Introduction
Percutaneous left atrial appendage occlusion (LAAO) may be technically challenging in very large left atrial appendages (LAAs) that exceed the maximal dimensions that can be occluded with a single LAAO device. In this setting, a double device technique may be employed1234. This study assessed the safety and feasibility of a double LAmbre technique in patients with wide-ostium LAAs, including all cases currently reported to the manufacturer worldwide.
Methods
Seven patients undergoing LAAO with the double LAmbre technique between 2019 and 2020 at four international hospitals that perform ≥50 LAAO procedures per year were included. Three of the cases had been previously reported individually in scientific literature567, but this is the first international case series on double LAmbre. Inclusion criteria consisted of a landing zone (LZ) >32 mm and/or an ostium >40 mm on the largest dimension on preprocedural transoesophageal echocardiography (TEE) or cardiac computed tomography, which were confirmed at the beginning of the procedure. No patient was excluded because of a failed LAAO procedure. LAAO was performed in a single procedure, with simultaneous or consecutive device deployment and was guided by TEE or angiography alone, based on the operator’s experience.
Technical success was defined as complete LAA occlusion with no peri-device leak >5 mm on colour Doppler TEE and no device-related complications8. All patients were followed in the outpatient clinic for ≥3 months. Outcome variables were prospectively recorded at each site, according to the Munich consensus document on definitions and endpoints in LAAO8. These included device dislocation or embolisation, device-related thrombosis (DRT), peri-device leakage, pericardial effusion, acute kidney injury, stroke, systemic embolism, major bleeding (Bleeding Academic Research Consortium [BARC] types 3-5) and death.
For statistical analysis, categorical variables were expressed as frequencies and percentages, and continuous variables as median and interquartile range (IQR: 25-75).
The study protocol complied with the Declaration of Helsinki and all subjects provided informed consent prior to the procedure.
Results
Baseline clinical characteristics and procedural features of the seven patients in our case series are summarised in Table 1.
The median maximal LAA ostium and LZ diameters were 43 (41-48.4) mm and 38 (33-40) mm, respectively. Five (71.4%) procedures were guided by TEE under general anaesthesia, while 2 (28.6%) were performed with superficial sedation under fluoroscopic guidance alone, in a large-volume centre with extensive experience of performing LAAO without echocardiographic support. Procedural steps are described in the Central illustration and Moving image 1-Moving image 4. Procedural success was attained in all cases, with ≤1 device repositioning manoeuvres in 5 (71.4%) patients (Supplementary Figure 1). Although it would have been theoretically possible to seal 2 of the LAAs in our series with a single device (one with a 34 mm AMPLATZER Amulet [Abbott] and another with a 40 mm cover LAmbre [Lifetech Scientific]) (Figure 1), concerns about the feasibility and long-term results of this approach led to performance of a double device technique. No periprocedural or in-hospital complications occurred. Over a median follow-up period of 281 days (IQR: 92-515), one patient presented a BARC-3a haemorrhage and there was one non-cardiac death, resulting in 2 (28.6%) major adverse events. Follow-up TEE displayed a single case of minor (3 mm) peri-device leak in one patient; there were no device embolisations or DRT.
