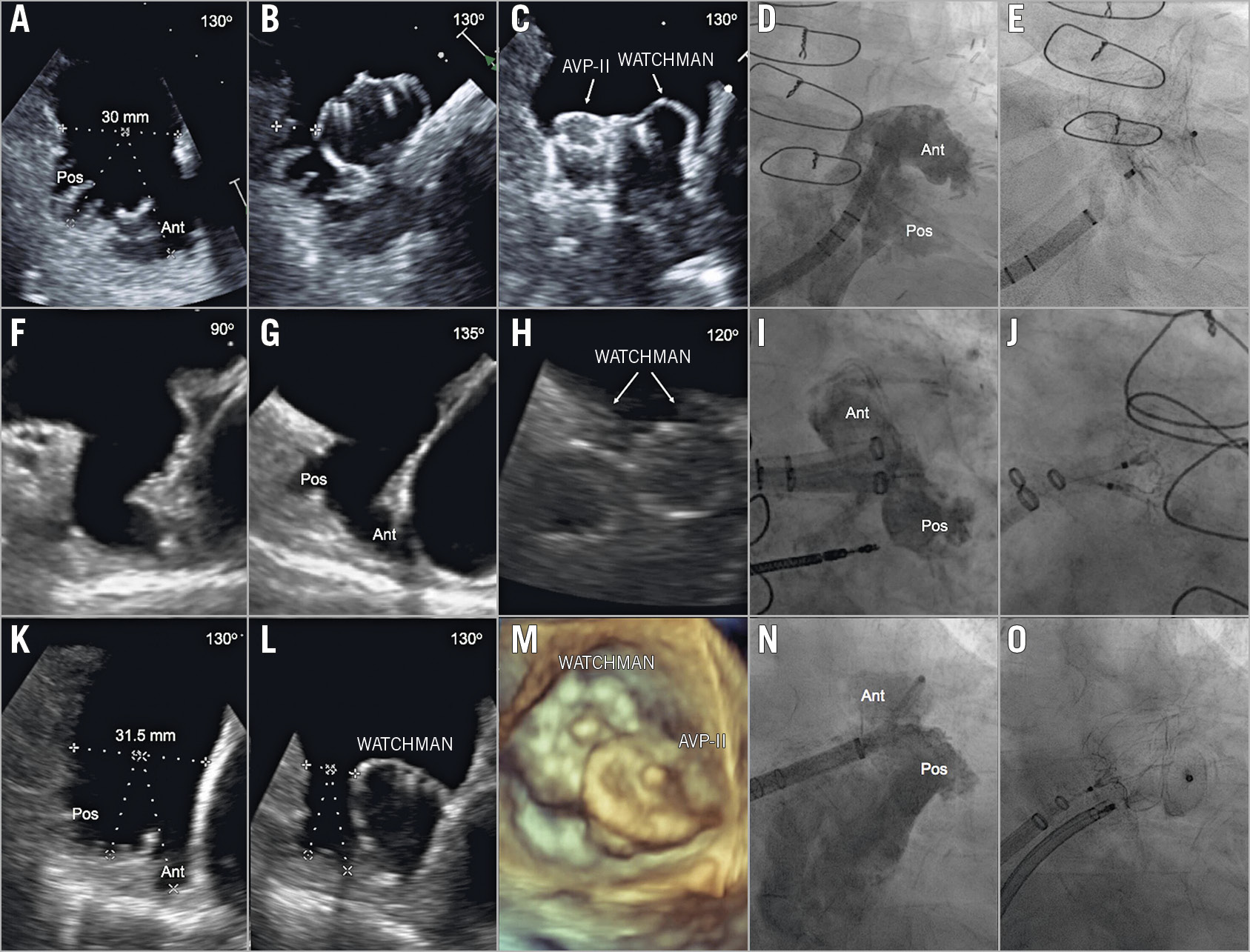
Figure 1. Case examples of double device closure in large or bilobar LAA anatomy. A) – E) Case 1. Sequential double device closure (WATCHMAN AVP II) for a large bilobar LAA. F) – J) Case 2. Simultaneous double device closure (two WATCHMANs) for a bilobar LAA with two discrete ostia. K) – O) Case 3. Simultaneous double device closure (WATCHMAN+AVP II) for a bilobar LAA. Ant: anterior; AVP: AMPLATZER Vascular Plug; Pos: posterior
The WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA) is the only approved left atrial appendage occlusion (LAAO) device in the USA1. Despite its good performance, this device suffers from important limitations in certain LAA anatomies. Double device closure has been reported for bilobar LAAs, but experience with this strategy remains limited2.
Among 319 consecutive patients undergoing LAAO, eight (2.5%) received double device closure (Figure 1). CHA2DS2-VASc score was 5.3±1.4 and HAS-BLED score was 3.0±1.5. The minimal and maximal orifice diameters were 20.8±6.5 mm and 26.7±6.7 mm, respectively. WATCHMAN was the first device in all patients, while the second device was the AMPLATZER™ Vascular Plug II (AVP II; St. Jude Medical, St. Paul, MN, USA) in seven, and a WATCHMAN device in one (simultaneous deployment in five patients, and sequential in three).
Procedural success was 100%, with no procedural mortality or major complications. Oral anticoagulation versus dual antiplatelets post LAAO was prescribed in three and five patients, respectively. Follow-up imaging revealed no peri-device thrombus, but two patients had peri-device leaks (2.4 mm, and 3.2 mm). During midterm follow-up (median=142, range=67-539 days), one patient died due to intracranial haemorrhage while on dual antiplatelets. No other major adverse events were observed.
Several technical issues are worth highlighting. 1) Although sequential device deployment was safe, caution should be exercised in utilising this technique especially in patients who do not have discrete lobes or prominent dividing trabeculae, in whom a significant interaction between the devices might occur. 2) Both WATCHMAN and AVP II have a nitinol frame and a polyethylene terephthalate fabric; however, the fabric in the AVP II is internal while it is external in the WATCHMAN. The long-term impact of this device combination on endothelialisation and thrombogenicity requires further investigation. 3) Only the WATCHMAN device is commercially available in the USA. It is possible that the availability of other occluders would limit the need for the two-device technique.
In conclusion, double device LAAO may provide a feasible alternative stroke prevention strategy for patients with challenging LAA anatomies. The long-term safety and efficacy of this technique remain to be assessed.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