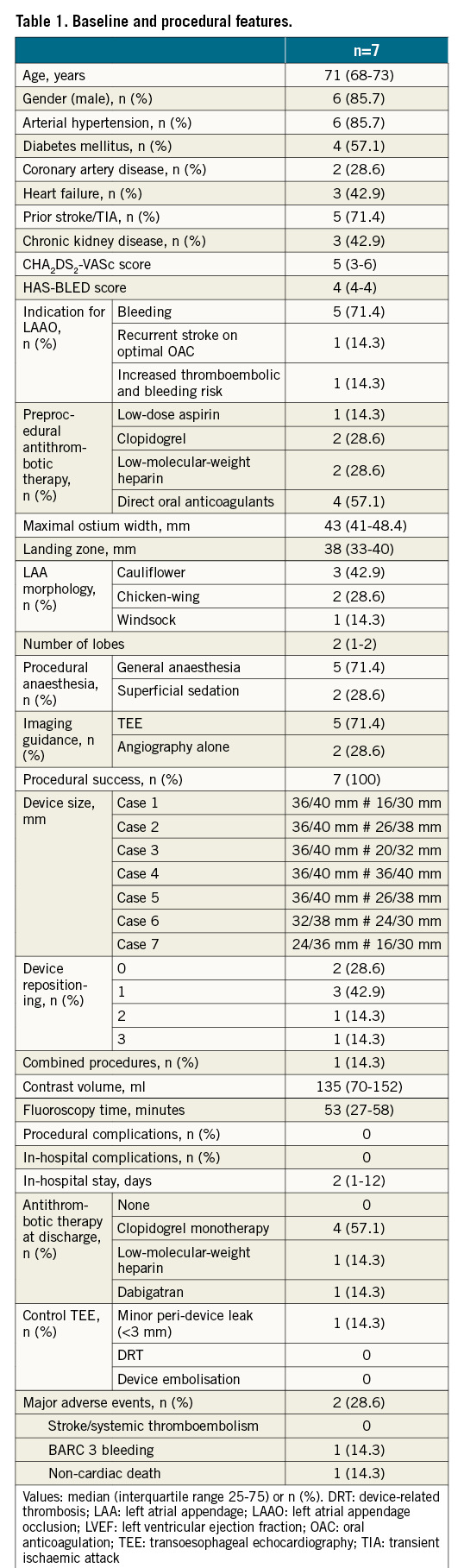
Central illustration. Procedural steps of LAAO employing the double LAmbre technique. Baseline LAA angiogram (A). Implantation of the first LAmbre device (size 24/36): the umbrella is implanted in the anterior portion of the LAA at the level of the landing zone (B), followed by subsequent deployment of the cover at the ostium level by unsheathing of the device (C & D). The delivery system of the first LAmbre device is exchanged for a 14 Fr steerable catheter over an extra-support guidewire located at the left superior pulmonary vein, allowing selective angiography of the posterior region of the LAA, still patent, by means of a 5 Fr multipurpose catheter (E). The delivery system of the second LAmbre is advanced through the steerable catheter over an extra-support guidewire (F) and the second LAmbre device (size 16/30) is deployed in a more posterior region of the LAA (G), achieving complete sealing of the LAA ostium (H). LAA: left atrial appendage; LAAO: left atrial appendage occlusion
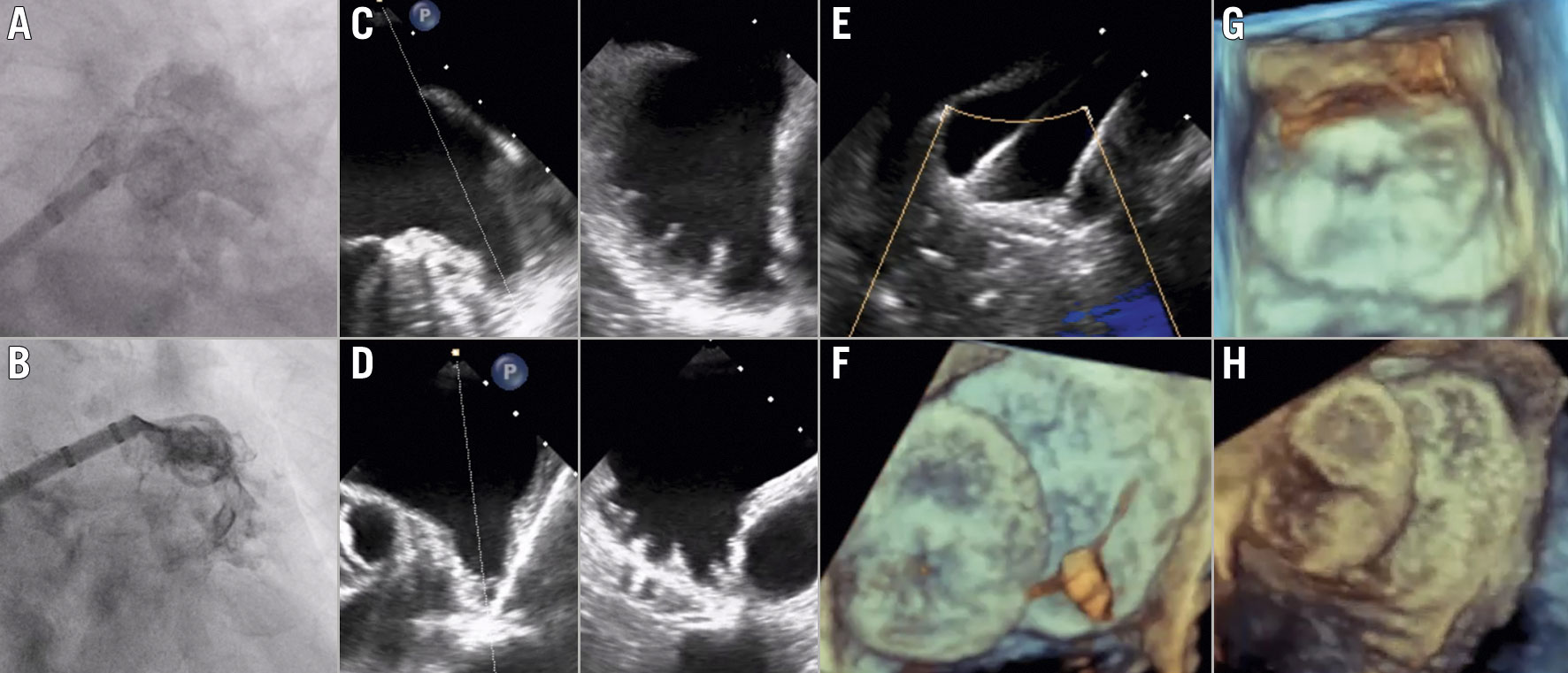
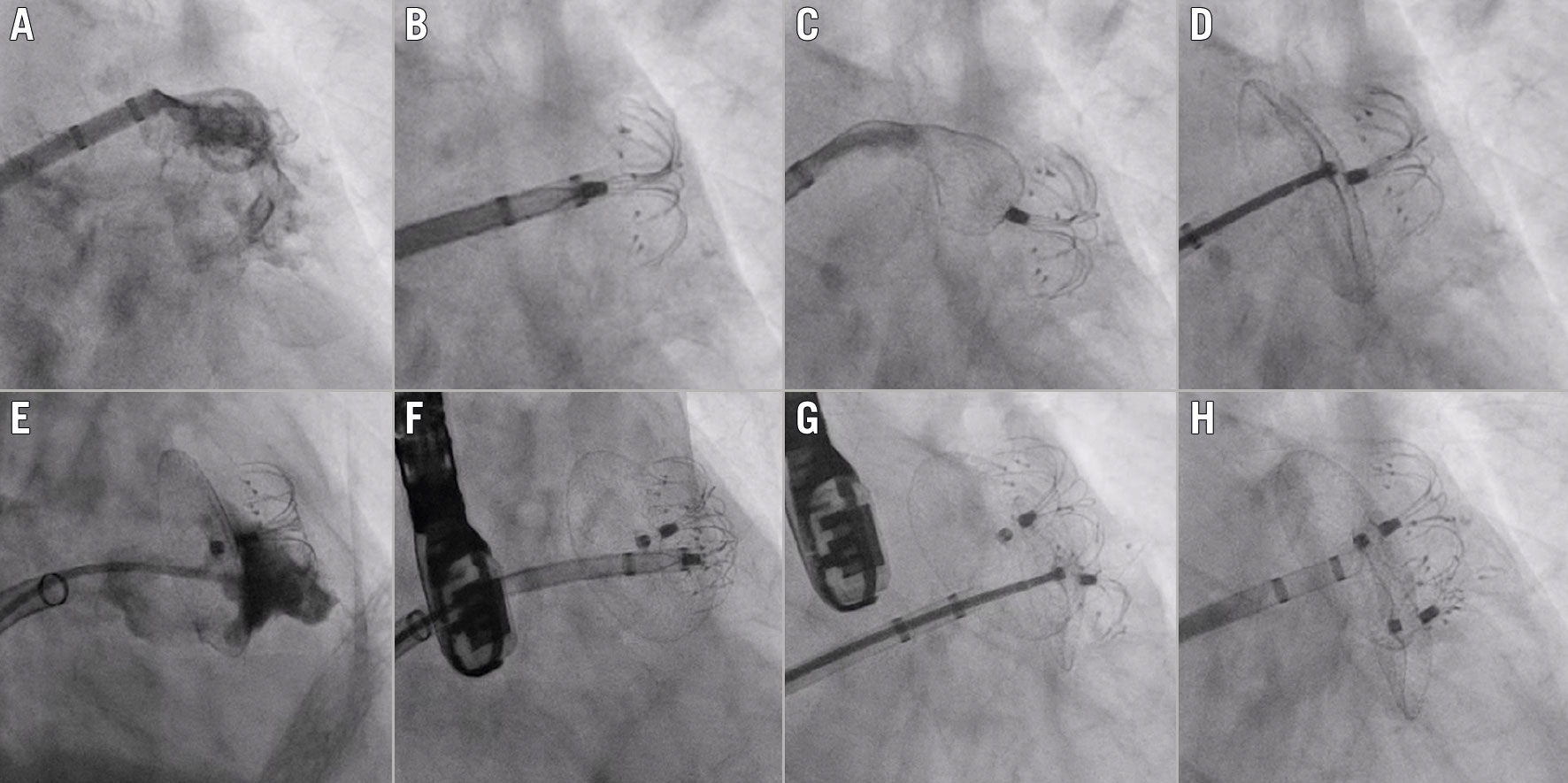
Figure 1. Procedural and follow-up multimodality imaging of LAAO employing the double LAmbre technique. Baseline left atrial appendage (LAA) angiograms (A & B) and preprocedural LAA anatomy on preprocedural transoesophageal echocardiography (TEE) of two patients in our case series (C & D). As depicted by intraprocedural TEE images, implantation of the two LAmbre devices was performed simultaneously in one case (E), while the other patient underwent consecutive device deployment (F). Follow-up TEE images of both cases depict complete LAA sealing with no device-related thrombus and no peri-device leaks (G & H).
Discussion
To the best of our knowledge, this is the first case series to assess the safety and feasibility of the double LAmbre technique in the largest LAAs that have undergone percutaneous LAAO described in scientific literature to date, well beyond the maximal dimensions that can be targeted with current dedicated devices.
In this study, LAAO with double LAmbre implantation was successful in all cases with no patient being excluded because of a failed LAAO procedure. No device dislodgement, embolisation or other periprocedural complications developed, supporting the safety of this technique. Favourable safety and efficacy outcomes were maintained over longer-term follow-up, with a single case of minor peri-device leak on control TEE and no systemic thromboembolisms.
A double device technique to attain complete sealing of very large LAAs has been previously reported with the AMPLATZER Cardiac Plug (ACP; Abbott)1 and WATCHMAN (Boston Scientific) devices23, but is subject to certain anatomical limitations, as detailed in Supplementary Table 1. In brief, adequate coupling of two ACP/Amulet devices can be demanding within single-lobe LAAs, given the composition of the device lobe, which is slightly more rigid than that of the LAmbre. As to the double WATCHMAN technique, it has been conceived for LAAs with well differentiated lobes that enable separate device implantation with minimal device interaction and it entails an inherent risk of residual flow between both devices, due to the absence of a proximal disc to seal the LAA ostium.
The double LAmbre technique offers several advantages over other double device techniques as it enables complete LAAO in shallow LAAs and provides complete sealing of the LAA ostium with the proximal device cover. Positioning of the umbrella in a sufficiently distal location within the LAA’s lobe to attain satisfactory device fixation, while also achieving optimal coupling of the umbrellas, can be particularly challenging in wide, shallow, single-lobed LAAs, but is facilitated by the highly flexible umbrella of the LAmbre as well as the compliant connection between the umbrella and cover. In addition, the fixation system, comprised of eight U-shaped claws and eight additional stabilisation hooks, provides great anchoring, thereby reducing the risk of device embolisation.
Another key step to attain success with the double LAmbre technique is selecting adequately sized devices. The umbrella size is selected based on the LZ diameter and, in general, a device with a larger umbrella will be deployed in combination with a device with a smaller umbrella. On the other hand, the size of the cover is calculated according to maximal ostial diameter, taking into account the degree of cover overlap, which is largely determined by the implantation location of the devices’ umbrellas within the LAA lobe(s). In procedures involving consecutive LAmbre deployment, the precise size of the cover of the second device will often be selected after positioning the first device. Besides, optimal device sizing is facilitated with the LAmbre, as it is available in numerous sizes which combine different measures of the umbrella and cover.
Nevertheless, the double LAmbre technique entails certain limitations. First, the covers of both devices might overlap on the left atrial side of the LAA ostium. The long-term impact of this disposition on endothelialisation and thrombogenicity requires further investigation, although no thrombi were reported during follow-up in our study. Second, use of the same transseptal puncture for deployment of two devices in cases undergoing simultaneous LAmbre implantation might condition the orientation and position of the second delivery sheath. Notwithstanding this, no patient required a second transseptal puncture in our sample.
Indeed, despite the encouraging results observed in this preliminary study, use of the double LAmbre technique remains an off-label indication beyond current clinical practice and should only be performed by highly experienced operators. Further larger-sized studies with longer follow-up periods are needed to establish firm conclusions on the safety, feasibility and clinical efficacy of the double LAmbre technique.
Limitations
The limited size of our sample, as well as the observational design of the study, entails the potential existence of confounding factors and selection and follow-up bias. Furthermore, we lacked a central core lab to assess imaging and procedural data and event adjudication was not crosschecked. Accordingly, definite conclusions regarding the clinical performance of LAAO with a double LAmbre technique cannot be established from this single case series.
Conclusions
LAAO with the double LAmbre technique appeared safe and feasible in this small sample of highly selected cases with very large LAAs. Longer-term safety and efficacy results in larger samples are required before widespread adoption of this technique.
Impact on daily practice
Left atrial appendage occlusion (LAAO) of very large left atrial appendages (LAAs) might prove technically challenging with a single dedicated device. LAAO with the double LAmbre technique is safe and technically feasible in very wide LAAs and provides complete sealing of the LAA, acutely and during follow-up, while effectively preventing thromboembolic events. LAAO with the double LAmbre technique offers technical advantages over a double WATCHMAN procedure in shallow and multilobe LAAs.
Conflict of interest statement
I. Cruz-González is a proctor for Lifetech Scientific (Shenzhen) Co., China, and was funded by ISCIII (PI19/00658), co-funded by ERDF, “A way to make Europe” and Gerencia Regional Salud de CyL (GRS 3031/A/19). X. Freixa is a proctor for Abbott Medical and Lifetech Scientific. Y-y. Lam is a clinical proctor for the LAmbre LAA closure system and a consultant for Lifetech Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Periprocedural transoesophageal echocardiography of Case 1, a patient with a large, shallow left atrial appendage that underwent left atrial appendage occlusion with the double LAmbre technique.
Moving image 2. Angiography imaging of Case 1 undergoing a double LAmbre procedure by means of consecutive device deployment through a single transseptal sheath.
Moving image 3. Periprocedural transoesophageal echocardiography of Case 2.
Moving image 4. Angiography imaging of Case 2 undergoing the double LAmbre procedure by means of simultaneous device deployment through two separate transseptal sheaths, advanced through a single transseptal puncture site.

